Co-Expression of Androgen Receptor and Cathepsin D Defines a Triple-Negative Breast Cancer Subgroup with Poorer Overall Survival
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Construction of Tissue Microarrays
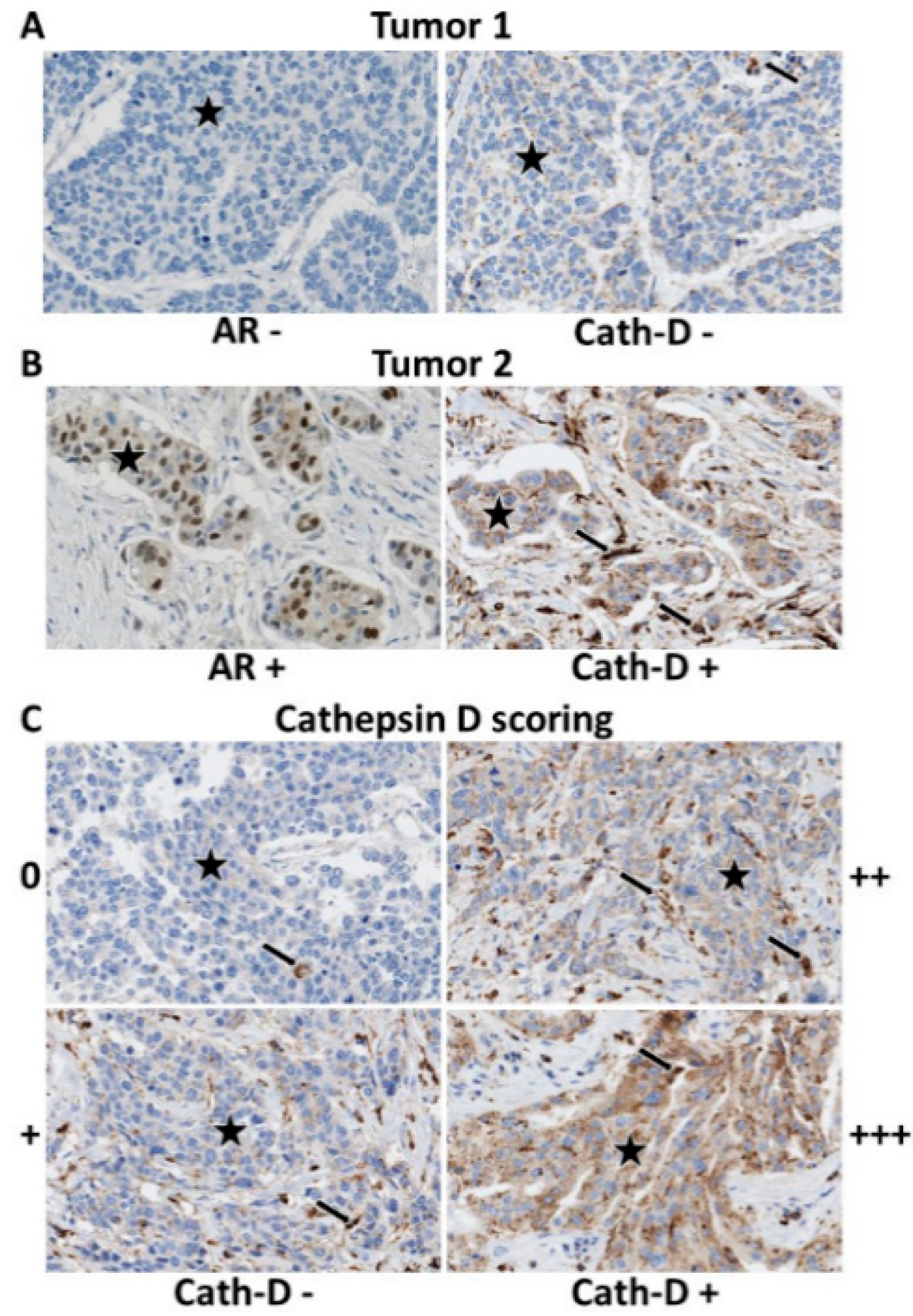
2.3. Tumor Microarray (TMA) Immunohistochemistry
2.4. Triple-Negative Breast Cancer (TNBC) Cell Lines, Enzalutamide Treatment, and Western Blotting
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Androgen Receptor (AR) Expression
3.3. AR and Cath-D Co-Expression
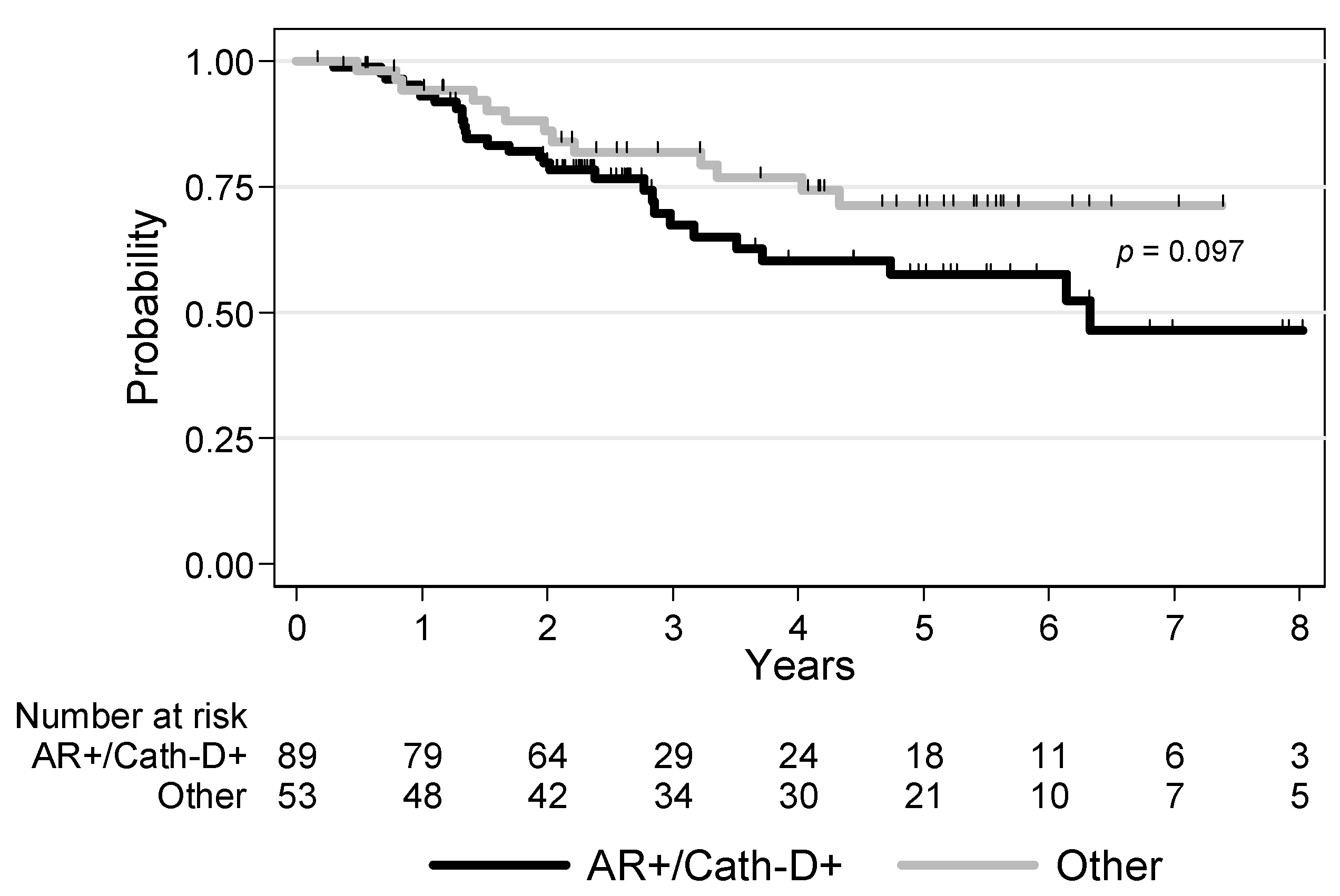
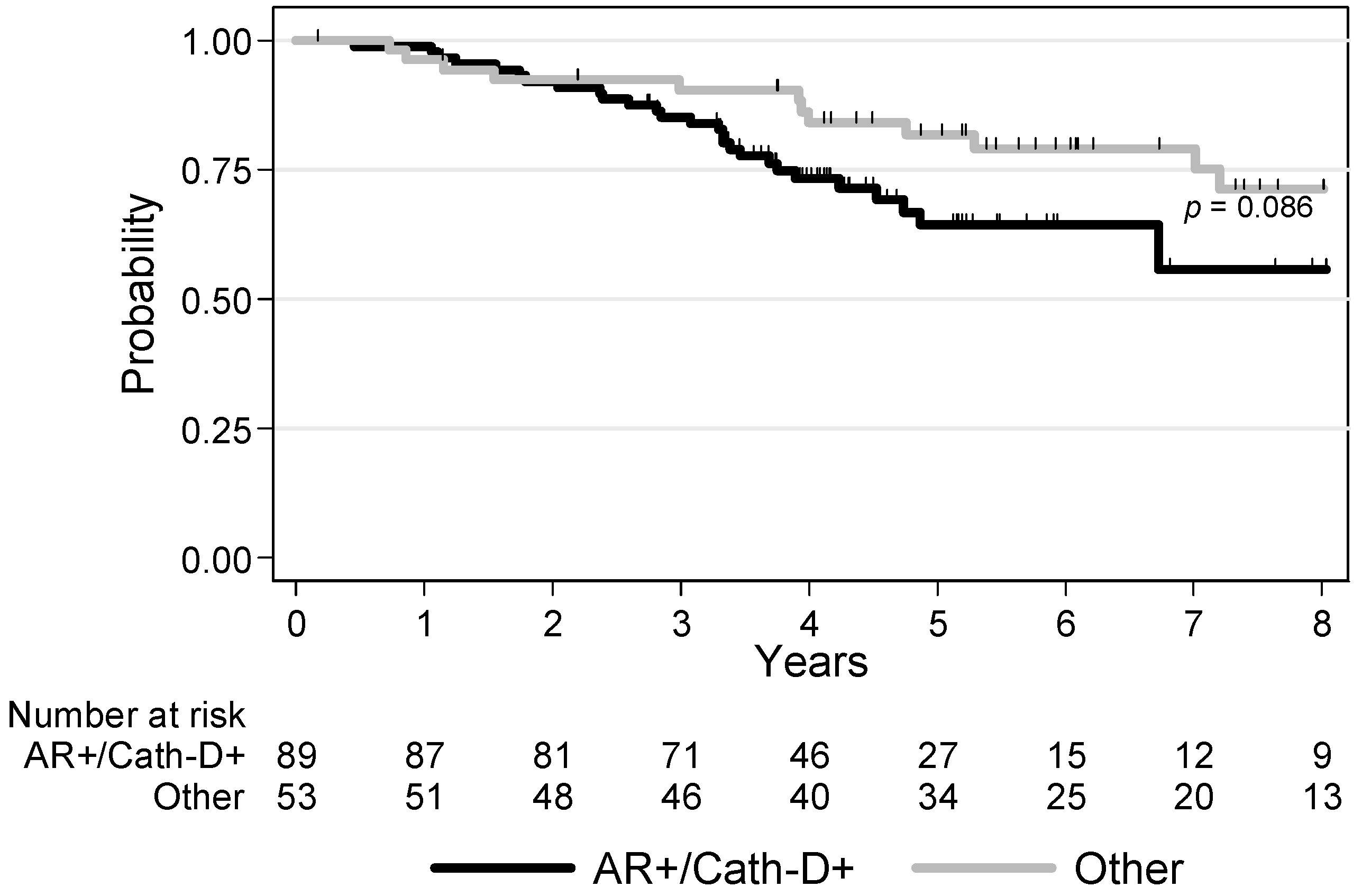
3.4. Survival Analyses
3.5. Effect of the AR Inhibitor Enzalutamide on Cath-D Expression and Secretion in TNBC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.M.; Yoda, T.; Takagi, K.; Miki, Y.; Suzuki, T.; Sasano, H. Androgen receptor in triple negative breast cancer. J. Steroid Biochem. Mol. Biol. 2013, 133, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Mao, Y.; Fei, X.C.; Shen, K.W. The impact of androgen receptor expression on breast cancer survival: A retrospective study and meta-analysis. PLoS ONE 2013, 8, e82650. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Klimov, S.; Mittal, K.; Krishnamurti, U.; Li, X.B.; Oprea-Ilies, G.; Wetherilt, C.S.; Riaz, A.; Aleskandarany, M.A.; Green, A.R.; et al. Prognostic Role of Androgen Receptor in Triple Negative Breast Cancer: A Multi-Institutional Study. Cancers 2019, 11, 995. [Google Scholar] [CrossRef]
- Kensler, K.H.; Poole, E.M.; Heng, Y.J.; Collins, L.C.; Glass, B.; Beck, A.H.; Hazra, A.; Rosner, B.A.; Eliassen, A.H.; Hankinson, S.E.; et al. Androgen Receptor Expression and Breast Cancer Survival: Results From the Nurses’ Health Studies. J. Natl. Cancer Inst. 2018. [Google Scholar] [CrossRef]
- Wang, C.; Pan, B.; Zhu, H.; Zhou, Y.; Mao, F.; Lin, Y.; Xu, Q.; Sun, Q. Prognostic value of androgen receptor in triple negative breast cancer: A meta-analysis. Oncotarget 2016, 7, 46482–46491. [Google Scholar] [CrossRef]
- Guiu, S.; Mollevi, C.; Charon-Barra, C.; Boissiere, F.; Crapez, E.; Chartron, E.; Lamy, P.J.; Gutowski, M.; Bourgier, C.; Romieu, G.; et al. Prognostic value of androgen receptor and FOXA1 co-expression in non-metastatic triple negative breast cancer and correlation with other biomarkers. Br. J. Cancer 2018, 119, 76–79. [Google Scholar] [CrossRef]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 884–890. [Google Scholar] [CrossRef]
- Bonnefoi, H.; Grellety, T.; Tredan, O.; Saghatchian, M.; Dalenc, F.; Mailliez, A.; L’Haridon, T.; Cottu, P.; Abadie-Lacourtoisie, S.; You, B.; et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1). Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2016, 27, 812–818. [Google Scholar] [CrossRef]
- Vignon, F.; Capony, F.; Chambon, M.; Freiss, G.; Garcia, M.; Rochefort, H. Autocrine growth stimulation of the MCF 7 breast cancer cells by the estrogen-regulated 52 K protein. Endocrinology 1986, 118, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Glondu, M.; Liaudet-Coopman, E.; Derocq, D.; Platet, N.; Rochefort, H.; Garcia, M. Down-regulation of cathepsin-D expression by antisense gene transfer inhibits tumor growth and experimental lung metastasis of human breast cancer cells. Oncogene 2002, 21, 5127–5134. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Benes, P.; Fusek, M. Procathepsin D in breast cancer: What do we know? Effects of ribozymes and other inhibitors. Cancer Gene Ther. 2002, 9, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Beaujouin, M.; Prebois, C.; Derocq, D.; Laurent-Matha, V.; Masson, O.; Pattingre, S.; Coopman, P.; Bettache, N.; Grossfield, J.; Hollingsworth, R.E.; et al. Pro-cathepsin D interacts with the extracellular domain of the beta chain of LRP1 and promotes LRP1-dependent fibroblast outgrowth. J. Cell Sci. 2010, 123, 3336–3346. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Matha, V.; Maruani-Herrmann, S.; Prebois, C.; Beaujouin, M.; Glondu, M.; Noel, A.; Alvarez-Gonzalez, M.L.; Blacher, S.; Coopman, P.; Baghdiguian, S.; et al. Catalytically inactive human cathepsin D triggers fibroblast invasive growth. J. Cell Biol. 2005, 168, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Berchem, G.; Glondu, M.; Gleizes, M.; Brouillet, J.P.; Vignon, F.; Garcia, M.; Liaudet-Coopman, E. Cathepsin-D affects multiple tumor progression steps in vivo: Proliferation, angiogenesis and apoptosis. Oncogene 2002, 21, 5951–5955. [Google Scholar] [CrossRef]
- Pranjol, M.Z.I.; Gutowski, N.J.; Hannemann, M.; Whatmore, J.L. Cathepsin D non-proteolytically induces proliferation and migration in human omental microvascular endothelial cells via activation of the ERK1/2 and PI3K/AKT pathways. Biochim. Et Biophys. Acta. Mol. Cell Res. 2018, 1865, 25–33. [Google Scholar] [CrossRef]
- Rochefort, H. Cathepsin D in breast cancer: A tissue marker associated with metastasis. Eur. J. Cancer 1992, 28A, 1780–1783. [Google Scholar] [CrossRef]
- Westley, B.R.; May, F.E. Prognostic value of cathepsin D in breast cancer. Br. J. Cancer 1999, 79, 189–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferrandina, G.; Scambia, G.; Bardelli, F.; Benedetti Panici, P.; Mancuso, S.; Messori, A. Relationship between cathepsin-D content and disease-free survival in node-negative breast cancer patients: A meta-analysis. Br. J. Cancer 1997, 76, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Foekens, J.A.; Look, M.P.; Bolt-de Vries, J.; Meijer-van Gelder, M.E.; van Putten, W.L.; Klijn, J.G. Cathepsin-D in primary breast cancer: Prognostic evaluation involving 2810 patients. Br. J. Cancer 1999, 79, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, Y.; Mansouri, H.; Laurent-Matha, V.; Alcaraz, L.B.; Roger, P.; Guiu, S.; Derocq, D.; Robin, G.; Michaud, H.A.; Delpech, H.; et al. Immunotherapy of triple-negative breast cancer with cathepsin D-targeting antibodies. J. Immunother. Cancer 2019, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Zhang, J.; Liang, G.; Ding, L.; He, Q.; Yang, B. Macrophage Polarization: Anti-Cancer Strategies to Target Tumor-Associated Macrophage in Breast Cancer. J. Cell. Biochem. 2017, 118, 2484–2501. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, J.; Karlsson, M.C. TGF-beta-induced epithelial-mesenchymal transition: A link between cancer and inflammation. Semin. Cancer Biol. 2012, 22, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qu, J.; Sun, Y.; Wang, J.; Liu, X.; Wang, F.; Zhang, H.; Wang, W.; Ma, X.; Gao, X.; et al. Prognostic significance of tumor-associated macrophages in breast cancer: A meta-analysis of the literature. Oncotarget 2017, 8, 30576–30586. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Miglietta, F.; Griguolo, G.; Guarneri, V.; Dieci, M.V. Programmed Cell Death Ligand 1 in Breast Cancer: Technical Aspects, Prognostic Implications, and Predictive Value. Oncologist 2019, 24, e1055–e1069. [Google Scholar] [CrossRef]
- Robinson, J.L.; Macarthur, S.; Ross-Innes, C.S.; Tilley, W.D.; Neal, D.E.; Mills, I.G.; Carroll, J.S. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J. 2011, 30, 3019–3027. [Google Scholar] [CrossRef]
- Cavailles, V.; Augereau, P.; Rochefort, H. Cathepsin D gene is controlled by a mixed promoter, and estrogens stimulate only TATA-dependent transcription in breast cancer cells. Proc. Natl. Acad. Sci. USA 1993, 90, 203–207. [Google Scholar] [CrossRef]
- Westley, B.R.; May, F.E. Oestrogen regulates cathepsin D mRNA levels in oestrogen responsive human breast cancer cells. Nucleic Acids Res. 1987, 15, 3773–3786. [Google Scholar] [CrossRef][Green Version]
- Dabrosin, C.; Johansson, A.C.; Ollinger, K. Decreased secretion of Cathepsin D in breast cancer in vivo by tamoxifen: Mediated by the mannose-6-phosphate/IGF-II receptor? Breast Cancer Res. Treat. 2004, 85, 229–238. [Google Scholar] [CrossRef]
- Coopman, P.; Garcia, M.; Brunner, N.; Derocq, D.; Clarke, R.; Rochefort, H. Anti-proliferative and anti-estrogenic effects of ICI 164,384 and ICI 182,780 in 4-OH-tamoxifen-resistant human breast-cancer cells. Int. J. Cancer 1994, 56, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Caiazza, F.; Murray, A.; Madden, S.F.; Synnott, N.C.; Ryan, E.J.; O’Donovan, N.; Crown, J.; Duffy, M.J. Preclinical evaluation of the AR inhibitor enzalutamide in triple-negative breast cancer cells. Endocr.-Relat. Cancer 2016, 23, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Barton, V.N.; Christenson, J.L.; Gordon, M.A.; Greene, L.I.; Rogers, T.J.; Butterfield, K.; Babbs, B.; Spoelstra, N.S.; D’Amato, N.C.; Elias, A.; et al. Androgen Receptor Supports an Anchorage-Independent, Cancer Stem Cell-like Population in Triple-Negative Breast Cancer. Cancer Res. 2017, 77, 3455–3466. [Google Scholar] [CrossRef] [PubMed]
- Bozovic-Spasojevic, I.; Zardavas, D.; Brohee, S.; Ameye, L.; Fumagalli, D.; Ades, F.; de Azambuja, E.; Bareche, Y.; Piccart, M.; Paesmans, M.; et al. The Prognostic Role of Androgen Receptor in Patients with Early-Stage Breast Cancer: A Meta-analysis of Clinical and Gene Expression Data. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 2702–2712. [Google Scholar] [CrossRef]
- Elebro, K.; Bendahl, P.O.; Jernstrom, H.; Borgquist, S. Androgen receptor expression and breast cancer mortality in a population-based prospective cohort. Breast Cancer Res. Treat. 2017, 165, 645–657. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Bianco-Miotto, T.; Jindal, S.; Butler, L.M.; Leung, S.; McNeil, C.M.; O’Toole, S.A.; Ebrahimie, E.; Millar, E.K.A.; Sakko, A.J.; et al. The Magnitude of Androgen Receptor Positivity in Breast Cancer Is Critical for Reliable Prediction of Disease Outcome. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 2328–2341. [Google Scholar] [CrossRef]
- Doane, A.S.; Danso, M.; Lal, P.; Donaton, M.; Zhang, L.; Hudis, C.; Gerald, W.L. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006, 25, 3994–4008. [Google Scholar] [CrossRef]
- Guerra, E.; Cimadamore, A.; Simeone, P.; Vacca, G.; Lattanzio, R.; Botti, G.; Gatta, V.; D’Aurora, M.; Simionati, B.; Piantelli, M.; et al. p53, cathepsin D, Bcl-2 are joint prognostic indicators of breast cancer metastatic spreading. BMC Cancer 2016, 16, 649. [Google Scholar] [CrossRef]
- Cavailles, V.; Garcia, M.; Rochefort, H. Regulation of cathepsin-D and pS2 gene expression by growth factors in MCF7 human breast cancer cells. Mol. Endocrinol. 1989, 3, 552–558. [Google Scholar] [CrossRef]
- Augereau, P.; Miralles, F.; Cavailles, V.; Gaudelet, C.; Parker, M.; Rochefort, H. Characterization of the proximal estrogen-responsive element of human cathepsin D gene. Mol. Endocrinol. 1994, 8, 693–703. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacobson-Raber, G.; Lazarev, I.; Novack, V.; Mermershtein, W.; Baumfeld, Y.; Geffen, D.B.; Sion-Vardy, N.; Ariad, S. The prognostic importance of cathepsin D and E-cadherin in early breast cancer: A single-institution experience. Oncol. Lett. 2011, 2, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Markicevic, M.; Kanjer, K.; Mandusic, V.; Buta, M.; Neskovic-Konstantinovic, Z.; Nikolic-Vukosavljevic, D. Cathepsin D as an indicator of clinical outcome in early breast carcinoma during the first 3 years of follow-up. Biomark. Med. 2013, 7, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Mazouni, C.; Fina, F.; Romain, S.; Ouafik, L.; Bonnier, P.; Brandone, J.M.; Martin, P.M. Epstein-Barr virus as a marker of biological aggressiveness in breast cancer. Br. J. Cancer 2011, 104, 332–337. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Z.; Chen, S.; Liu, Y.; Shao, Z. A prognostic model for triple-negative breast cancer patients based on node status, cathepsin-D and Ki-67 index. PLoS ONE 2013, 8, e83081. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Vangala, G.; Imhoff, F.M.; Squires, C.M.L.; Cridge, A.G.; Baird, S.K. Mesenchymal stem cell homing towards cancer cells is increased by enzyme activity of cathepsin D. Exp. Cell Res. 2019, 383, 111494. [Google Scholar] [CrossRef]
- Gui, P.; Ben-Neji, M.; Belozertseva, E.; Dalenc, F.; Franchet, C.; Gilhodes, J.; Labrousse, A.; Bellard, E.; Golzio, M.; Poincloux, R.; et al. The Protease-Dependent Mesenchymal Migration of Tumor-Associated Macrophages as a Target in Cancer Immunotherapy. Cancer Immunol. Res. 2018, 6, 1337–1351. [Google Scholar] [CrossRef]
- Kwilas, A.R.; Ardiani, A.; Gameiro, S.R.; Richards, J.; Hall, A.B.; Hodge, J.W. Androgen deprivation therapy sensitizes triple negative breast cancer cells to immune-mediated lysis through androgen receptor independent modulation of osteoprotegerin. Oncotarget 2016, 7, 23498–23511. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, F.; Huang, D.; Guan, X. Androgen blockade based clinical trials landscape in triple negative breast cancer. Biochim. Et Biophys. Acta. Rev. Cancer 2018, 1870, 283–290. [Google Scholar] [CrossRef]

| Clinical and Tumor Characteristics | Total Population n = 147 | AR+ TNBC n = 107 (72.8%) | AR− TNBC n = 40 (27.2%) | p Value |
|---|---|---|---|---|
| Age (years), median [min–max] <55 years ≥55 years | 61.6 [30.2–98.6] 50 (34.0%) 97 (66.0%) | 61.4 [30.2–98.6] 36 (33.6%) 71 (66.4%) | 63.2 [30.8–86.3] 14 (35.0%) 26 (65.0%) | 0.877 |
| Tumor size T1 T2 T3/T4 | 52 (35.4%) 78 (53.1%) 17 (11.5%) | 44 (41.1%) 53 (49.5%) 10 (9.4%) | 8 (20.0%) 25 (62.5%) 7 (17.5%) | 0.044 |
| Node status N− N+ | 90 (61.2%) 57 (38.8%) | 60 (56.1%) 47 (47.9%) | 30 (75.0%) 10 (25.0%) | 0.036 |
| Histological grade (SBR) 1–2 3 | 16 (11.0%) 129 (89.0%) | 15 (14.1%) 91 (85.9%) | 1 (2.6%) 38 (97.4%) | 0.048 |
| Histology Ductal Lobular Other | 124 (86.2%) 10 (6.9%) 10 (6.9%) | 89 (85.6%) 9 (8.7%) 6 (5.7%) | 35 (87.5%) 1 (2.5%) 4 (10.0%) | 0.312 |
| Adjuvant chemotherapy No Yes | 47 (32.0%) 100 (68.0%) | 34 (31.8%) 73 (68.2%) | 13 (32.5%) 27 (67.5%) | 0.933 |
| Basal-like phenotype Yes No | 90 (61.6%) 56 (38.4%) | 64 (60.4%) 42 (39.6%) | 26 (65.0%) 14 (35.0%) | 0.608 |
| TIL density [0–2] 3 | 105 (74.5%) 36 (25.5%) | 73 (73.3%) 28 (27.7%) | 32 (80.0%) 8 (20.0%) | 0.343 |
| PDL-1 expression in tumor cells <1% ≥1% | 45 (33.1%) 91 (66.9%) | 28 (29.2%) 68 (70.8%) | 17 (42.5%) 23 (57.5%) | 0.132 |
| PD-1 expression in TILs 0 1 2 3 | 18 (13.0%) 29 (20.9%) 74 (53.1%) 18 (13.0%) | 13 (13.0%) 20 (20.0%) 58 (58.0%) 9 (9.0%) | 5 (12.8%) 9 (23.1%) 16 (41.0%) 9 (23.1%) | 0.115 |
| Cath-D expression in tumor cells No (score 0/+) Yes (score ++/+++) | 24 (16.9%) 118 (83.1%) | 13 (12.7%) 89 (87.3%) | 11 (27.5%) 29 (72.5%) | 0.035 |
| Macrophages (inflammation) 0/+1 2 3 | 25 (17.6%) 44 (31.0%) 73 (51.4%) | 18 (17.5%) 38 (36.9%) 47 (45.6%) | 7 (18.0%) 6 (15.3%) 26 (66.7%) | 0.036 |
| Clinical and Tumor Characteristics | Total Sample n = 147 | AR+/Cath-D+ TNBC n = 89 (62.7%) | Other TNBC Types n = 53 (37.3%) | p Value |
|---|---|---|---|---|
| Age (years), median [min–max] <55 years ≥55 years | 61.6 [30.2–98.6] 50 (34.0%) 97 (66.0%) | 61.6 [30.2–98.6] 28 (31.5%) 61 (68.5%) | 60.7 [30.8–86.3] 20 (37.7%) 33 (62.3%) | 0.445 |
| Tumor size T1 T2 T3/T4 | 52 (35.4%) 78 (53.1%) 17 (11.5%) | 37 (41.6%) 44 (49.4%) 8 (9.0%) | 13 (24.5%) 32 (6.4%) 8 (15.1%) | 0.101 |
| Node status N− N+ | 90 (61.2%) 57 (38.8%) | 48 (53.9%) 41 (46.1%) | 38 (71.7%) 15 (28.3%) | 0.036 |
| Histological grade (SBR) 1–2 3 | 16 (11.0%) 129 (89.0%) | 13 (13.6%) 76 (86.4%) | 2 (3.8%) 50 (96.2%) | 0.062 |
| Histology Ductal Lobular Other | 124 (86.2%) 10 (6.9%) 10 (6.9%) | 75 (86.2%) 6 (6.9%) 6 (6.9%) | 45 (86.5%) 3 (5.8%) 4 (7.7%) | 0.955 |
| Adjuvant chemotherapy No Yes | 47 (32.0%) 100 (68.0%) | 28 (31.5%) 61 (68.5%) | 17 (32.1%) 36 (67.9%) | 0.939 |
| Basal-like phenotype Yes No | 90 (61.6%) 56 (38.4%) | 53 (60.2%) 35 (39.8%) | 35 (66.0%) 18 (34.0%) | 0.490 |
| TIL density [0–2] 3 | 105 (74.5%) 36 (25.5%) | 65 (76.5%) 20 (23.5%) | 39 (73.6%) 14 (26.4%) | 0.702 |
| PDL-1 expression in tumor cells <1% ≥1% | 45 (33.1%) 91 (66.9%) | 23 (28.1%) 59 (71.9%) | 21 (39.6%) 32 (60.4%) | 0.161 |
| PD-1 expression in TILs 0 1 2 3 | 18 (13.0%) 29 (20.9%) 74 (53.1%) 18 (13.0%) | 12 (14.3%) 16 (19.0%) 47 (56.0%) 9 (10.7%) | 5 (9.6%) 12 (23.1%) 26 (50.0%) 9 (17.3%) | 0.556 |
| Macrophages 0/+1 2 3 | 25 (17.6%) 44 (31.0%) 73 (51.4%) | 15 (17.4%) 32 (37.2%) 39 (45.4%) | 9 (17.7%) 9 (17.7%) 33 (64.6%) | 0.041 |
| Clinical and Tumor Characteristics | Univariate Analysis HR 95% CI | Multivariate Analysis HR 95% CI |
|---|---|---|
| Age <55 years ≥55 years | 1 1.68 [0.83–3.41] p = 0.138 | |
| Tumor size T1 T2 T3/T4 | 1 1.66 [0.74–3.74] 4.69 [1.88–11.7] p = 0.004 | 1.47 [0.63–3.40] 3.34 [1.20–9.28] p = 0.056 |
| Node status N− N+ | 1 2.67 [1.43–4.98] p = 0.002 | 1 1.90 [0.94–3.85] p = 0.074 |
| Histological grade (SBR) 1–2 3 | 1 0.80 [0.36–1.82] p = 1.82 | |
| Histology Ductal Lobular Other | 1 1.50 [0.59–3.84] 0.39 [0.05–2.83] p = 0.371 | |
| Adjuvant chemotherapy No Yes | 1 0.41 [0.22–0.75] p = 0.005 | 1 0.40 [0.21–0.74] p = 0.004 |
| Basal-like phenotype Yes No | 1 1.63 [0.89–2.98] p = 0.117 | |
| AR AR+ AR− | 1 0.57 [0.27–1.20] p = 0.122 | |
| AR/Cath-D co-expression AR+/Cath-D+ Other profiles | 1 0.58 [0.30–1.12] p = 0.097 | 1 0.56 [0.28–1.12] p = 0.093 |
| TIL density [0–2] 3 | 1 0.46 [0.19–1.09] p = 0.054 | |
| PDL-1 expression in tumor cells <1% ≥1% | 1 0.74 [0.39–1.40] p = 0.360 | |
| PD-1 expression in TILs 0 1 2 3 | 1 1.30 [0.41–4.08] 1.23 [0.42–3.57] 0.80 [0.2–3.20] p = 0.821 | |
| Cath-D expression in tumor cells No (score 0/+) Yes (score ++/+++) | 1 1.44 [0.61–3.43] p = 0.386 | |
| Macrophages 0/+1 2 3 | 1 1.98 [0.78–4.97] 1.09 [0.43–2.74] p = 0.148 |
| Clinical and Tumor Characteristics | Univariate Analysis HR 95% CI | Multivariate Analysis HR 95% CI |
|---|---|---|
| Age <55 years ≥55 years | 1 2.52 [1.12–5.66] p = 0.013 | |
| Tumor size T1 T2 T3/T4 | 1 3.02 [1.16–7.86] 6.17 [2.11–18.0] p = 0.002 | 1 2.31 [0,86–6.23] 4.61 [1.46–14.5] p = 0.024 |
| Node status N− N+ | 1 2.38 [1.31–4.33] p = 0.004 | 1 1.82 [0.92–3.58] p = 0.082 |
| Histological grade (SBR) 1–2 3 | 1 1.05 [0.44–2.88] p = 0.920 | |
| Histology Ductal Lobular Other | 1 0.70 [0.21–2.27] 0.88 [0.27–2.85] p = 0.810 | |
| Adjuvant chemotherapy No Yes | 1 0.34 [0.19–0.62] p = 0.004 | 1 0.29 [0.16–0.55] p < 0.001 |
| Basal-like phenotype Yes No | 1 1.20 [0.67–2.17] p = 0.541 | |
| AR AR+ AR− | 1 0.62 [0.31–1.24] p = 0.163 | |
| AR/Cath-D co-expression AR+/Cath-D+ Other profiles | 1 0.58 [0.31–1.09] p = 0.086 | 1 0.49 [0.25–0.96] p = 0.034 |
| TIL density [0–2] 3 | 1 0.54 [0.24–1.21] p = 0.111 | |
| PDL-1 expression in tumor cells <1% ≥1% | 1 0.91 [0.49–1.70] p = 0.770 | |
| PD-1 expression in TILs 0 1 2 3 | 1 0.69 [0.25–1.95] 1.05 [0.43–2.57] 0.72 [0.22–2.38] p = 0.689 | |
| Cath-D expression in tumor cells No (score 0/+) Yes (score ++/+++) | 1 1.28 [0.57–2.88] p = 0.534 | |
| Macrophages 0/+1 2 3 | 1 1.12 [0.51–2.44] 0.64 [0.30–1.38] p = 0.219 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansouri, H.; Alcaraz, L.B.; Mollevi, C.; Mallavialle, A.; Jacot, W.; Boissière-Michot, F.; Simony-Lafontaine, J.; Laurent-Matha, V.; Roger, P.; Liaudet-Coopman, E.; et al. Co-Expression of Androgen Receptor and Cathepsin D Defines a Triple-Negative Breast Cancer Subgroup with Poorer Overall Survival. Cancers 2020, 12, 1244. https://doi.org/10.3390/cancers12051244
Mansouri H, Alcaraz LB, Mollevi C, Mallavialle A, Jacot W, Boissière-Michot F, Simony-Lafontaine J, Laurent-Matha V, Roger P, Liaudet-Coopman E, et al. Co-Expression of Androgen Receptor and Cathepsin D Defines a Triple-Negative Breast Cancer Subgroup with Poorer Overall Survival. Cancers. 2020; 12(5):1244. https://doi.org/10.3390/cancers12051244
Chicago/Turabian StyleMansouri, Hanane, Lindsay B. Alcaraz, Caroline Mollevi, Aude Mallavialle, William Jacot, Florence Boissière-Michot, Joelle Simony-Lafontaine, Valérie Laurent-Matha, Pascal Roger, Emmanuelle Liaudet-Coopman, and et al. 2020. "Co-Expression of Androgen Receptor and Cathepsin D Defines a Triple-Negative Breast Cancer Subgroup with Poorer Overall Survival" Cancers 12, no. 5: 1244. https://doi.org/10.3390/cancers12051244
APA StyleMansouri, H., Alcaraz, L. B., Mollevi, C., Mallavialle, A., Jacot, W., Boissière-Michot, F., Simony-Lafontaine, J., Laurent-Matha, V., Roger, P., Liaudet-Coopman, E., & Guiu, S. (2020). Co-Expression of Androgen Receptor and Cathepsin D Defines a Triple-Negative Breast Cancer Subgroup with Poorer Overall Survival. Cancers, 12(5), 1244. https://doi.org/10.3390/cancers12051244






