Can 18F-NaF PET/CT before Autologous Stem Cell Transplantation Predict Survival in Multiple Myeloma?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. PET/CT Data Acquisition
2.3. PET/CT Data Analysis
2.4. Clinical Parameters, Bone Marrow Plasma Cell Infiltration and Fluorescence In Situ Hybridization
2.5. Statistical Analysis
3. Results
3.1. Patient Cohort
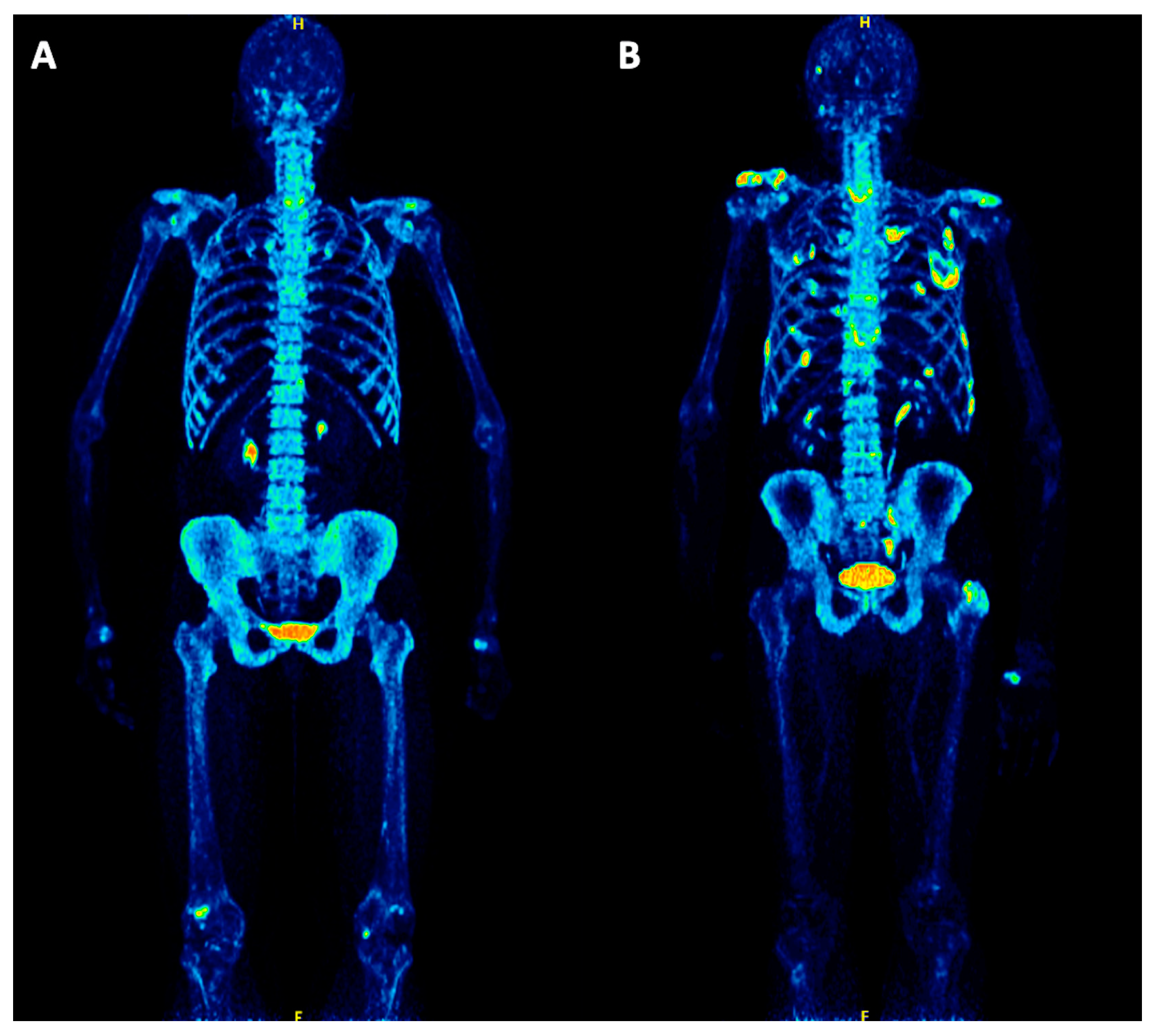
3.2. 18F-NaF PET/CT Findings
3.3. Correlation between 18F-NaF PET/CT Parameters, Bone Marrow Plasma Cell Infiltration and Cytogenetics
3.4. Correlation between 18F-NaF PET/CT and PFS
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zamagni, E.; Patriarca, F.; Nanni, C.; Zannetti, B.; Englaro, E.; Pezzi, A.; Tacchetti, P.; Buttignol, S.; Perrone, G.; Brioli, A.; et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood 2011, 118, 5989–5995. [Google Scholar] [CrossRef] [PubMed]
- Bartel, T.B.; Haessler, J.; Brown, T.L.; Shaughnessy, J.D., Jr.; van Rhee, F.; Anaissie, E.; Alpe, T.; Angtuaco, E.; Walker, R.; Epstein, J.; et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood 2009, 114, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- van Lammeren-Venema, D.; Regelink, J.C.; Riphagen, I.I.; Zweegman, S.; Hoekstra, O.S.; Zijlstra, J.M. 18F-fluoro-deoxyglucose positron emission tomography in assessment of myeloma-related bone disease: A systematic review. Cancer 2012, 118, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Terpos, E.; Nanni, C.; Moreau, P.; Lentzsch, S.; Zweegman, S.; Hillengass, J.; Engelhardt, M.; Usmani, S.Z.; Vesole, D.H.; et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: A consensus statement by the International Myeloma Working Group. Lancet. Oncol. 2017, 18, e206–e217. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet. Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Rasche, L.; Angtuaco, E.; McDonald, J.E.; Buros, A.; Stein, C.; Pawlyn, C.; Thanendrarajan, S.; Schinke, C.; Samant, R.; Yaccoby, S.; et al. Low expression of hexokinase-2 is associated with false-negative FDG-positron emission tomography in multiple myeloma. Blood 2017, 130, 30–34. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Goldschmidt, H.; Dimitrakopoulou-Strauss, A. Positron Emission Tomography (PET) Radiopharmaceuticals in Multiple Myeloma. Molecules 2019, 25, 134. [Google Scholar] [CrossRef]
- Zamagni, E.; Cavo, M. The role of imaging techniques in the management of multiple myeloma. Br. J. Haematol. 2012, 159, 499–513. [Google Scholar] [CrossRef]
- Nanni, C.; Versari, A.; Chauvie, S.; Bertone, E.; Bianchi, A.; Rensi, M.; Bellò, M.; Gallamini, A.; Patriarca, F.; Gay, F.; et al. Interpretation criteria for FDG PET/CT in multiple myeloma (IMPeTUs): Final results. IMPeTUs (Italian myeloma criteria for PET USe). Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 712–719. [Google Scholar] [CrossRef]
- Hawkins, R.A.; Choi, Y.; Huang, S.C.; Hoh, C.K.; Dahlbom, M.; Schiepers, C.; Satyamurthy, N.; Barrio, J.R.; Phelps, M.E. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J. Nucl. Med. 1992, 33, 633–642. [Google Scholar]
- Grant, F.D.; Fahey, F.H.; Packard, A.B.; Davis, R.T.; Alavi, A.; Treves, S.T. Skeletal PET with 18 F-fluoride: Applying new technology to an old tracer. J. Nucl. Med. 2008, 49, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Czernin, J.; Satyamurthy, N.; Schiepers, C. Molecular mechanisms of bone 18F-NaF deposition. J. Nucl. Med. 2010, 51, 1826–1829. [Google Scholar] [CrossRef] [PubMed]
- Segall, G.; Delbeke, D.; Stabin, M.G.; Even-Sapir, E.; Fair, J.; Sajdak, R.; Smith, G.T. SNM. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J. Nucl. Med. 2010, 51, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, M.; Mottaghy, F.M.; Payche, F.; Behrendt, F.F.; Van den Wyngaert, T.; Fogelman, I.; Strobel, K.; Celli, M.; Fanti, S.; Giammarile, F.; et al. (18)F-NaF PET/CT: EANM procedure guidelines for bone imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1767–1777. [Google Scholar] [CrossRef]
- Hillner, B.E.; Siegel, B.A.; Hanna, L.; Duan, F.; Quinn, B.; Shields, A.F. 18F-fluoride PET used for treatment monitoring of systemic cancer therapy: Results from the National Oncologic PET Registry. J. Nucl. Med. 2015, 56, 222–228. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Goldschmidt, H.; Hose, D.; Pan, L.; Cheng, C.; Kopka, K.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. PET/CT studies of multiple myeloma using (18) F-FDG and (18) F-NaF: Comparison of distribution patterns and tracers’ pharmacokinetics. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1343–1353. [Google Scholar] [CrossRef]
- Ak, İ.; Onner, H.; Akay, O.M. Is there any complimentary role of F-18 NaF PET/CT in detecting of osseous involvement of multiple myeloma? A comparative study for F-18 FDG PET/CT and F-18 FDG NaF PET/CT. Ann. Hematol. 2015, 94, 1567–1575. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Hillengass, J.; Goldschmidt, H.; Anwar, H.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Quantitative analysis of 18F-NaF dynamic PET/CT cannot differentiate malignant from benign lesions in multiple myeloma. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 148–156. [Google Scholar]
- Sachpekidis, C.; Hillengass, J.; Goldschmidt, H.; Wagner, B.; Haberkorn, U.; Kopka, K.; Dimitrakopoulou-Strauss, A. Treatment response evaluation with 18F-FDG PET/CT and 18F-NaF PET/CT in multiple myeloma patients undergoing high-dose chemotherapy and autologous stem cell transplantation. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 50–62. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Tateishi, U.; Shizukuishi, K.; Shishikura, A.; Yamazaki, E.; Shibata, H.; Yoneyama, T.; Ishigatsubo, Y.; Inoue, T. Role of 18F-fluoride PET/CT in the assessment of multiple myeloma: Initial experience. Ann. Nucl. Med. 2013, 27, 78–83. [Google Scholar] [CrossRef]
- Wang, Y.; Yee, A.J.; Sirard, C.; Landau, S.; Raje, N.; Mahmood, U. Sodium fluoride PET imaging as a quantitative pharmacodynamic biomarker for bone homeostasis during anti-DKK1 therapy for multiple myeloma. Blood Cancer J. 2017, 7, e615. [Google Scholar] [CrossRef] [PubMed]
- Withofs, N.; Beguin, Y.; Cousin, F.; Tancredi, T.; Simoni, P.; Alvarez-Miezentseva, V.; De Prijck, B.; Hafraoui, K.; Bonnet, C.; Baron, F.; et al. Dual-tracer PET/CT scan after injection of combined [18 F]NaF and [18 F]FDG outperforms MRI in the detection of myeloma lesions. Hematol. Oncol. 2019, 37, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zirakchian Zadeh, M.; Ostergaard, B.; Mehdizadeh Seraj, S.; Ayubcha, C.; Raynor, W.; Gerke, O.; Constantinescu, C.; Werner, T.; Zhuang, H.; Hoilund-Carlsen, P.F.; et al. Evaluation of myeloma bone disease by means of 18F-sodium fluoride PET/CT. J. Nucl. Med. 2019, 60, 24. [Google Scholar]
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br. J. Haematol. 2003, 121, 749–757. [Google Scholar] [CrossRef]
- Goldschmidt, H.; Mai, E.K.; Dürig, J.; Scheid, C.; Weisel, K.C.; Kunz, C.; Bertsch, U.; Hielscher, T.; Munder, M.; Lindemann, H.-W.; et al. Response-Adapted Lenalidomide Maintenance in Newly Diagnosed, Transplant-Eligible Multiple Myeloma: Results from the Multicenter Phase III GMMG-MM5 Trial. Blood 2017, 130 (Suppl. 1), 400. Available online: http://www.bloodjournal.org/content/130/Suppl_1/400 (accessed on 24 June 2018).
- Sachpekidis, C.; Merz, M.; Kopp-Schneider, A.; Jauch, A.; Raab, M.S.; Sauer, S.; Hillengass, J.; Goldschmidt, H.; Dimitrakopoulou-Strauss, A. Quantitative dynamic 18F-fluorodeoxyglucose positron emission tomography/computed tomography before autologous stem cell transplantation predicts survival in multiple myeloma. Haematologica. 2019, 104, e420–e423. [Google Scholar] [CrossRef]
- Dimitrakopoulou-Strauss, A.; Pan, L.; Strauss, L.G. Quantitative approaches of dynamic FDG-PET and PET/CT studies (dPET/CT) for the evaluation of oncological patients. Cancer Imaging 2012, 12, 283–289. [Google Scholar] [CrossRef]
- PMOD Technologies. Available online: http://www.pmod.com/files/download/v31/doc/pbas/4729.htm (accessed on 16 January 2020).
- Mikolajczyk, K.; Szabatin, M.; Rudnicki, P.; Grodzki, M.; Burger, C. A Java environment for medical image data analysis: Initial application for brain PET quantitation. Med. Inform. 1998, 23, 207–214. [Google Scholar] [CrossRef]
- Burger, C.; Buck, A. Requirements and implementations of a flexible kinetic modeling tool. J. Nucl. Med. 1997, 38, 1818–1823. [Google Scholar]
- Pan, L.; Cheng, C.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Machine learning-based kinetic modeling: A robust and reproducible solution for quantitative analysis of dynamic PET data. Phys. Med. Biol. 2017, 62, 3566–3581. [Google Scholar] [CrossRef]
- Ohtake, T.; Kosaka, N.; Watanabe, T.; Yokoyama, I.; Moritan, T.; Masuo, M.; Iizuka, M.; Kozeni, K.; Toshimitsu Momose, T.; Oku, S.; et al. Noninvasive method to obtain input function for measuring tissue glucose utilization of thoracic and abdominal organs. J. Nucl. Med. 1991, 32, 1432–1438. [Google Scholar] [PubMed]
- Dimitrakopoulou-Strauss, A.; Pan, L.; Sachpekidis, C. Kinetic modeling and parametric imaging with dynamic PET for oncological applications: General considerations, current clinical applications, and future perspectives. Eur. J. Nucl. Med. Mol. Imaging. 2020. [Google Scholar] [CrossRef]
- Dimitrakopoulou-Strauss, A.; Strauss, L.G.; Mikolajczyk, K.; Burger, C.; Lehnert, T.; Bernd, L.; Ewerbeck, V. On the fractal nature of positron emission tomography (PET) studies. World J. Nucl. Med. 2003, 4, 306–313. Available online: https://inis.iaea.org/search/search.aspx?orig_q = RN:34087347 (accessed on 12 January 2020).
- Sachpekidis, C.; Mai, E.K.; Goldschmidt, H.; Hillengass, J.; Hose, D.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. (18)F-FDG dynamic PET/CT in patients with multiple myeloma: Patterns of tracer uptake and correlation with bone marrow plasma cell infiltration rate. Clin. Nucl. Med. 2015, 40, e300–e307. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Neben, K.; Lokhorst, H.M.; Jauch, A.; Bertsch, U.; Hielscher, T.; van der Holt, B.; Salwender, H.; Blau, I.W.; Weisel, K.; Pfreundschuh, M.; et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood 2012, 119, 940–948. [Google Scholar] [CrossRef]
- Lapa, C.; Knop, S.; Schreder, M.; Rudelius, M.; Knott, M.; Jörg, G.; Samnick, S.; Herrmann, K.; Buck, A.K.; Einsele, H.; et al. 11C-Methionine-PET in Multiple Myeloma: Correlation with Clinical Parameters and Bone Marrow Involvement. Theranostics 2016, 6, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Schreder, M.; Lückerath, K.; Samnick, S.; Rudelius, M.; Buck, A.K.; Kortüm, K.M.; Einsele, H.; Rosenwald, A.; Knop, S. [11 C]Methionine emerges as a new biomarker for tracking active myeloma lesions. Br. J. Haematol. 2018, 181, 701–703. [Google Scholar] [CrossRef]
- Lapa, C.; Garcia-Velloso, M.J.; Lückerath, K.; Samnick, S.; Schreder, M.; Otero, P.R.; Schmid, J.S.; Herrmann, K.; Knop, S.; Buck, A.K.; et al. 11C-Methionine-PET in Multiple Myeloma: A Combined Study from Two Different Institutions. Theranostics 2017, 7, 2956–2964. [Google Scholar] [CrossRef]
- Da Vià, M.C.; Solimando, A.G.; Garitano-Trojaola, A.; Barrio, S.; Munawar, U.; Strifler, S.; Haertle, L.; Rhodes, N.; Teufel, E.; Vogt, C.; et al. CIC Mutation as a Molecular Mechanism of Acquired Resistance to Combined BRAF-MEK Inhibition in Extramedullary Multiple Myeloma with Central Nervous System Involvement. Oncologist 2020, 25, 112–118. [Google Scholar] [CrossRef]
- Philipp-Abbrederis, K.; Herrmann, K.; Knop, S.; Schottelius, M.; Eiber, M.; Lückerath, K.; Pietschmann, E.; Habringer, S.; Gerngroß, C.; Franke, K.; et al. In vivo molecular imaging of chemokine receptor CXCR4 expression in patients with advanced multiple myeloma. EMBO. Mol. Med. 2015, 7, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Cao, X.; Luo, Y.; Li, J.; Feng, J.; Li, F. Chemokine receptor-4 targeted PET/CT with 68Ga-Pentixafor in assessment of newly diagnosed multiple myeloma: Comparison to 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Herrmann, K.; Schirbel, A.; Hänscheid, H.; Lückerath, K.; Schottelius, M.; Kircher, M.; Werner, R.A.; Schreder, M.; Samnick, S.; et al. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed Multiple Myeloma. Theranostics 2017, 7, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Varettoni, M.; Corso, A.; Pica, G.; Mangiacavalli, S.; Pascutto, C.; Lazzarino, M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: A longitudinal study on 1003 consecutive patients. Ann. Oncol. 2010, 21, 325–330. [Google Scholar] [CrossRef]
- Hall, M.N.; Jagannathan, J.P.; Ramaiya, N.H.; Shinagare, A.B.; Van den Abbeele, A.D. Imaging of extraosseous myeloma: CT, PET/CT, and MRI features. AJR. Am. J. Roentgenol. 2010, 195, 1057–1065. [Google Scholar] [CrossRef]
- Turkbey, B.; Tan, E.; Korde, N.; Kwok, M.; Manasanch, E.E.; Tageja, N.; Mailankody, S.; Roschewski, M.; Mulquin, M.; Carpenter, A.; et al. Bone marrow abnormalities and early bone lesions in multiple myeloma and its precursor disease: A prospective study using functional and morphologic imaging. Leuk. Lymphoma. 2016, 57, 1114–1121. [Google Scholar] [CrossRef][Green Version]
- Esteve, F.; Roodman, G.D. Pathophysiology of myeloma bone disease. Best Pract Res. Clin. Haematol. 2007, 20, 613–624. [Google Scholar] [CrossRef]
- Nakuz, T.S.; Millinger, F.P.; El-Rabadi, K.; Weber, M.; Pichler, V.; Wadsak, W.; Mitterhauser, M.; Haug, A.; Hacker, M.; Karanikas, G.; et al. Characterization of Bone Lesions in Myeloma Before and During Anticancer Therapy Using 18F-FDG-PET/CT and 18F-NaF-PET/CT. Anticancer Res. 2019, 39, 1943–1952. [Google Scholar] [CrossRef]
- Zirakchian Zadeh, M.; Østergaard, B.; Raynor, W.Y.; Revheim, M.E.; Seraj, S.M.; Acosta-Montenegro, O.; Ayubcha, C.; Yellanki, D.P.; Al-Zaghal, A.; Nielsen, A.L.; et al. Comparison of 18F-sodium fluoride uptake in the whole bone, pelvis, and femoral neck of multiple myeloma patients before and after high-dose therapy and conventional-dose chemotherapy [published online ahead of print, 2020 Apr 3]. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Z.; Berg, E.; Judenhofer, M.S.; Liu, W.; Xu, T.; Ding, Y.; Lv, Y.; Dong, Y.; Deng, Z.; et al. Total-Body Dynamic Reconstruction and Parametric Imaging on the uEXPLORER. J. Nucl. Med. 2020, 61, 285–291. [Google Scholar] [CrossRef]



| Baseline Characteristics | n |
|---|---|
| Median age, years | 59.9 [38.4–73.5] |
| Gender | |
| Male | 31 (66%) |
| Female | 16 (34%) |
| Median albumin, g/dL | 4.2 [0.5–5.0] |
| Median β2-microglobulin, mg/L | 2.8 [1.1–37.0] |
| Median LDH, u/L | 184.0 [117.0–283.0] |
| Median bone marrow plasma cell infiltration | 32% [1–92%] |
| Cytogenetic abnormalities | |
| High-risk [del(17p) and/or t(4;14) and/or t(14;16)] | 8 (20.0%) |
| Standard risk | 32 (80.0%) |
| ISS | |
| 1 | 26 (61.9%) |
| 2 | 12 (28.6%) |
| 3 | 4 (9.5%) |
| R-ISS | |
| 1 | 14 (38.9%) |
| 2 | 20 (55.6%) |
| 3 | 2 (5.6%) |
| Parameters | Reference Tissue (os ilium) | MM Lesions | ||||||
|---|---|---|---|---|---|---|---|---|
| Median | Mean | SD | n | Median | Mean | SD | n | |
| SUVaverage | 8.1 | 8.2 | 2.5 | 47 | 14.4 | 20.1 | 15.8 | 14 |
| SUVmax | 12.9 | 13.5 | 4.5 | 47 | 24.4 | 35.2 | 28.9 | 14 |
| VB | 0.004 | 0.03 | 0.08 | 47 | 0.003 | 0.03 | 0.04 | 10 |
| K1 | 0.25 | 0.27 | 0.11 | 47 | 0.24 | 0.30 | 0.16 | 10 |
| k3 | 0.05 | 0.05 | 0.15 | 47 | 0.24 | 0.27 | 0.19 | 10 |
| Influx (Ki) | 0.07 | 0.07 | 0.03 | 45 | 0.08 | 0.13 | 0.09 | 10 |
| FD | 1.38 | 1.37 | 0.05 | 47 | 1.40 | 1.38 | 0.08 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachpekidis, C.; Kopp-Schneider, A.; Merz, M.; Jauch, A.; Raab, M.-S.; Goldschmidt, H.; Dimitrakopoulou-Strauss, A. Can 18F-NaF PET/CT before Autologous Stem Cell Transplantation Predict Survival in Multiple Myeloma? Cancers 2020, 12, 1335. https://doi.org/10.3390/cancers12051335
Sachpekidis C, Kopp-Schneider A, Merz M, Jauch A, Raab M-S, Goldschmidt H, Dimitrakopoulou-Strauss A. Can 18F-NaF PET/CT before Autologous Stem Cell Transplantation Predict Survival in Multiple Myeloma? Cancers. 2020; 12(5):1335. https://doi.org/10.3390/cancers12051335
Chicago/Turabian StyleSachpekidis, Christos, Annette Kopp-Schneider, Maximilian Merz, Anna Jauch, Marc-Steffen Raab, Hartmut Goldschmidt, and Antonia Dimitrakopoulou-Strauss. 2020. "Can 18F-NaF PET/CT before Autologous Stem Cell Transplantation Predict Survival in Multiple Myeloma?" Cancers 12, no. 5: 1335. https://doi.org/10.3390/cancers12051335
APA StyleSachpekidis, C., Kopp-Schneider, A., Merz, M., Jauch, A., Raab, M.-S., Goldschmidt, H., & Dimitrakopoulou-Strauss, A. (2020). Can 18F-NaF PET/CT before Autologous Stem Cell Transplantation Predict Survival in Multiple Myeloma? Cancers, 12(5), 1335. https://doi.org/10.3390/cancers12051335






