Abstract
Lymphoedema is a well-known concern for cancer survivors. A crucial issue in lymphoedema is that we cannot predict who will be affected, and onset can occur many years after initial cancer treatment. The variability of time between cancer treatment and lymphoedema onset is an unexplained mystery. Retrospective cohort studies have investigated the risk factors for lymphoedema development, with extensive surgery and the combination of radiation and surgery identified as common high-risk factors. However, these studies could not predict lymphoedema risk in each individual patient in the early stages, nor could they explain the timing of onset. The study of anatomy is one promising tool to help shed light on the pathophysiology of lymphoedema. While the lymphatic system is the area least investigated in the field of anatomical science, some studies have described anatomical changes in the lymphatic system after lymph node dissection. Clinical imaging studies in lymphangiography, lymphoscintigraphy and indocyanine green (ICG) fluorescent lymphography have reported post-operative anatomical changes in the lymphatic system, including dermal backflow, lymphangiogenesis and creation of alternative pathways via the deep and torso lymphatics, demonstrating that such dynamic anatomical changes contribute to the maintenance of lymphatic drainage pathways. This article presents a descriptive review of the anatomical and imaging studies of the lymphatic system in the normal and post-operative conditions and attempts to answer the questions of why some people develop lymphoedema after cancer and some do not, and what causes the variability in lymphoedema onset timing.
1. Introduction
Lymphoedema is a well-known side-effect of post-cancer treatment. The standard treatment for lymphoedema includes the daily wearing of a compression garment on the affected body part to prevent the progression of the disease [1]. Management recommendations are normally provided by an allied health professional who is accredited as a lymphoedema therapist. Surgical interventions can be employed to reduce patient discomfort, but they cannot provide a cure for lymphoedema at present [2,3]. Unlike cancer, lymphoedema is not a life-threatening condition, but its impact is great, causing life-long physical, psychological and economic distress for those who suffer from it [4].
One of the crucial issues in lymphoedema is that oncologists cannot predict which patients will suffer from the disease in the future and which will be spared. Lymphoedema can develop at any time from six months to many years after completion of cancer treatment, and, thus, the onset is variable [5]. Although cancer survivors are relieved to have their cancer cured, they are still under threat of possibly developing lymphoedema afterwards. Retrospective cohort studies have investigated the risk factors for lymphoedema and have identified extensive surgery that includes radical lymph node dissection, the combination of radiotherapy and surgery, and obesity as three high-risk factors [6,7,8]. The results of these studies helped to provide a ratio of odds for lymphoedema development, but they are of no help to patients at an individual level.
The study of lymphatic anatomy is considered to be one of the promising tools to help explain the pathophysiology of lymphoedema. The lymphatic system is a part of the vascular system, but it has been far less studied than the blood circulatory system. The role of the lymphatic system is to collect and transport interstitial tissue fluid and protein substances that have escaped from the blood capillaries. The dysfunction of the lymphatic system causes oedema in the affected regions. Cancer cells also travel via the lymphatic system, and the process of cancer cells reaching the regional lymph nodes and taking root there is known as lymphogenous metastasis. Hence, the lymphatic system is commonly targeted in cancer treatment to prevent the disease from spreading. In this way, cancer treatment is a direct cause of lymphoedema.
Previous anatomical studies have focused on the development of a lymphoedema model in animals and patients affected by lymphoedema, but less attention has been paid to patients who survived cancer and did not develop lymphoedema afterwards [9,10,11,12]. I speculate that the anatomy of the lymphatics changes in all patients after cancer treatment, but for the majority of them, those changes do not reach the threshold for developing into lymphoedema. Recently, near-infrared indocyanine green (ICG) fluorescence lymphography has been widely used to provide new imaging data for the assessment of lymphoedema [13,14,15,16,17].
This article presents a review of studies into the anatomical changes that occur in the lymphatic system post-lymph node dissection and discusses anatomical theories as to why some patients develop lymphoedema and others do not.
2. Normal Lymphatic Anatomy
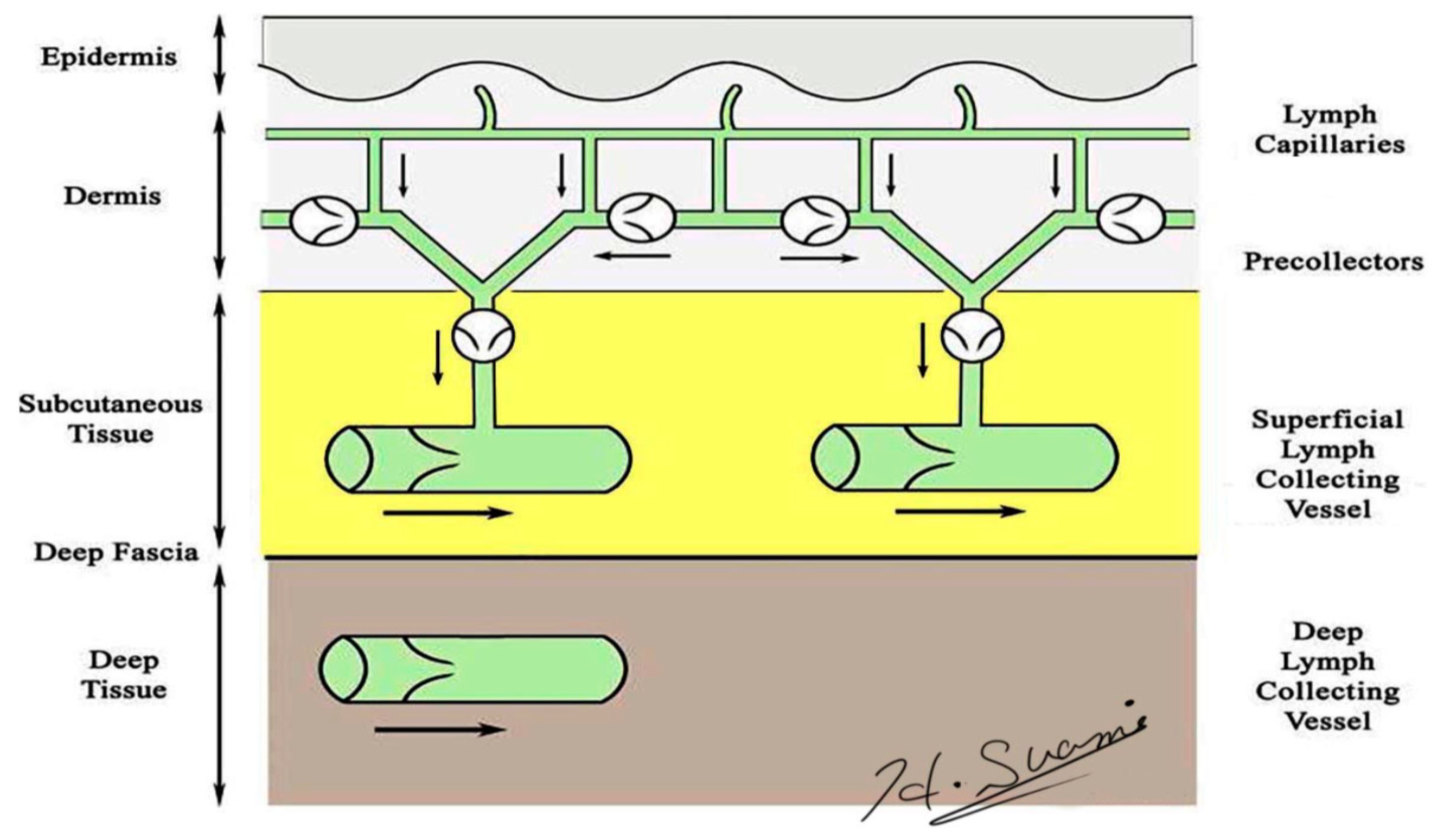
Precise knowledge of normal lymphatic anatomy provided the baseline information needed to identify the changes to lymphatic structures resulting from lymph node dissection. Lymphatic vessels originate as avalvular lymphatic capillaries in the superficial dermis and connect to valvular pre-collectors in the deep dermis. Pre-collectors merge with each other and change course to run vertically from the skin to the deep layer. These vertical vessels connect to the superficial lymph-collecting vessels running horizontally in the subcutaneous layer. The deep lymph-collecting vessels run alongside the major arteries below the deep fascia (Figure 1) [18,19]. The perforating lymphatic vessels are branches of the deep lymph-collecting vessels that run along the perforating arteries above the deep fascia. For example, breast cancer can metastasize to the internal mammary lymph nodes via the perforating lymphatic vessels. Both the superficial lymph-collecting vessels and the perforating lymphatic vessels exist in the subcutaneous fat layer above the deep fascia, but there are no connections between them. In general, the superficial and deep lymph-collecting vessels are independent of each other, and they only unite in the proximal body region, such as level-two nodes in the axilla or intrapelvic nodes.
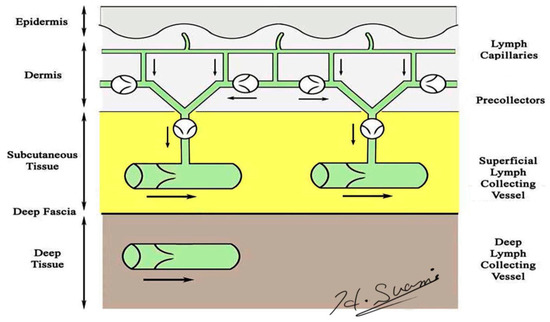
Figure 1.
Schematic diagram of the lymphatic system. (Reproduced with the permission of Hiroo Suami).
In lymphoedema, excess lymph fluid and proliferation of adipose tissue occur predominantly in the subcutaneous fat layer above the deep fascia [20,21]. These findings indicate that dysfunction of the superficial lymphatic system can be considered a major cause of lymphoedema. The superficial lymph-collecting vessels are independent of each other and may diverge and converge at various points. Interconnections between vessels are uncommon and, therefore, do not form a network (Figure 2) [18].
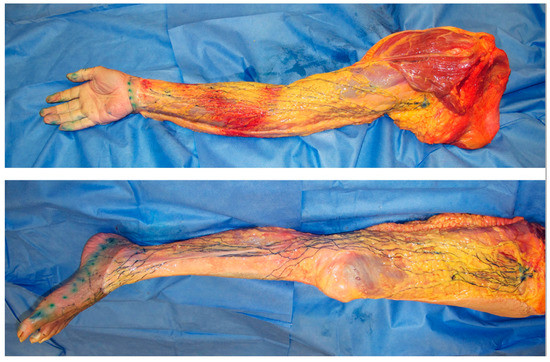
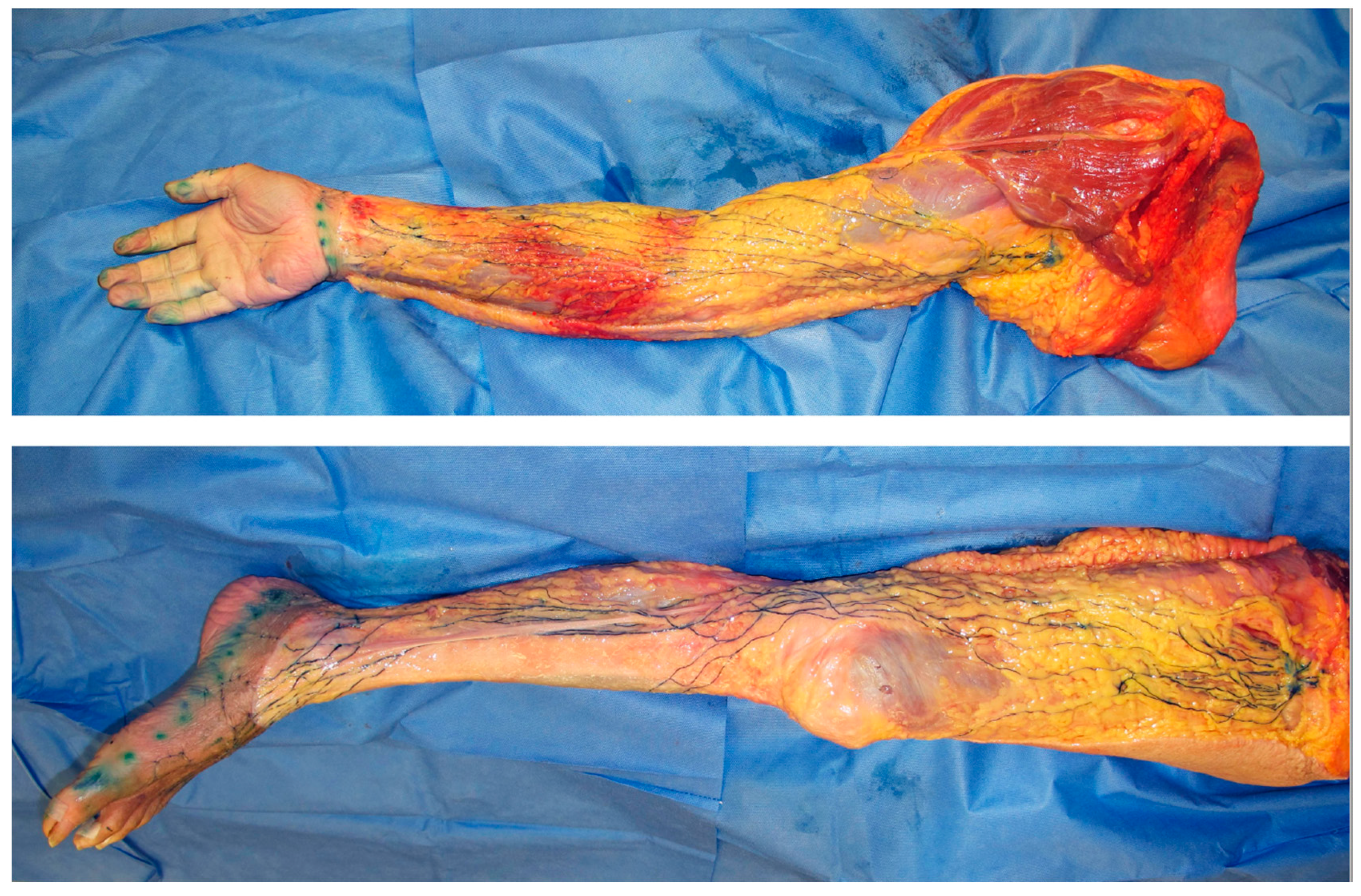
Figure 2.
Dissection photos of the superficial lymph-collecting vessels and their corresponding lymph nodes in the upper and lower limbs visualized using the microinjection technique. The lymphatic vessels were injected with blue acrylic dye, and the skin and adipose tissue were removed just above the vessels. Arrows indicate the sentinel node/s of the upper limb (white) and lower limb (black).
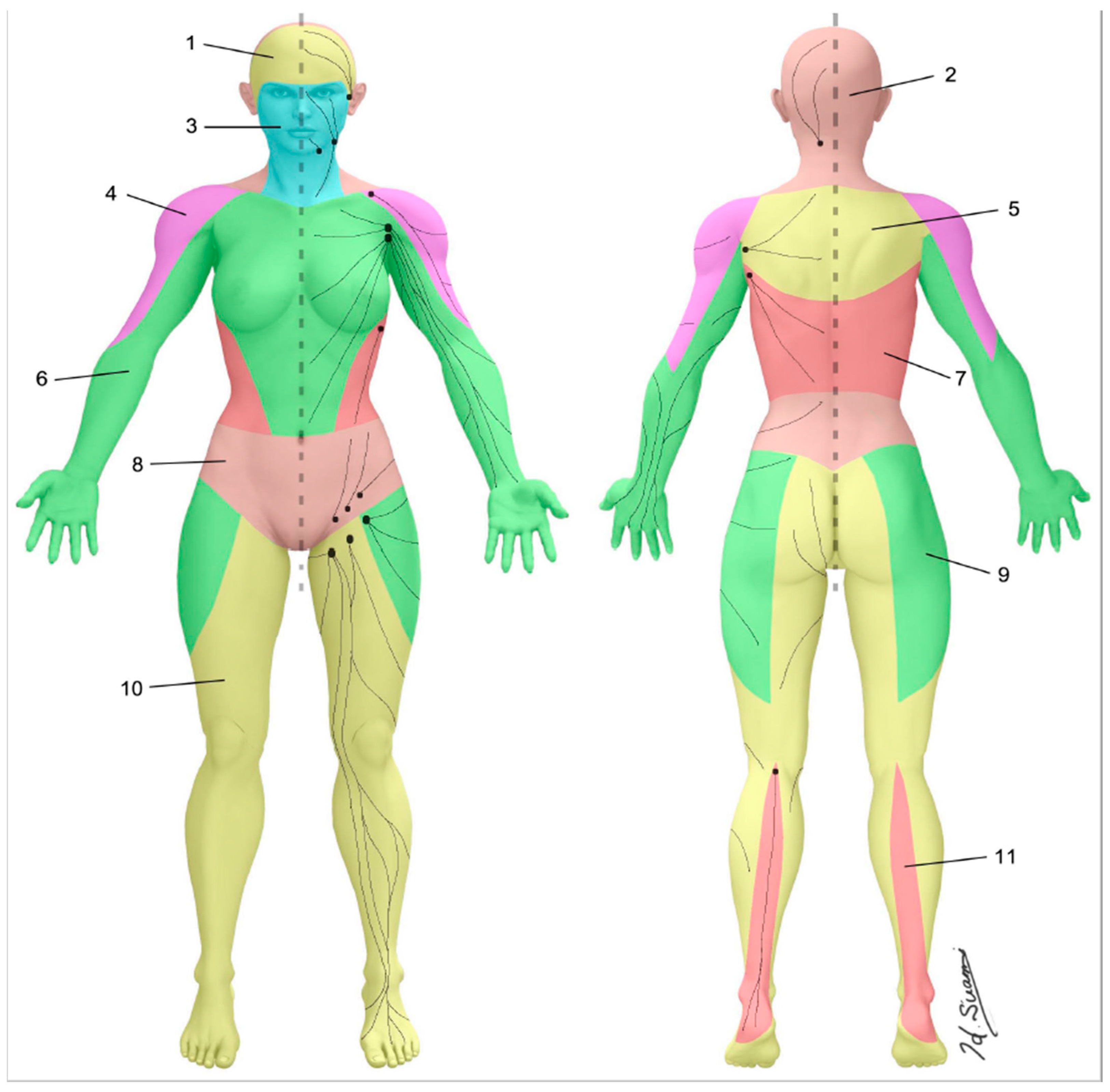
The skin can be divided into superficial lymphatic territories called “lymphosomes” (Figure 3) [22,23]. Both the upper and lower limb are demarcated into two lymphosomes, one dominant and one supplementary. In the upper limb, the dominant lymphatic territory drains to the axillary nodes. The supplementary territory covers the lateral upper arm. It bypasses the axillary nodes and drains to the supraclavicular nodes [24,25]. In the lower limb, the dominant lymphatic territory drains to the superficial inguinal nodes. The supplementary territory covers the calf region and drains to the popliteal node on its way to the deep inguinal lymph nodes via the deep lymph-collecting vessels in the thigh [26,27].
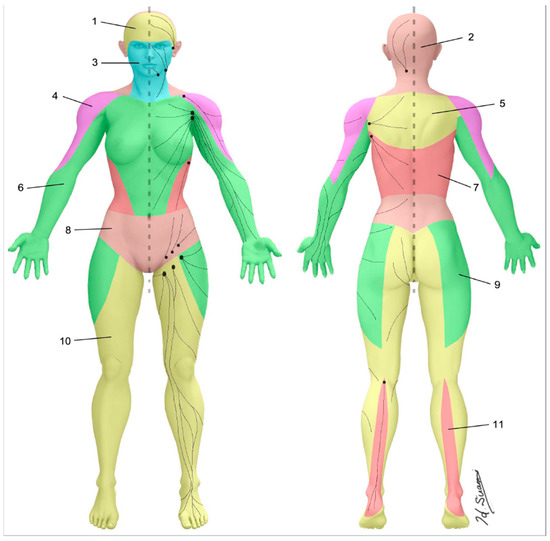
Figure 3.
Lymphosomes of the body. The lymphatic territories are demarcated according to their corresponding lymphatic basins: (1) temporal, (2) occipital, (3) submental, (4) subclavicular, (5) subscapular, (6) lateral axillary, (7) pectoral, (8) superior inguinal, (9) lateral inguinal, (10) inferior inguinal, (11) popliteal. (Reproduced with permission of Hiroo Suami).
There are two types of lymph nodes, known as regional and interval nodes. The axillary and inguinal nodes are regional nodes, and the popliteal and epitrochlear nodes are interval nodes [28]. The difference between regional and interval nodes is demonstrated by the number of afferent lymphatic vessels that connect to them. Multiple afferent lymphatic vessels connect to the regional nodes, but only one or two afferent vessels connect to interval nodes.
The focus in lymphoedema is generally the lymphatics of the upper or lower limb, and the torso is often overlooked. The lymphatics in the torso are divided by the sagittal midline and horizontal line at the umbilical level [29]. Each quadrant drains to the ipsilateral axillary or inguinal lymph nodes. The superficial lymph-collecting vessels in the torso are also affected by lymph node dissection because the axilla and inguinal regions are not the only regions to which drainage from the limbs occurs.
3. Anatomical Changes in Lymphoedema
3.1. Dermal Backflow
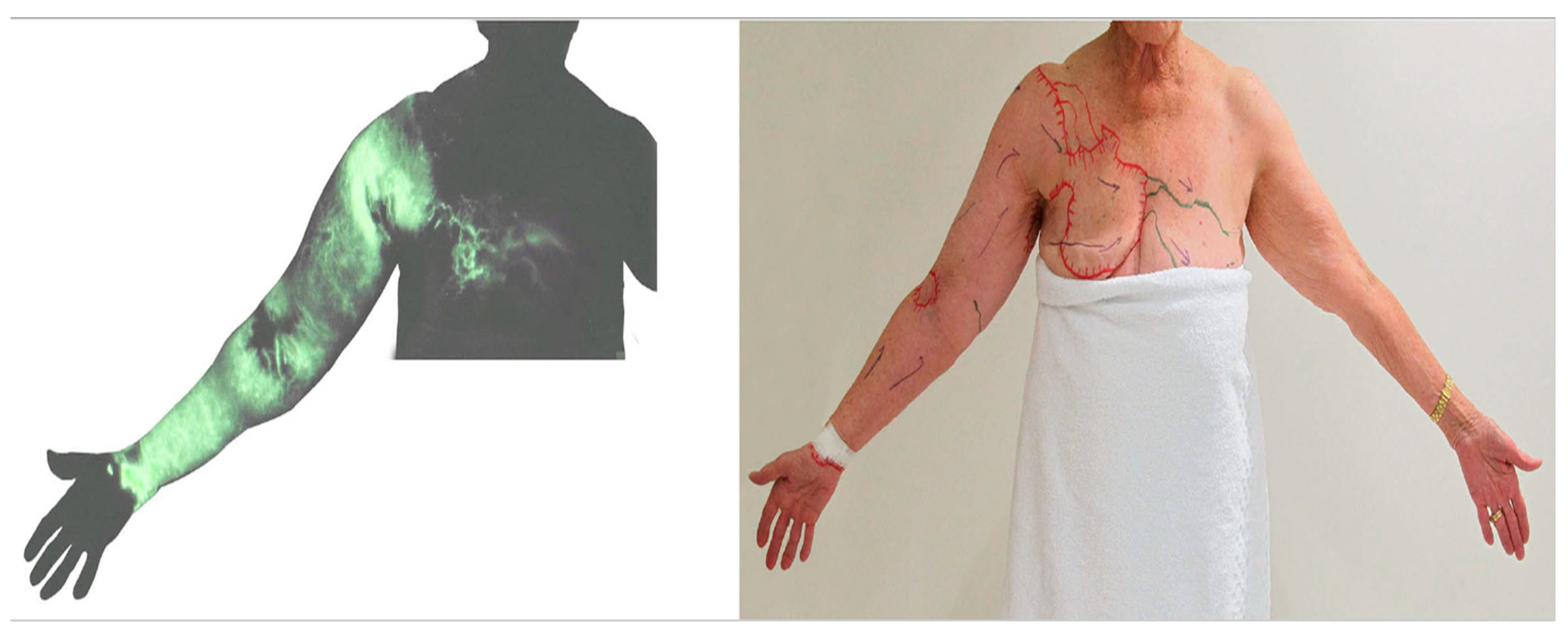
The presence of dermal backflow is a specific imaging criterion for the diagnosis of lymphoedema. This indicator was originally used in lymphangiography [30]. When radiocontrast was injected into a superficial lymph-collecting vessel in the dorsal hand or foot in patients with lymphoedema, the injected contrast overflowed to fill the dermal lymphatics. The identification of dermal backflow has also been utilized in lymphoscintigraphy and ICG lymphography to diagnose lymphoedema [13,14,15,16,17,31].
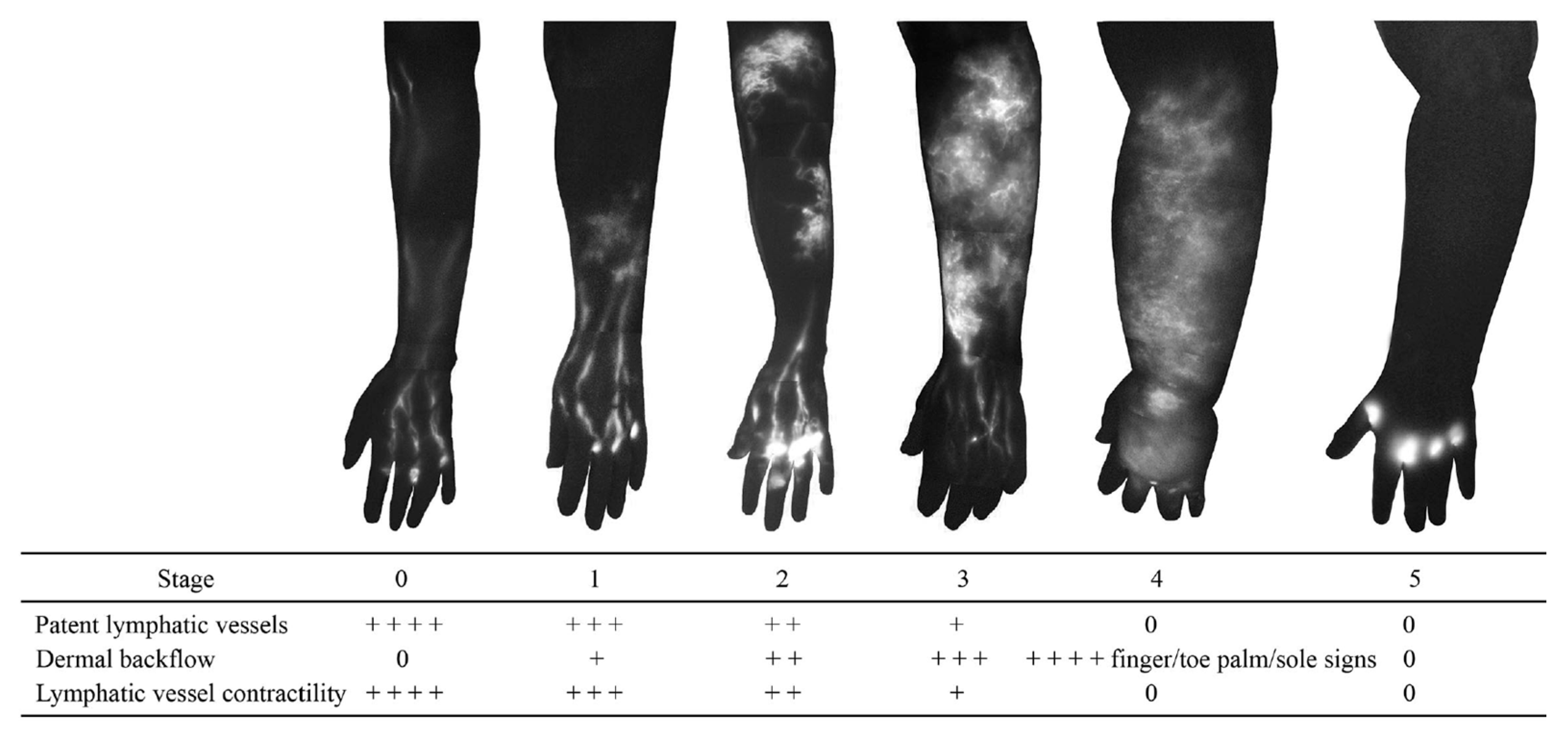
Dermal backflow is composed of dilated dermal lymphatic capillaries and pre-collectors [32]. It develops when the obstruction of the superficial lymph-collecting vessels causes the valves in the pre-collectors to become incompetent. Dermal backflow is considered to be the body’s anatomical response to lymphatic obstruction by creating alternative pathways to transport lymph fluid [33]. The amount of dermal backflow in the limbs correlates with the severity of lymphoedema (Figure 4) [34,35].
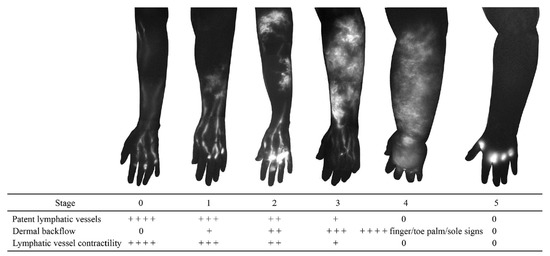
Figure 4.
Indocyanine green lymphography staging scale. Stage 0 represents normal lymphatics, and stages 1 to 5 represent increasing lymphoedema severity. There is no dye movement at Stage 5. (Reproduced from [35] with permission).
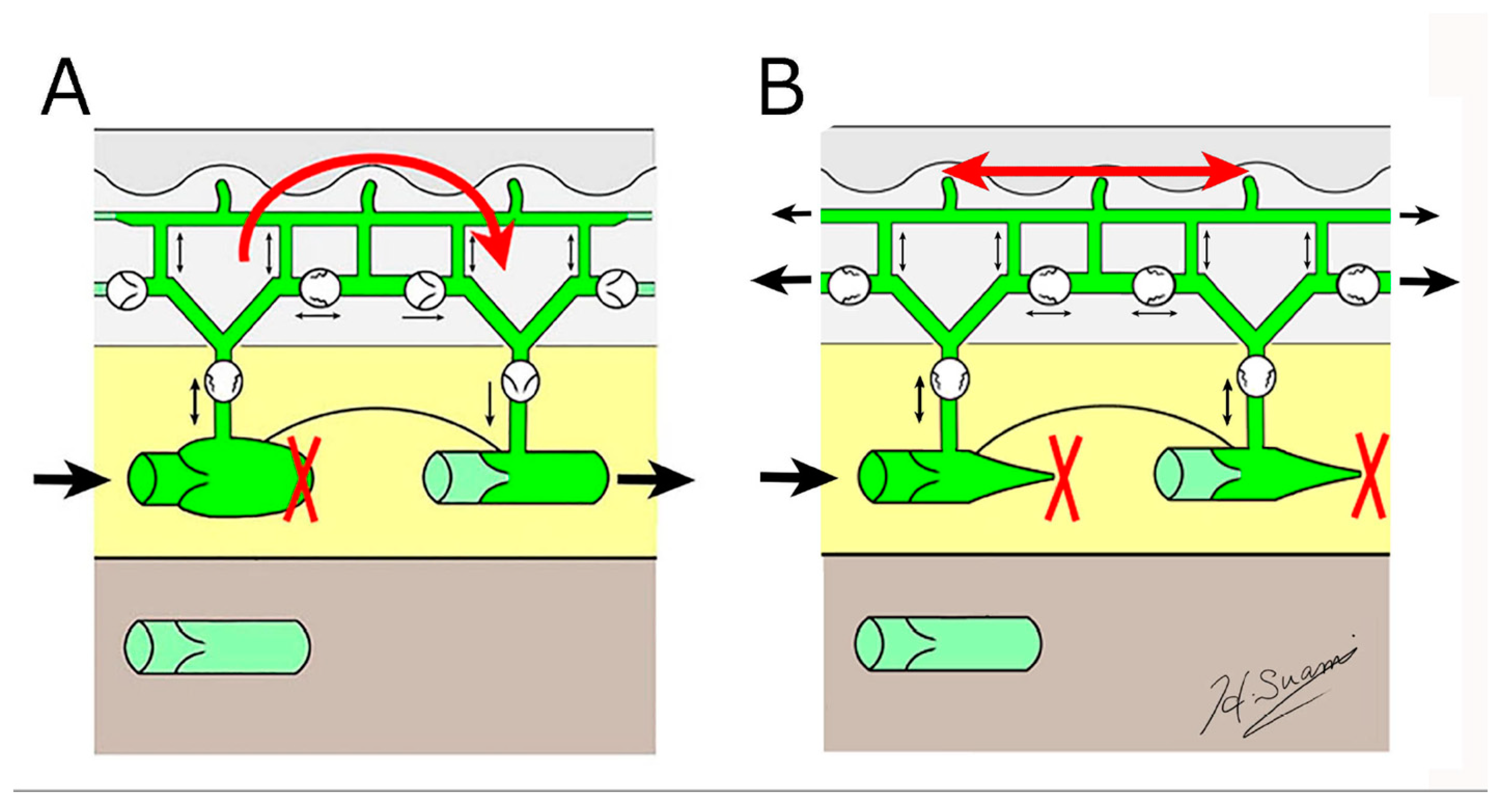
Dermal backflow has two functions in regulating lymph transport in lymphoedema (Figure 5).
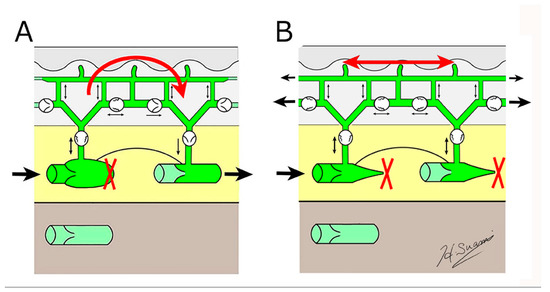
Figure 5.
Schematic diagrams showing the structural changes in lymphoedema. (A) dermal backflow forms a bridge between obstructed and patent lymphatic vessels, and (B) dermal backflow serves as a substitute route to transport lymph fluid following the obstruction of the superficial collecting vessels through fibrosis. (Reproduced with permission of Hiroo Suami).
Firstly, if patent, superficial lymph-collecting vessels remain in the affected limb, it bridges the gap between the non-patent and patent vessels. Secondly, if all the superficial lymph-collecting vessels have deteriorated, the dermal backflow becomes the only pathway to drain lymph fluid from the affected area.
The lymph capillaries and pre-collectors in the dermis are several times smaller than the lymph-collecting vessels [19], so if dermal backflow intervenes in the process of lymphatic drainage, lymph flow is restricted. Consequently, dermal backflow is considered a negative pathological change in lymphoedema because patients feel discomfort, and it causes skin induration in the affected area due to restricted flow. However, in anatomical terms, dermal backflow is a positive change as it enables lymph drainage to be maintained by creating an alternative pathway.
3.2. Lymphangiogenesis
The lymphatic system can regenerate after lymph node dissection. Animal studies found that although lymph nodes themselves did not regenerate, the lymphatic vessels could regenerate to develop new lymph drainage pathways (Figure 6) [36,37]. The types of vessels able to regenerate were both the fine lymphatic capillaries and also the larger lymph-collecting vessels. In these studies, lymphangiogenesis started at the site of severed, afferent lymphatic vessels, with the newly regenerated vessels connecting to the remaining lymph nodes to maintain the lymph drainage pathways.
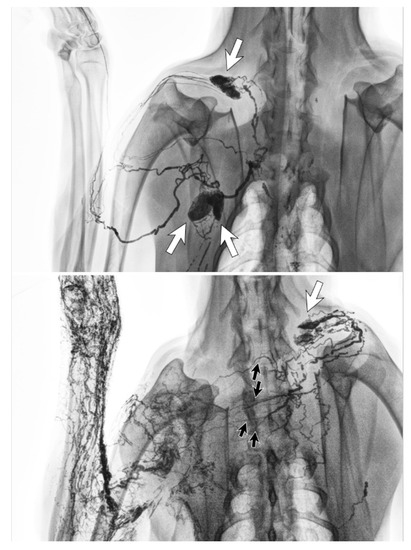
Figure 6.
Lymphangiography images of a canine before lymph node dissection surgery (top) and six months afterwards (bottom). The lymphatic vessels from the left forelimb crossed the front midline and connected to the right cervical lymph node (white arrow) via regenerated lymphatic vessels (black arrows). (Reproduced from [36] with permission.).
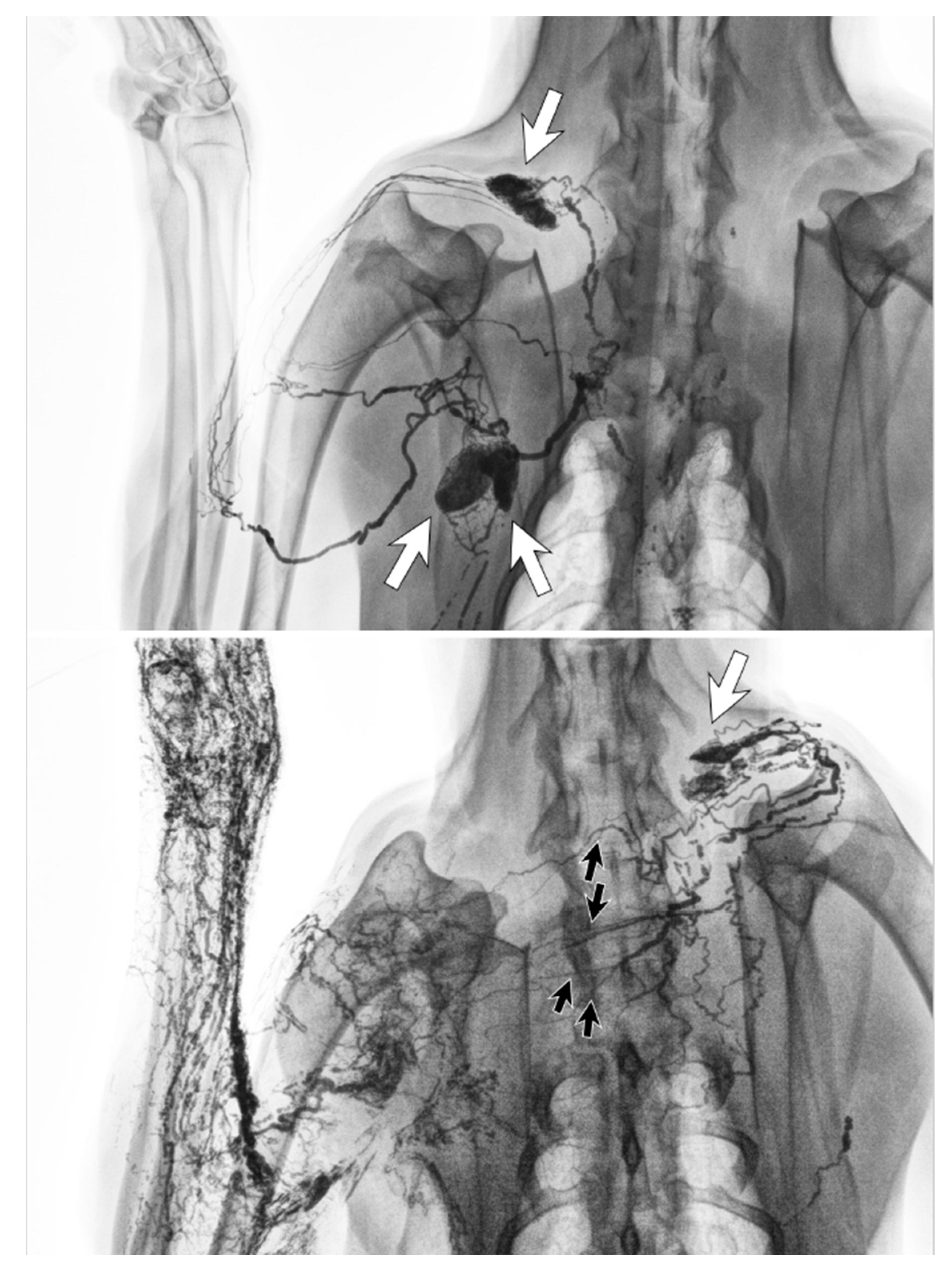
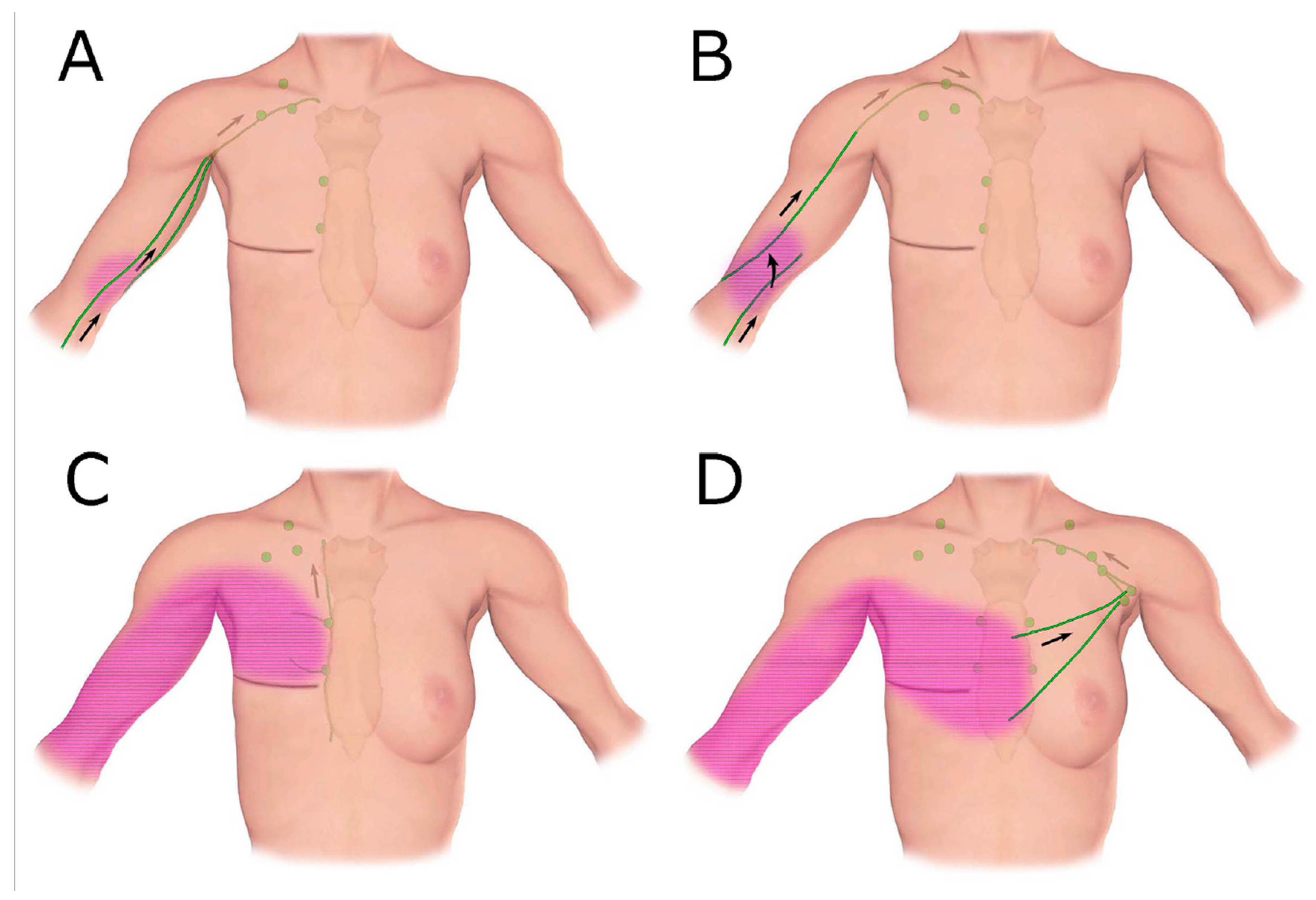
Clinical imaging studies using lymphangiography, lymphoscintigraphy and ICG lymphography also found that the lymphatic system in the affected limb could reconnect to the remaining lymph nodes through lymphangiogenesis [38,39,40,41,42]. The location of the remaining nodes varied from the ipsilateral axillary, supraclavicular, internal mammary and contralateral axillary nodes (Figure 7) [42].
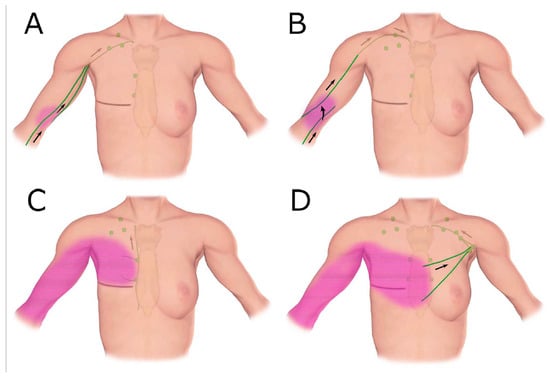
Figure 7.
Schematic diagrams show patterns of lymphatic drainage in upper extremity lymphoedema. (A) The ipsilateral axillary region, (B) the clavicular region, (C) the parasternal region, (D) The contralateral axillary region. (Reproduced from [42] with permission.).
3.3. Detour via the Deep Lymphatic System
When a patient undergoes lymph node dissection and does not develop lymphoedema later, the common assumption is that a part of the original lymphatic pathway is intact. For example, breast surgeons do not skeletonize the axillary vein during axillary node dissection, because anecdotal experience suggests an increasing chance of lymphoedema development. However, anatomical studies indicate that both the breast and arm sentinel nodes were located in level-one nodes in the lateral axillary region [43]. Although the deep lymph-collecting vessels may be preserved by the remaining soft tissue around the axillary vein, axillary node dissection always creates a surgical gap in the lymphatic pathway from the arm. This suggests that there is another mechanism at play that is preventing the development of lymphoedema in these patients.
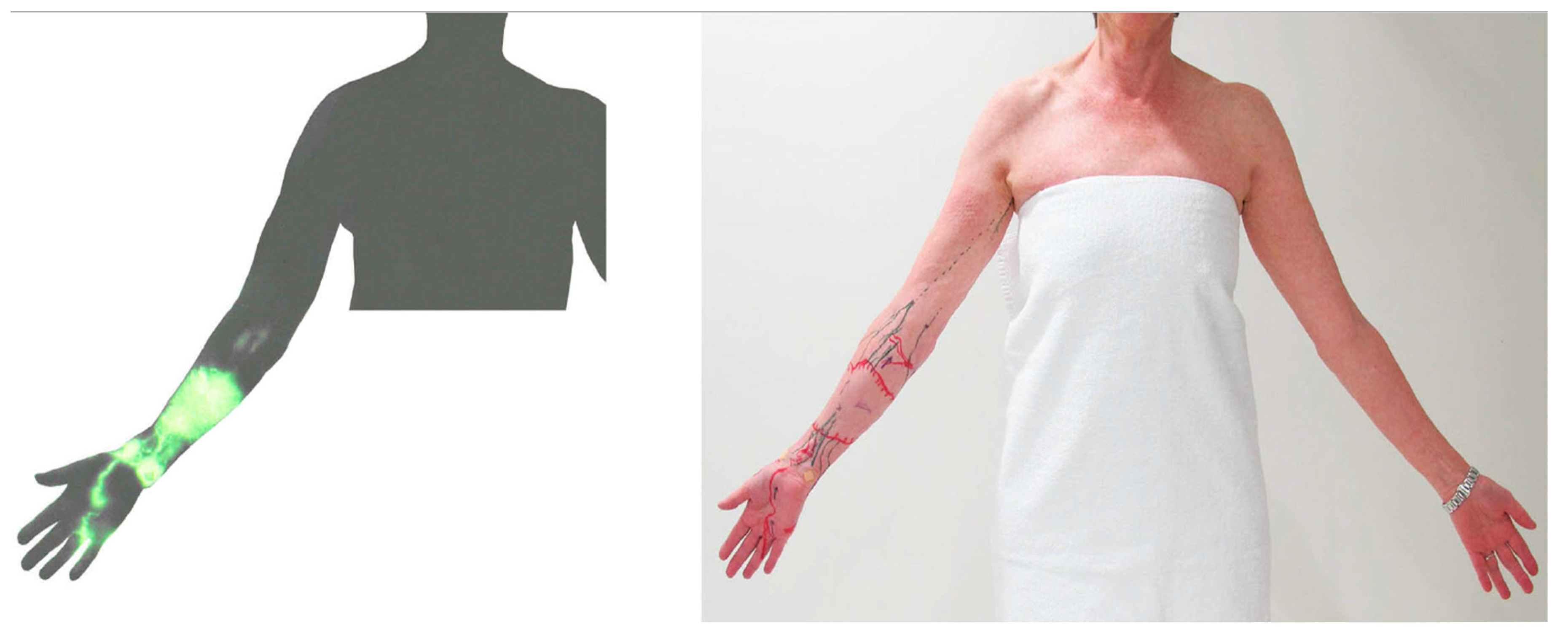
We have previously reported that the superficial and deep lymph-collecting vessels are independent of each other in normal anatomy [18,19]. However, post-operative lymphatic anatomy changes significantly from the original anatomy to maintain lymphatic drainage pathways. ICG lymphography in some lymphoedema patients identified that the superficial lymph-collecting vessels connected to the deep lymph collecting vessels at the medial elbow (Figure 8). A cadaver study using post-axillary node dissection also found the same unusual connection [28]. It appears that the preservation of the deep lymphatic pathway during axillary node dissection may help to establish connections between the superficial and deep lymphatic systems and serve as a detour route to prevent the progression of lymphoedema.

Figure 8.
Indocyanine green (ICG) lymphography image (left) and tracing photo (right) in a patient with breast cancer-related lymphoedema. The ICG signal disappeared at the elbow and reappeared in the axilla. This suggests a connection between superficial and deep lymphatics.
3.4. Detour via the Lymphatics in The Torso
The lymphatic system in the torso can be affected by lymph node dissection, with either breast or abdominal oedema identified. Newer techniques have been developed to reduce the extent of breast surgery, and breast-conserving therapy is becoming a more popular option in early breast cancer [44]. Breast-conserving therapy and oncoplastic surgery can achieve better cosmetic outcomes [45]. However, combined lymph node dissection and radiation therapy compromises lymphatic drainage from the remaining breast tissue and gives rise to the possibility of developing breast oedema [46].
Breast and abdominal oedema can be independent of, or associated with, lymphoedema in the limbs. In some cases of advanced breast cancer-related lymphoedema, dermal backflow covered the whole arm and extended to the front chest. The tracer drained to the internal mammary lymph nodes via the perforating lymphatic vessels or crossed the front sagittal midline and drained to the contralateral axillary nodes via normal lymphatics in the unaffected breast (Figure 9) [17].
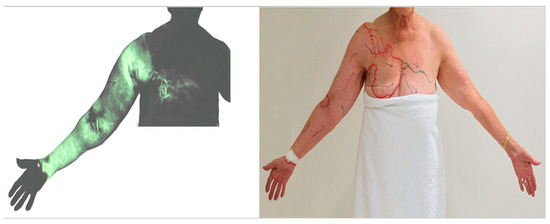
Figure 9.
ICG lymphography image (left) and tracing photo (right) in a patient with breast cancer-related lymphoedema. Dermal backflow in the right arm extended to the right front chest and then connected to the left axillary lymph node via the superficial lymph-collecting vessels in the left chest. (Reproduced from [17] with permission).
In these cases, the lymphatic system in the torso worked as a collateral lymphatic drainage pathway from the affected arm. Similar anatomical changes were observed in leg lymphoedema. Advanced unilateral leg lymphoedema sometimes drained to the contralateral inguinal nodes via the lymphatics in the lower abdomen. The possible interaction of the lymphatic system between the limbs and torso should be considered in post-lymph node dissection because limb lymphoedema can cause breast and abdominal oedema.
4. Anatomical Theories of Cancer-Related Lymphoedema
Knowledge of normal lymphatic anatomy and an understanding of how it changes post-operatively may shed light on the pathophysiology of lymphoedema. Olszewski conducted lymphangiography studies to investigate post-operative lymphoedema in animal models [47]. His results found that post-operative lymphatic anatomy was significantly changed from the original anatomy, although clinical lymphoedema did not develop immediately, or sometimes at all. When lymphoedema developed after surgery, the time from anatomical structural change to the onset of clinical symptoms took from a minimum 7–8 months to several years in some cases. The anatomical changes included dilatation of both dermal lymphatics and lymph-collecting vessels. Below, this manuscript will describe a framework for the different stages of lymphatic vessel change after lymph node dissection.
4.1. Lymph Node Dissection
When axillary lymph node dissection is performed, the superficial lymphatic drainage pathway from the arm is interrupted and a surgical break is created because arm sentinel nodes are located in level-one nodes in the lateral axilla [18]. The deep lymphatic system may also be interrupted when level-two nodes are removed. If the patient has supplementary drainage pathways to the supraclavicular node in the lateral upper arm, they will be outside the sphere of dissection and will be able to keep draining lymph fluid [24,25].
When the break occurs, lymph fluid leaks from the stump of the lymph-collecting vessels, and the surgical drains remove the pooled fluid in the defective tissue. Lymphoedema does not develop immediately because excess fluid is removed from the wound.
4.2. Lymphangiogenesis
Lymphangiogenesis occurs during the wound-healing process to restore the break caused by surgery. New lymphatics are regenerated from the distal stumps of the arm lymph-collecting vessels and connect to the remaining lymph nodes [36]. The patient may notice some arm swelling, but the symptoms gradually subside. The initial process to restore lymphatic pathways in this way may take around one month [47].
4.3. Latent Phase
The regenerated lymphatics develop from immature vessels into stable, mature vessels. If this process is successful, lymphoedema may not develop at all. Scar formation, delayed wound-healing with seroma, post-operative irradiation, and cellulitis are the factors that are considered to adversely impact the maturation process and may cause regression of the immature vessels.
4.4. Development of Lymphoedema
If the temporarily restored lymphatics lose function, lymphoedema will develop. Obstruction of the superficial lymph-collecting vessels causes incompetent valves to form in the pre-collectors, resulting in dermal backflow, forming a connection between patent and non-patent lymph-collecting vessels [32,42]. If the deep lymph-collecting vessels are intact, the obstructed, superficial lymph-collecting vessels may create a detour to the deep lymphatics (Figure 8). The previous dominant pathway to the axilla may still work, but the restriction of lymph flow will cause dermal backflow in the distal arm. Alternative pathways to the other lymph nodes will develop to maintain lymph drainage in the lymphoedematous limb (Figure 9) [42].
Olszewski’s study found that the gap in time between lymph node dissection and lymphoedema development may be explained by the length of time taken for the initial bridging process through lymphangiogenesis to occur, followed by the regression of the regenerated lymphatics [47]. Akita’s prospective cohort study of ICG lymphography in breast cancer patients found that dermal backflow could be identified in patients in the latent phase of lymphoedema, where no clinical symptoms were evident [48]. This study suggested that ICG lymphography could be used to screen for breast cancer-related lymphoedema at the subclinical stage.
Several ideas have been proposed to prevent the development of lymphoedema. One is the vascular endothelial growth factor C (VGEF-C) drug that has been developed to the clinical trial stage [49]. Another is Boccardo’s creation of a microsurgical lymphovenous bypass during axillary dissection to protect the lymph drainage pathways from the arm and promote healing [50]. A third approach is a synthetic fibrous thread called a “BioBridge” that has been developed to facilitate the process of lymphatic regeneration [51]. These new procedures may prevent the regression of newly regenerated lymphatic vessels during the latent phase.
The exact factors that determine which patients will develop lymphoedema after cancer treatment have not yet been identified. However, medical imaging plays an important role in screening patients before lymphoedema develops, and techniques to do this are advancing all the time. Anatomical theories are also vital in promoting understanding about what structural changes occur post-surgery and how these changes correlate with lymphoedema development.
5. Conclusions
Lymphoedema is a debilitating condition that affects many people who have survived cancer. However, we are still unable at present to identify who among them will develop lymphoedema and who will not. This paper has reported on the anatomical changes that occur after surgery and how they contribute to the maintenance of lymphatic drainage pathways. We have also described how the mechanism of lymphangiogenesis works to bridge the gap in the lymphatics caused by surgery and demonstrated that regression of the immature lymphatic vessels may explain the delayed onset of cancer-related lymphoedema.
Funding
This research received no external funding.
Acknowledgments
The authors thank Belinda Thompson and Philippa Sutton for editorial assistance with the preparation of this article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Lee, B.B.; Andrade, M.; Antignani, P.L.; Boccardo, F.; Bunke, N.; Campisi, C.; Damstra, R.; Flour, M.; Forner-Cordero, I.; Gloviczki, P.; et al. Diagnosis and treatment of primary lymphedema. Consensus document of the International Union of Phlebology (IUP)-2013. Int. Angiol. 2013, 32, 541–574. [Google Scholar] [PubMed]
- Mehrara, B.; Zampell, J.C.; Suami, H.; Chang, D.W. Surgical Management of Lymphedema: Past, Present, and Future. Lymphat. Res. Biol. 2011, 9, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Chang, D.W. Overview of Surgical Treatments for Breast Cancer–Related Lymphedema. Plast. Reconstr. Surg. 2010, 126, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Boyages, J.; Xu, Y.; Kalfa, S.; Koelmeyer, L.A.; Parkinson, B.; Mackie, H.; Viveros, H.; Gollan, P.; Taksa, L. Financial cost of lymphedema borne by women with breast cancer. Psycho-Oncology 2016, 26, 849–855. [Google Scholar] [CrossRef] [PubMed]
- McDuff, S.G.; Mina, A.I.; Brunelle, C.L.; Salama, L.; Warren, L.E.; Abouegylah, M.; Swaroop, M.; Skolny, M.N.; Asdourian, M.; Gillespie, T.; et al. Timing of Lymphedema After Treatment for Breast Cancer: When Are Patients Most At Risk? Int. J. Radiat. Oncol. 2019, 103, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.; Chang, J.; Choi, S.; Kim, Y. Risk of Lymphedema Following Contemporary Treatment for Breast Cancer: An Analysis of 7,426 Consecutive Patients from a Multidisciplinary Perspective. Int. J. Radiat. Oncol. 2019, 105, E52. [Google Scholar] [CrossRef]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 2018, 7, 379–403. [Google Scholar] [CrossRef]
- Zou, L.; Liu, F.-H.; Shen, P.-P.; Hu, Y.; Liu, X.-Q.; Xu, Y.-Y.; Pen, Q.-L.; Wang, B.; Zhu, Y.-Q.; Tian, Y. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: A 2-year follow-up prospective cohort study. Breast Cancer 2018, 25, 309–314. [Google Scholar] [CrossRef]
- Frueh, F.S.; Gousopoulos, E.; Rezaeian, F.; Menger, M.D.; Lindenblatt, N.; Giovanoli, P.; Information, P.E.K.F.C. Animal models in surgical lymphedema research—A systematic review. J. Surg. Res. 2016, 200, 208–220. [Google Scholar] [CrossRef]
- Hadamitzky, C.; Pabst, R. Acquired Lymphedema: An Urgent Need for Adequate Animal Models. Cancer Res. 2008, 68, 343–345. [Google Scholar] [CrossRef]
- Witte, C.L.; Witte, M.H.; Unger, E.C.; Williams, W.H.; Bernas, M.J.; McNeill, G.C.; Stazzone, A.M. Advances in Imaging of Lymph Flow Disorders. Radiographics 2000, 20, 1697–1719. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.F.; Lu, Q.; Jiang, Z.-H.; Wang, C.-G.; Zhou, J.-G. Anatomic and functional evaluation of the lymphatics and lymph nodes in diagnosis of lymphatic circulation disorders with contrast magnetic resonance lymphangiography. J. Vasc. Surg. 2009, 49, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Unno, N.; Inuzuka, K.; Suzuki, M.; Yamamoto, N.; Sagara, D.; Nishiyama, M.; Konno, H. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J. Vasc. Surg. 2007, 45, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Chang, D.W.; Yamada, K.; Kimata, Y. Use of Indocyanine Green Fluorescent Lymphography for Evaluating Dynamic Lymphatic Status. Plast. Reconstr. Surg. 2011, 127, 74e–76e. [Google Scholar] [CrossRef]
- Aldrich, M.B.; Guilliod, R.; Fife, C.E.; Maus, E.A.; Smith, L.; Rasmussen, J.C.; Sevick-Muraca, E.M. Lymphatic abnormalities in the normal contralateral arms of subjects with breast cancer-related lymphedema as assessed by near-infrared fluorescent imaging. Biomed. Opt. Express 2012, 3, 1256–1265. [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Doi, K.; Oshima, A.; Ogata, F.; Mihara, M.; Koshima, I.; Mundinger, G.S. Characteristic Indocyanine Green Lymphography Findings in Lower Extremity Lymphedema: The Generation of a Novel Lymphedema Severity Staging System Using Dermal Backflow Patterns. Plast. Reconstr. Surg. 2011, 127, 1979–1986. [Google Scholar] [CrossRef]
- Suami, H.; Heydon-White, A.; Mackie, H.; Czerniec, S.; Koelmeyer, L.A.; Boyages, J. A new indocyanine green fluorescence lymphography protocol for identification of the lymphatic drainage pathway for patients with breast cancer-related lymphoedema. BMC Cancer 2019, 19, 985–987. [Google Scholar] [CrossRef]
- Suami, H.; Kato, S. Anatomy of the Lymphatic System and Its Structural Disorders in Lymphoedema, 2nd ed.; Lee, B.B., Rockson, S.G., Bergan, J., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 57–78. [Google Scholar]
- Suami, H.; Taylor, G.I.; Pan, W.-R. The Lymphatic Territories of the Upper Limb: Anatomical Study and Clinical Implications. Plast. Reconstr. Surg. 2007, 119, 1813–1822. [Google Scholar] [CrossRef]
- Brorson, H.; Ohlin, K.; Olsson, G.; Nilsson, M. Adipose Tissue Dominates Chronic Arm Lymphedema Following Breast Cancer: An Analysis Using Volume Rendered CT Images. Lymphat. Res. Biol. 2006, 4, 199–210. [Google Scholar] [CrossRef]
- Brorson, H.; Ohlin, K.; Olsson, G.; Karlsson, M.K. Breast Cancer-Related Chronic Arm Lymphedema Is Associated with Excess Adipose and Muscle Tissue. Lymphat. Res. Biol. 2009, 7, 3–10. [Google Scholar] [CrossRef]
- Suami, H. Lymphosome concept: Anatomical study of the lymphatic system. J. Surg. Oncol. 2016, 115, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, M.F.; Suami, H. Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Semin. Plast. Surg. 2018, 32, 005–011. [Google Scholar] [CrossRef] [PubMed]
- Kubik, S. The role of the lateral upper arm bundle and the lymphatic watersheds in the formation of collateral pathways in lymphedema. Acta Biol. Acad. Sci. Hung. 1980, 31, 191–200. [Google Scholar] [PubMed]
- Johnson, A.R.; Bravo, M.G.; James, T.A.; Suami, H.; Lee, B.T.; Singhal, D. The All but Forgotten Mascagni–Sappey Pathway: Learning from Immediate Lymphatic Reconstruction. J. Reconstr. Microsurg. 2019, 36, 028–031. [Google Scholar] [CrossRef]
- Shinaoka, A.; Koshimune, S.; Yamada, K.; Kumagishi, K.; Suami, H.; Kimata, Y.; Ohtsuka, A. Correlations between Tracer Injection Sites and Lymphatic Pathways in the Leg. Plast. Reconstr. Surg. 2019, 144, 634–642. [Google Scholar] [CrossRef]
- Shinaoka, A.; Koshimune, S.; Suami, H.; Yamada, K.; Kumagishi, K.; Boyages, J.; Kimata, Y.; Ohtsuka, A. Lower-Limb Lymphatic Drainage Pathways and Lymph Nodes: A CT Lymphangiography Cadaver Study. Radiology 2019, 294, 223–229. [Google Scholar] [CrossRef]
- Suami, H.; Pan, W.-R.; Taylor, G.I. Changes in the Lymph Structure of the Upper Limb after Axillary Dissection: Radiographic and Anatomical Study in a Human Cadaver. Plast. Reconstr. Surg. 2007, 120, 982–991. [Google Scholar] [CrossRef]
- Sappey, M.P.C. Amatomie, Physiologie, Pathologie des Vaisseaux lymphatiques Consideres Chez l’Homme et les Vertebres; A Delahaye & E Lecroisnier: Paris, France, 1874. [Google Scholar]
- Kinmonth, J.B. The Lymphatics: Diseases, Lymphography and Surgery; Edward Arnold: London, UK, 1972. [Google Scholar]
- Weissleder, H.; Weissleder, R. Lymphedema: Evaluation of qualitative and quantitative lymphoscintigraphy in 238 patients. Radiology 1988, 167, 729–735. [Google Scholar] [CrossRef]
- Suami, H.; Pan, W.-R.; I Taylor, G. The lymphatics of the skin filled by a dermal backflow: An observation in a scarred cadaver leg. Lymphology 2007, 40, 122–126. [Google Scholar]
- Chang, D.W.; Suami, H. Discussion: Comparison of indocyanine green lymphographic findings with the conditions of collecting lymphatic vessels of limbs in patients with lymphedema. Plast. Reconstr. Surg. 2013, 132, 1619–1621. [Google Scholar] [CrossRef]
- Chang, D.W.; Suami, H.; Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast. Reconstr. Surg. 2013, 132, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Suami, H.; Hanasono, M.M.; Womack, V.A.; Wong, F.C.; Chang, E.I. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J. Surg. Oncol. 2016, 115, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Scaglioni, M.F.; Dixon, K.A.; Tailor, R.C. Interaction between vascularized lymph node transfer and recipient lymphatics after lymph node dissection-a pilot study in a canine model. J. Surg. Res. 2016, 204, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Yamashita, S.; Soto-Miranda, M.A.; Chang, D.W. Lymphatic Territories (Lymphosomes) in a Canine: An Animal Model for Investigation of Postoperative Lymphatic Alterations. PLoS ONE 2013, 8, e69222. [Google Scholar] [CrossRef] [PubMed]
- Tsangaris, N.T.; Yutzy, C.V. A lymphangiographic study of postmastectomy lymphedema. Surg. Gynecol. Obstet. 1966, 123, 1228–1232. [Google Scholar]
- Abe, R. A Study on the Pathogenesis of Postmastectomy Lymphedema. Tohoku J. Exp. Med. 1976, 118, 163–171. [Google Scholar] [CrossRef]
- Bourgeois, P.; Frühling, J.; Henry, J. Jacques Postoperative axillary lymphoscintigraphy in the management of breast cancer. Int. J. Radiat. Oncol. 1983, 9, 29–32. [Google Scholar] [CrossRef]
- Szuba, A.; Chachaj, Z.; Koba-Wszedybylb, M.; Hawro, R.; Jasinski, R.; Tarkowski, R.; Szewczyk, K.; Bebenek, M.; Forgacz, J.; Jodkowska, A.; et al. Axillary lymph nodes and arm lymphatic drainage pathways are spared during routine complete axillary clearance in majority of women undergoing breast cancer surgery. Lymphology 2011, 44, 103–112. [Google Scholar]
- Suami, H.; Koelmeyer, L.A.; Mackie, H.; Boyages, J. Patterns of lymphatic drainage after axillary node dissection impact arm lymphoedema severity: A review of animal and clinical imaging studies. Surg. Oncol. 2018, 27, 743–750. [Google Scholar] [CrossRef]
- Suami, H.; O’neill, J.K.; Pan, W.-R.; Taylor, G.I. Superficial Lymphatic System of the Upper Torso: Preliminary Radiographic Results in Human Cadavers. Plast. Reconstr. Surg. 2008, 121, 1231–1239. [Google Scholar] [CrossRef]
- Lagendijk, M.; Van Maaren, M.C.; Saadatmand, S.; Strobbe, L.J.; Poortmans, P.M.; Koppert, L.B.; Tilanus-Linthorst, M.M.; Siesling, S. Breast conserving therapy and mastectomy revisited: Breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int. J. Cancer 2017, 142, 165–175. [Google Scholar] [CrossRef]
- Berry, M.; Fitoussi, A.; Curnier, A.; Couturaud, B.; Salmon, R. Oncoplastic breast surgery: A review and systematic approach. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Belsack, D.; Buyl, R.; Ruggiero, L.; Breucq, C.; De Mey, J.; Lievens, P.; Lamote, J. Ultrasound elastography as an objective diagnostic measurement tool for lymphoedema of the treated breast in breast cancer patients following breast conserving surgery and radiotherapy. Radiol. Oncol. 2012, 46, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, W. Pathophysiological and clinical observations of obstructive lymphedema of the limbs. In Lymphedema; Clodius, L., Ed.; Georg Thieme Verlag: Stuttgar, Germany, 1977; pp. 79–102. [Google Scholar]
- Akita, S.; Nakamura, R.; Yamamoto, N.; Tokumoto, H.; Ishigaki, T.; Yamaji, Y.; Sasahara, Y.; Kubota, Y.; Mitsukawa, N.; Satoh, K. Early Detection of Lymphatic Disorder and Treatment for Lymphedema following Breast Cancer. Plast. Reconstr. Surg. 2016, 138, 192e–202e. [Google Scholar] [CrossRef] [PubMed]
- Tervala, T.V.; Hartiala, P.; Tammela, T.; Visuri, M.T.; Ylä-Herttuala, S.; Alitalo, K.; Saarikko, A.M. Growth factor therapy and lymph node graft for lymphedema. J. Surg. Res. 2015, 196, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, F.; Casabona, F.; Decian, F.; Friedman, D.; Murelli, F.; Puglisi, M.; Campisi, C.; Molinari, L.; Spinaci, S.; Dessalvi, S.; et al. Lymphatic Microsurgical Preventing Healing Approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: Over 4 years follow-up. Microsurgery 2014, 34, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, D.H.; Inchauste, S.; Zelones, J.; Nguyen, D. The role of adjunct nanofibrillar collagen scaffold implantation in the surgical management of secondary lymphedema: Review of the literature and summary of initial pilot studies. J. Surg. Oncol. 2019, 121, 121–128. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).