Sorafenib for the Treatment of Unresectable Hepatocellular Carcinoma: Preliminary Toxicity and Activity Data in Dogs
Abstract
:1. Introduction
2. Results
2.1. Dogs and Tumor Characteristics
2.2. Treatment and Toxicity
2.3. Response Rate and Outcome
3. Discussion
4. Material and Methods
4.1. Inclusion and Exclusion Criteria
4.2. Treatment Protocol
4.3. Safety Monitoring and Efficacy of Treatment
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Patnaik, A.K.; Hurvitz, A.I.; Lieberman, P.H.; Johnson, G.F. Canine hepatocellular carcinoma. Vet. Pathol. 1981, 18, 427–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liptak, J.M.; Dernell, W.S.; Monnet, E.; Powers, B.E.; Bachand, A.M.; Kenney, J.G.; Withrow, S.J. Massive hepatocellular carcinoma in dogs: 48 cases, (1992–2002). Am. Vet. Med. Assoc. 2004, 225, 1225–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosovsky, J.E.; Matthiesen, D.T. Results of partial hepatectomy in 18 dogs with hepatocellular, carcinoma. J. Am. Anim. Hosp. Assoc. 1989, 25, 203–206. [Google Scholar]
- Marin, J.J.; Romero, M.R.; Briz, O. Molecular bases of liver cancer refractoriness to pharmacological, treatment. Curr. Med. Chem. 2010, 17, 709–740. [Google Scholar] [CrossRef] [PubMed]
- Elpiner, A.K.; Brodsky, E.M.; Hazzah, T.N.; Post, G.S. Single-agent gemcitabine chemotherapy in dogs with hepatocellular carcinomas. Vet. Comp. Oncol. 2011, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, P.A.; Dervisis, N.G.; Cadile, C.D.; Sarbu, L.; Kitchell, B.E. Combined gemcitabine carboplatin therapy for carcinomas in dogs. J. Vet. Intern. Med. 2009, 23, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kareva, I.; Waxman, D.L.; Klement, G.L. Metronomic chemotherapy: An attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity minimize therapeutic resistance. Cancer Lett. 2015, 358, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Lana, S.; U’ren, L.; Plaza, S.; Elmslie, R.; Gustafson, D.; Morley, P.; Dow, S. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in, dogs. Vet. Intern. Med. 2007, 21, 764–769. [Google Scholar] [CrossRef]
- Elmslie, R.E.; Glawe, P.; Dow, S.W. Metronomic therapy with cyclophosphamide piroxicam effectively delays tumor recurrence in dogs with incompletely resected soft tissue sarcomas. J. Vet. Intern. Med. 2008, 22, 1373–1379. [Google Scholar] [CrossRef]
- London, C.A.; Gardner, H.L.; Mathie, T.; Stingle, N.; Portela, R.; Pennell, M.L.; Clifford, C.A.; Rosenberg, M.P.; Vail, D.M.; Williams, L.E.; et al. Impact of toceranib/piroxicam/cyclophosphamide maintenance therapy on outcome of dogs with appendicular osteosarcoma following amputation carboplatin chemotherapy: A multi-institutional, study. PLoS ONE 2015, 10, e0124889. [Google Scholar] [CrossRef]
- Finotello, R.; Henriques, J.; Sabattini, S.; Stefanello, D.; Felisberto, R.; Pizzoni, S.; Ferrari, R.; Marconato, L. A retrospective analysis of chemotherapy switch suggests improved outcome in surgically removed biologically aggressive canine haemangiosarcoma. Vet. Comp. Oncol. 2017, 15, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.P.; Orbell, G.; Cave, N.; Munday, J.S. Does thalidomide prolong survival in dogs with splenic haemangiosarcoma? Small Anim. Pract. 2018, 59, 85–91. [Google Scholar] [CrossRef] [PubMed]
- de Campos, C.B.; Lavalle, G.E.; Fialho Ligório, S.; Camargo Nunes, F.; Carneiro, R.A.; Amorim, R.L.; Cassali, G.D. Absence of significant adverse events following thalidomide administration in bitches diagnosed with mammary gland carcinomas. Vet. Rec. 2016, 179, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancedda, S.; Marconato, L.; Meier, V.; Laganga, P.; Roos, M.; Leone, V.F.; Rossi, F.; Bley, C.R. Hypofractionated radiotherapy for macroscopic canine soft tissue sarcoma: A retrospective study of 50 cases treated with a 5x6 Gy protocol with or without metronomic chemotherapy. Vet. Radiol. Ultrasound 2016, 57, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polton, G.; Finotello, R.; Sabattini, S.; Rossi FLaganga, P.; Vasconi, M.E.; Barbanera, A.; Stiborova, K.; Rohrer Bley, C.; Marconato, L. Survival analysis of dogs with advanced primary lung carcinoma treated by metronomic cyclophosphamide piroxicam thalidomide. Vet. Comp. Oncol. 2018, 16, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- De Matteis, S.; Ragusa, A.; Marisi, G.; De Domenico, S.; Gardini, A.C.; Bonafè, M.; Giudetti, A.M. Abberant metabolism in hepatocellular carcinoma provides diagnostic therapeutic, opportunities. Oxid. Med. Cell Longev. 2018, 2018, 7512159. [Google Scholar] [CrossRef] [Green Version]
- Casadei-Gardini, A.; Del Coco, L.; Marisi, G.; Conti, F.; Rovesti, G.; Ulivi, P.; Canale, M.; Frassineti, G.L.; Foschi, F.G.; Longo, S.; et al. 1H-NMR based serum metabolomics highlights different specific biomarkers between early advanced hepatocellular carcinoma, stages. Cancers (Basel) 2020, 12, 241. [Google Scholar] [CrossRef] [Green Version]
- De Matteis, S.; Scarpi, E.; Granato, A.M.; Vespasiani-Gentilucci, U.; La Barba, G.; Foschi, F.G.; Bandini, E.; Ghetti, M.; Marisi, G.; Cravero, P.; et al. Role of SIRT-3, p-mTOR and HIF-1α in Hepatocellular carcinoma patients affected by metabolic dysfunctions and in chronic treatment with metformin. Int. J. Mol. Sci. 2019, 20, 1503. [Google Scholar] [CrossRef] [Green Version]
- Fiume, L.; Manerba, M.; Vettraino, M.; Di Stefano, G. Effect of sorafenib on the energy metabolism of hepatocellular carcinoma cells. Eur. J. Pharmacol. 2011, 670, 39–43. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, Y.K.; Park, S.Y.; Jang, S.Y.; Lee, J.Y.; Ham, H.J.; Kim, B.G.; Jeon, H.J.; Kim, J.H.; Kim, J.G.; et al. PPARδ reprograms glutamine metabolism in sorafenib-resistant HCC. Mol. Cancer Res. 2017, 15, 1230–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassim, S.; Raymond, V.A.; Lacoste, B.; Lapierre, P.; Bilodeau, M. Metabolite profiling identifies a signature of tumorigenicity in hepatocellular carcinoma. Oncotarget 2018, 9, 26868–26883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strumberg, D.; Clark, J.W.; Awada, A.; Moore, M.J.; Richly, H.; Hendlisz, A.; Hirte, H.W.; Eder, J.P.; Lenz, H.J.; Schwartz, B. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: A review of four phase I trials in patients with advanced refractory solid tumors. Oncologist 2007, 12, 426–437. [Google Scholar] [CrossRef] [Green Version]
- Wolfesberger, B.; Tonar, Z.; Gerner, W.; Skalicky, M.; Heiduschka, G.; Egerbacher, M.; Thalhammer, J.G.; Walter, I. Pharmacologic inhibition of MEK signaling prevents growth of canine hemangiosarcoma. Mol. Cancer Ther. 2013, 12, 1701–1714. [Google Scholar]
- Wolfesberger, B.; Tonar, Z.; Gerner, W.; Skalicky, M.; Heiduschka, G.; Egerbacher, M.; Thalhammer, J.G.; Walter, I. The tyrosine kinase inhibitor sorafenib decreases cell number and induces apoptosis in a canine osteosarcoma cell line. Res. Vet. Sci 2010, 88, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Chien, D.S. BAY 43-9006: Preclinical data. Curr. Pharm. Des. 2002, 8, 2255–2257. [Google Scholar] [CrossRef] [PubMed]
- European Medical Agency [Webpage on the Internet]. Initial Marketing Authorization Study of Nexavar. 2007. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000690/human_med_000929.jsp&mid=WC0b01ac058001d124 (accessed on 4 March 2017).
- Foskett, A.; Manley, C.; Naramore, R.; Gordon, I.K.; Stewart, B.M.; Khanna, C. Tolerability of oral sorafenib in pet dogs with a diagnosis of cancer. Vet. Med. (Auckl.) 2017, 8, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Casadei Gardini, A.; Foca, F.; Scartozzi, M.; Silvestris, N.; Tamburini, E.; Faloppi, L.; Brunetti, O.; Rudnas, B.; Pisconti, S.; Valgiusti, M.; et al. Metronomic capecitabine versus best supportive care as second-line treatment in hepatocellular carcinoma: A retrospective study. Sci. Rep. 2017, 7, 42499. [Google Scholar] [CrossRef] [Green Version]
- Trevisani, F.; Brandi, G.; Garuti, F.; Barbera, M.A.; Tortora, R.; Casadei Gardini, A.; Granito, A.; Tovoli, F.; De Lorenzo, S.; Inghilesi, A.L.; et al. Metronomic capecitabine as second-line treatment for hepatocellular carcinoma after sorafenib discontinuation. J. Cancer Res. Clin. Oncol. 2018, 144, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Marisi, G.; Cucchetti, A.; Ulivi, P.; Canale, M.; Cabibbo, G.; Solaini, L.; Foschi, F.G.; De Matteis, S.; Ercolani, G.; Valgiusti, M.; et al. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J. Gastroenterol. 2018, 24, 4152–4163. [Google Scholar] [CrossRef]
- Di Costanzo, G.G.; Casadei Gardini, A.; Marisi, G.; Foschi, F.G.; Scartozzi, M.; Granata, R.; Faloppi, L.; Cascinu, S.; Silvestris, N.; Brunetti, O.; et al. Validation of a simple scoring system to predict sorafenib effectiveness in patients with hepatocellular carcinoma. Target. Oncol. 2017, 12, 795–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veterinary Co-operative Oncology Group. Veterinary Co-operative oncology group- common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet. Comp. Oncol. 2004, 2, 194–213. [Google Scholar]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumours in dogs (v1.0): A veterinary cooperative oncology group (VCOG) consensus document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]

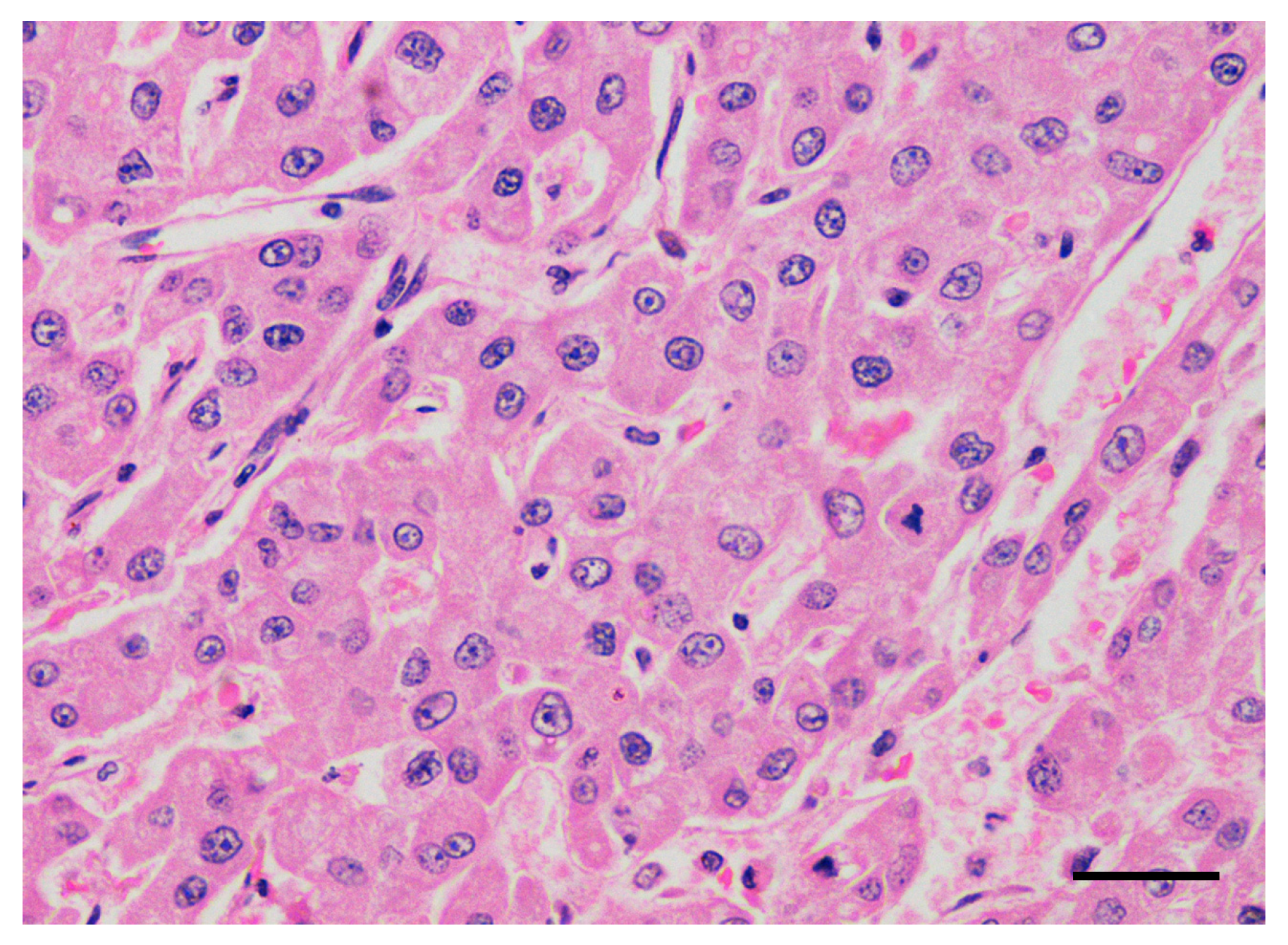
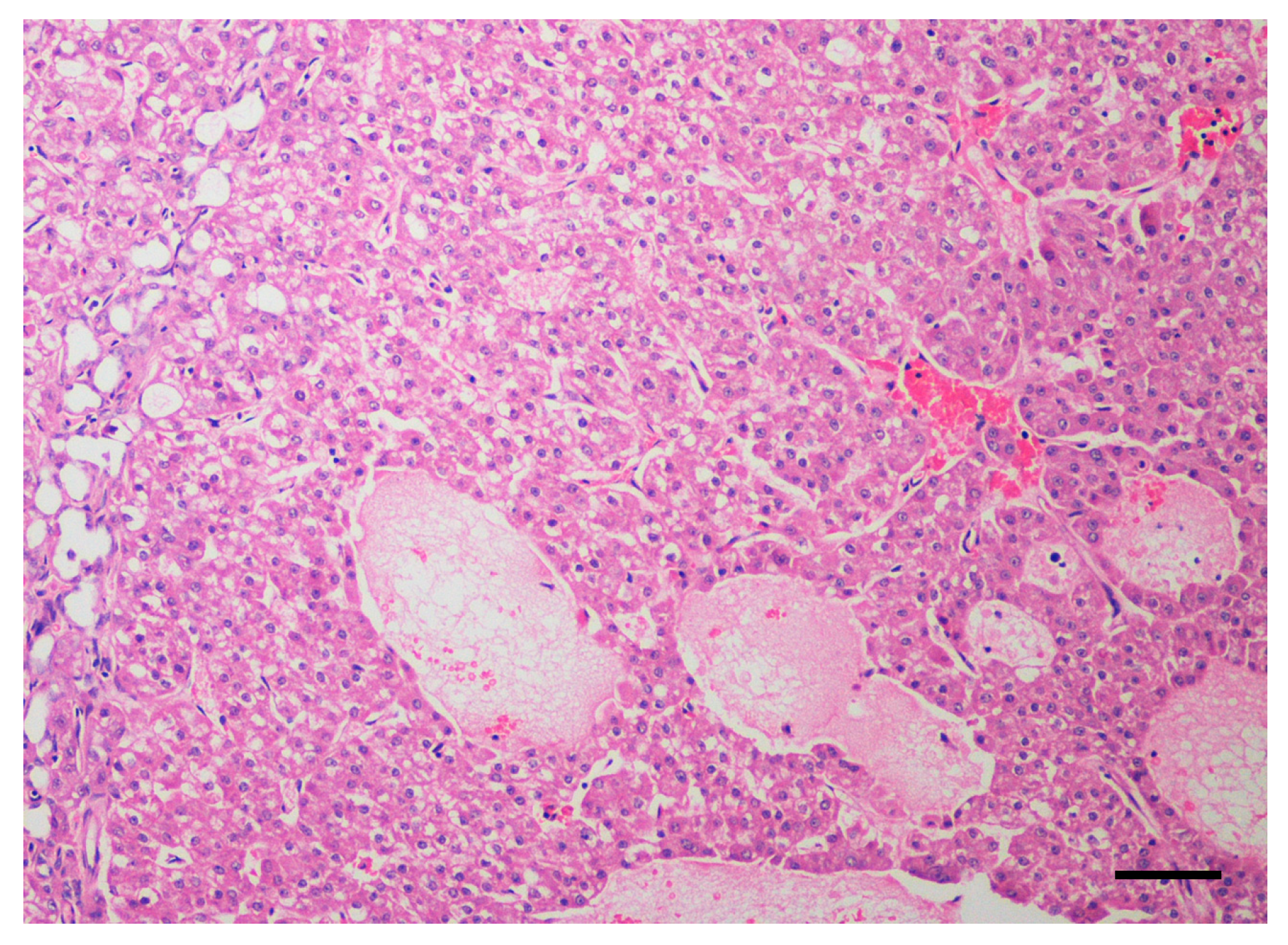

| Breed | Sex | Age | Weight | Clinical Stage | Altered Liver Enzymes | Surgical Debulking | Treatment Protocol | Treatment Toxicity | Antitumor Response, TTP | OS (Cause of Death) |
|---|---|---|---|---|---|---|---|---|---|---|
| Golden retriever | M | 11 | 41.1 | T2N0M1 | Yes | No | Sorafenib | G1 erythema and alopecia | PR, 363 | 784 (cancer) |
| Golden retriever | SF | 5 | 27.8 | T1N2M0 | No | Yes | Sorafenib | G1 hyperpigmentation; G1 diarrhea | CR, 1579 (no progression) | 1706 (alive) |
| Yorkshire terrier | SF | 10 | 3.6 | T2N1M0 | Yes | Yes | Sorafenib | G2 alopecia | PR, 1250 (no progression) | 1398 (alive) |
| Bloodhound | F | 8 | 20.5 | T3N1M1 | No | No | Sorafenib | None | PR, 228 (no progression) | 255 (leishmaniasis) |
| Cross-breed | M | 11 | 10 | T3N0M0 | Yes | No | Sorafenib | None | SD, 168 | 361 (cancer) |
| Pomeranian | SF | 13 | 10.1 | T2N1M0 | Yes | No | Sorafenib, Then MC crossover | None | SD, 230 | 327 (cancer) |
| Poodle | F | 13 | 2.7 | T2N0M1 | No | No | Sorafenib, Then MC crossover | None | SD, 205 | 279 (cancer) |
| Shih-tzu | SF | 11 | 6.7 | T3N1M1 | Yes | Yes | MC | None | PD, 27 | 27 (cancer) |
| Beagle | SF | 11 | 13.1 | T3N1M0 | Yes | No | MC | None | SD, 703 | 1390 (cancer) |
| Scottish terrier | SF | 7 | 10.6 | T2N0M1 | Yes | No | MC | G1 gastrointestinal; G1 renal | SD, 35 | 196 (cancer) |
| French bouledogue | SF | 6 | 11.7 | T3N0M0 | No | No | MC | G1 hemorrhagic cystitis | SD, 219 | 390 (cancer) |
| Jack Russell Terrier | M | 12 | 8.1 | T3N0M0 | Yes | No | MC | None | PD, 1 | 19 (cancer) |
| German Shepherd | F | 11 | 29.6 | T3N1M0 | No | No | MC | G2 gastrointestinal | PD, 1 | 32 (cancer) |
| Variable | Sorafenib (n = 7) | Metronomic Chemotherapy (n = 6) | p |
|---|---|---|---|
| Sex | >0.999 | ||
| Male | 2 | 1 | |
| Female | 5 | 5 | |
| Age a | >0.999 | ||
| ≤11 Years | 5 | 5 | |
| >11 Years | 2 | 1 | |
| Weight a | 0.592 | ||
| ≤10.6 kg | 4 | 2 | |
| >10.6 kg | 3 | 4 | |
| Surgical Debulking | >0.999 | ||
| No | 5 | 5 | |
| Yes | 2 | 1 | |
| Clinical Stage | >0.999 | ||
| III | 2 | 1 | |
| IV | 5 | 5 | |
| Hepatic Enzymes | >0.999 | ||
| Within Normal Limits | 3 | 2 | |
| Altered | 4 | 4 | |
| Treatment Toxicity | >0.099 | ||
| No | 4 | 3 | |
| Yes | 3 | 3 |
| Variable | Time to Progression | Overall Survival | ||
|---|---|---|---|---|
| Median (95% CI) | p | Median (95% CI) | p | |
| Sex | 0.301 | 0.474 | ||
| Male | 168 (0–435) | 327 (172–481) | ||
| Female | 219 (184–253) | 361 (0–908) | ||
| Age a | 0.235 | 0.059 | ||
| ≤11 Years | 219 (0–488) | 390 (0–979) | ||
| >11 Years | 205 (0–531) | 212 (0–695) | ||
| Weight a | 0.237 | 0.194 | ||
| ≤10.6 kg | 168 (0–509) | 279 (66–492) | ||
| >10.6 kg | 363 (81–645) | 784 (0–1606) | ||
| Surgical Debulking | 0.107 | 0.104 | ||
| No | 205 (126–284) | 327 (213–441) | ||
| Yes | not reached | not reached | ||
| Clinical Stage | 0.102 | 0.330 | ||
| III | 168 (0–435) | 361 (0–908) | ||
| IV | 230 (18–442) | 327 (0–1030) | ||
| Hepatic Enzymes | 0.643 | 0.675 | ||
| Within Normal Limits | 219 (189–249) | 390 (93–687) | ||
| Altered | 168 (0–438) | 327 (98–556) | ||
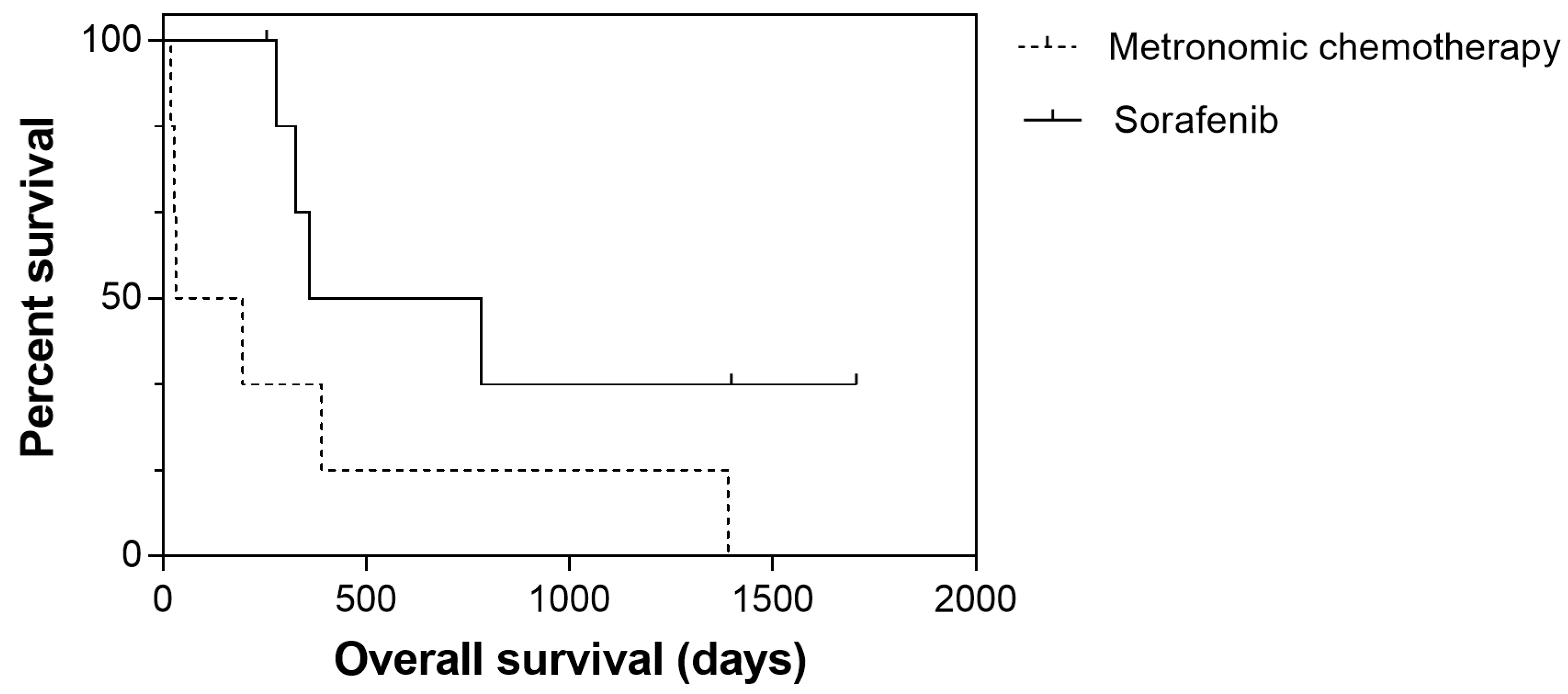
| Treatment | 0.044 * | 0.079 | ||
| Metronomic Chemotherapy | 27 (0–68) | 32 (0–235) | ||
| Sorafenib | 363 (191–535) | 361 (0–909) | ||
| Treatment Toxicity | 0.357 | 0.194 | ||
| No | 205 (110–300) | 327 (1–653) | ||
| Yes | 219 (0–613) | 390 (0–1096) | ||
| Primary tumor (T) |
| T0: no evidence of primary tumor |
| T1: solitary tumor of any size involving one lobe |
| T2: multiple tumors of any size involving multiple lobes |
| T3: tumor(s) with direct invasion of adjacent organs |
| Regional lymph nodes (N) |
| N0: no regional lymph nodes metastasis |
| N1: regional lymph node metastasis |
| N2: distant lymph node metastasis |
| Distant metastasis (M) |
| M0: no distant metastasis |
| M1: distant metastasis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marconato, L.; Sabattini, S.; Marisi, G.; Rossi, F.; Leone, V.F.; Casadei-Gardini, A. Sorafenib for the Treatment of Unresectable Hepatocellular Carcinoma: Preliminary Toxicity and Activity Data in Dogs. Cancers 2020, 12, 1272. https://doi.org/10.3390/cancers12051272
Marconato L, Sabattini S, Marisi G, Rossi F, Leone VF, Casadei-Gardini A. Sorafenib for the Treatment of Unresectable Hepatocellular Carcinoma: Preliminary Toxicity and Activity Data in Dogs. Cancers. 2020; 12(5):1272. https://doi.org/10.3390/cancers12051272
Chicago/Turabian StyleMarconato, Laura, Silvia Sabattini, Giorgia Marisi, Federica Rossi, Vito Ferdinando Leone, and Andrea Casadei-Gardini. 2020. "Sorafenib for the Treatment of Unresectable Hepatocellular Carcinoma: Preliminary Toxicity and Activity Data in Dogs" Cancers 12, no. 5: 1272. https://doi.org/10.3390/cancers12051272





