Galectins and Ovarian Cancer
Abstract
:1. Introduction
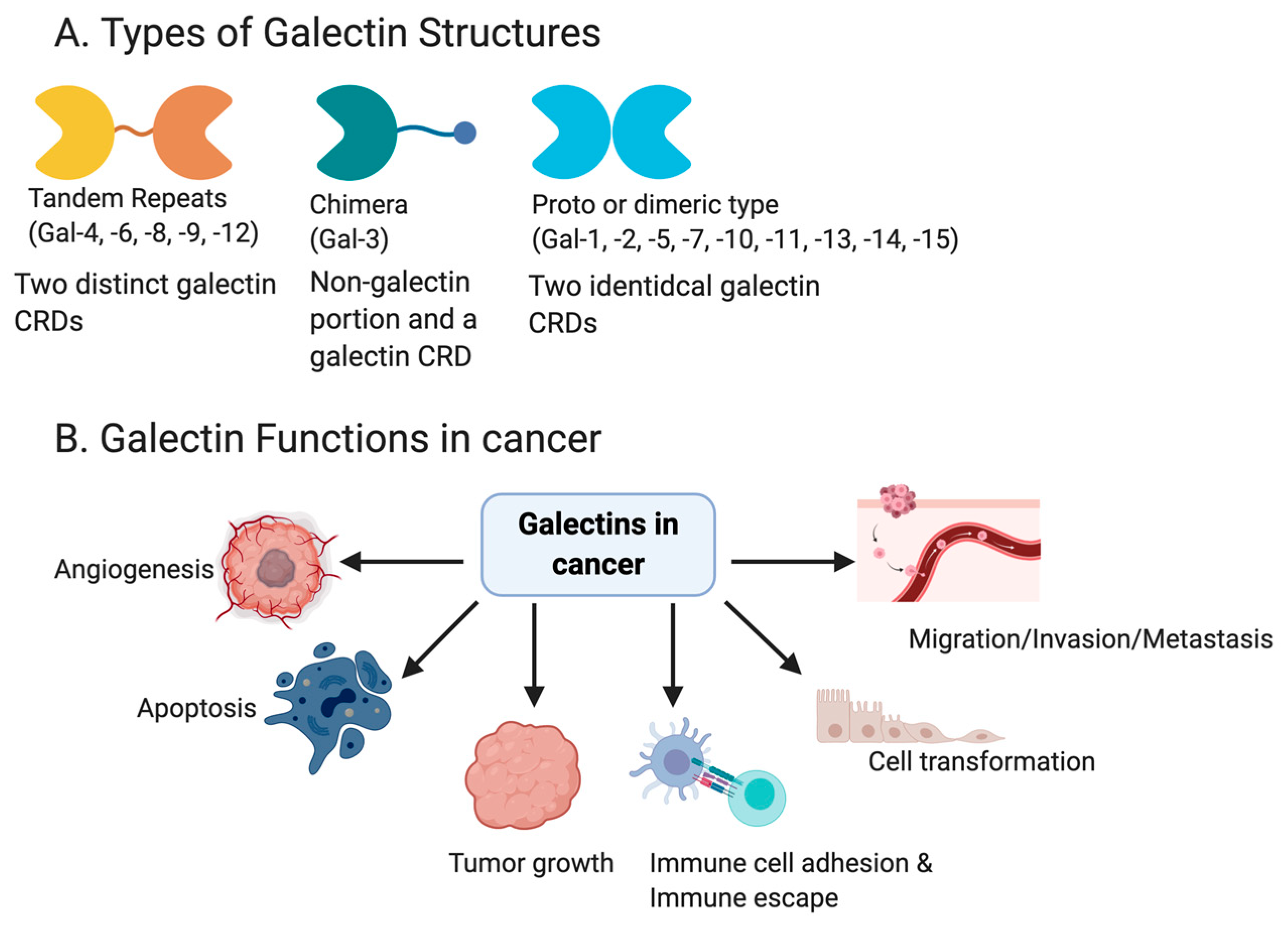
1.1. General Role of Galectins
1.2. Galectin Mediated Immune Functions
1.3. Galectins and Cancer
1.4. Ovarian Cancer and the Implication of Galectins in Its Pathology
2. Galectin-1 (LGALS1)
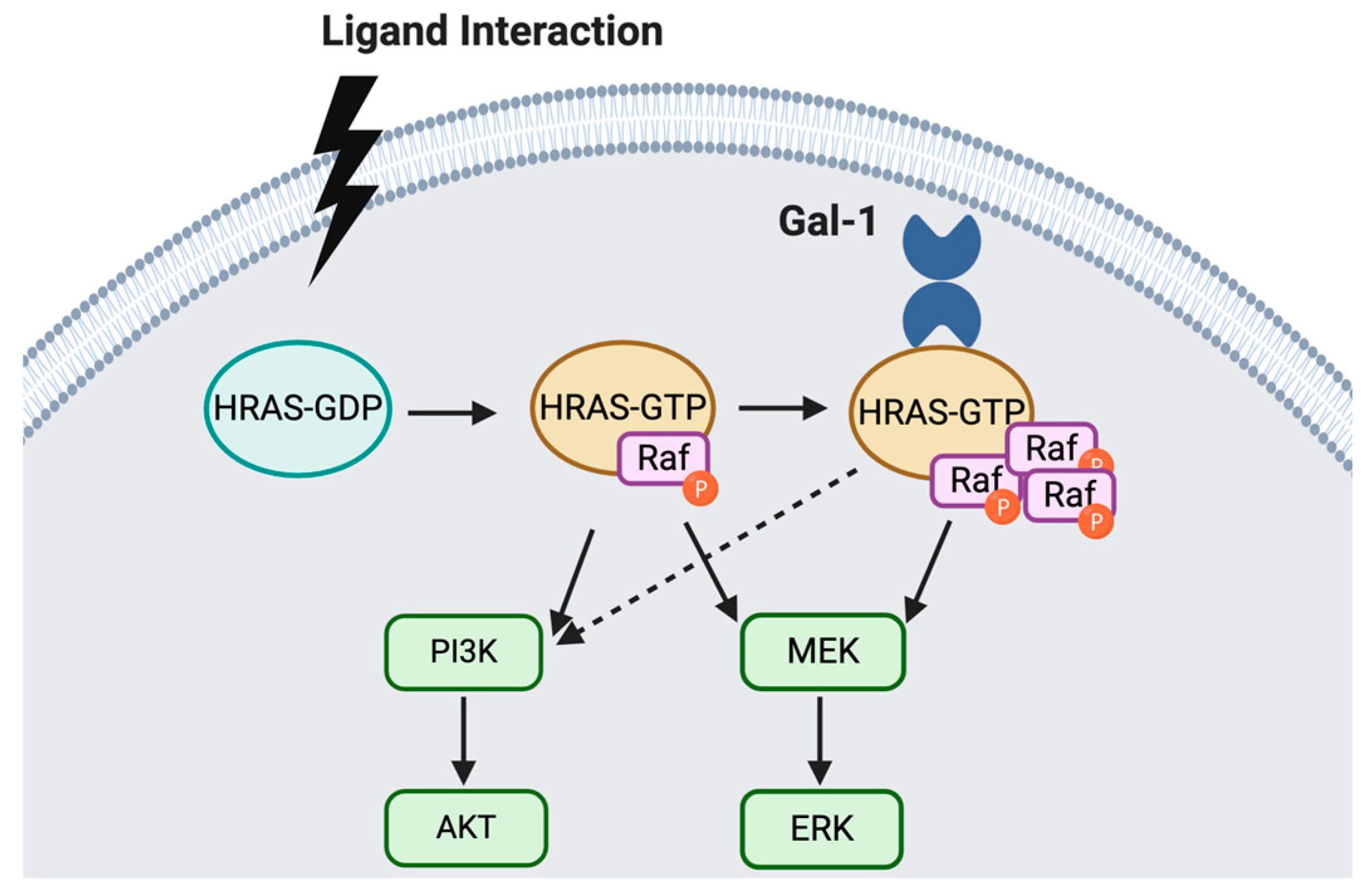
2.1. General Functional Roles of Galectin-1
2.2. Gal-1 Expression and Localization in Ovarian Cancer
2.3. Gal-1 Function in Ovarian Cancer
2.4. Clinical Implication of Gal-1 as a Biomarker
3. Galectin 3-LGALS3
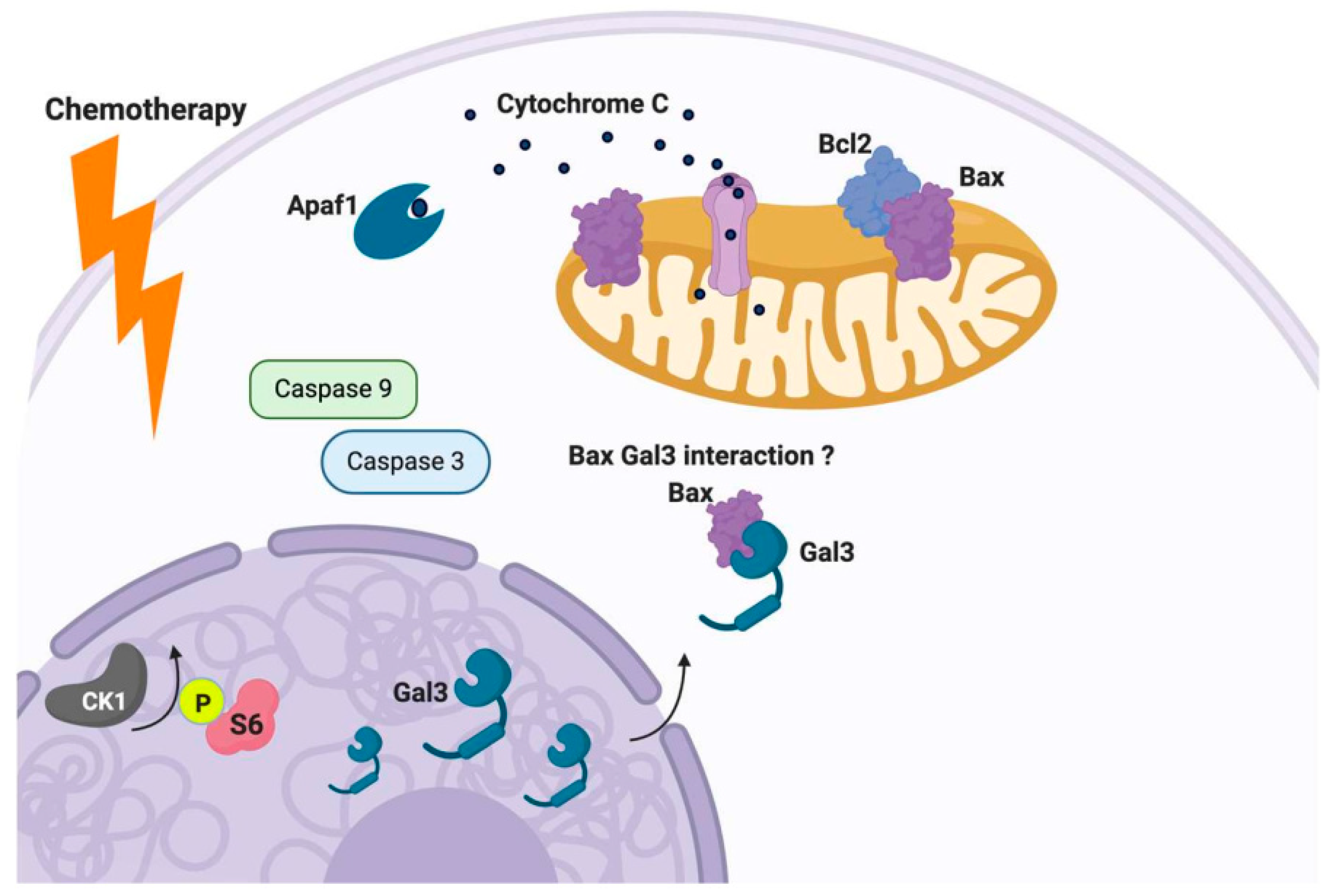
3.1. General Function of Gal-3
3.2. Galectin-3 Expression, Localization and Relevance as a Biomarker of Ovarian Cancer
3.3. Function of Gal-3 in Ovarian Cancer
4. Galectin 7 (LGALS7)
4.1. General Function of Gal-7
4.2. Galectin-7 Expression, Localization and Relevance as a Biomarker of Ovarian Cancer
4.3. Gal-7 Function in Ovarian Cancer
5. Galectin-8 (LGALS8)
5.1. General Function of Gal-8
5.2. Gal-8 Expression and Localization in Ovarian Cancer
5.3. Relevance of Gal-8 as a Biomarker for Ovarian Cancer
6. Galectin-9 (LGALS9)
6.1. General Function of Gal-9
6.2. Gal-9 Expression and Localization in Ovarian Cancer
6.3. Gal-9 Function in Ovarian Cancer
6.4. Relevance of Gal-9 as a Biomarker of Ovarian Cancer
7. Therapeutic Targeting of Galectins
7.1. General Strategies for Disrupting Galectin Mediated Effects
7.2. Specific Targeting of Galectins in Cancer
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKT | Protein kinase-B |
| Bcl-2 | B cell lymphoma-2 |
| BH3 | Bcl-2 homology 3 |
| BrdU | Bromodeoxyuridine |
| CA125 | Cancer antigen 125 |
| CBD | Collagen-binding domains |
| CCK-8 | Cell counting kit 8 |
| CRD | Carbohydrate recognition domain |
| CTLA-4 | Cytotoxic T-lymphocyte associated factor-4 |
| Cyt C | Cytochrome C |
| DBF | Dibenzofuran |
| DDR | DNA damage response |
| DFS | Disease free survival |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial mesenchymal transition |
| ELISA | Enzyme-linked immunosorbent assay |
| FITC | Fluorescein isothiocyanate |
| Gal-1 | Galectin-1 |
| Gal-11 | Galectin-11 |
| Gal-13 | Galectin-13 |
| Gal-14 | Galectin-14 |
| Gal-15 | Galectin-15 |
| Gal-2 | Galectin-2 |
| Gal-3 | Galectin-3 |
| Gal-5 | Galectin-5 |
| Gal-7 | Galectin-7 |
| Gal-8 | Galectin-8 |
| Gal-9 | Galectin-9 |
| HIV | Human immunodeficiency virus |
| HGSC | High grade serous cancer |
| IL-17 | Interleukin-17 |
| Il-2 | Interleukin-2 |
| JNK1 | C-jun-NH2-terminal kinase 1 |
| LPS | Lipopolysaccharide |
| MALP2 | Macrophage-activating lipopetide-2 |
| MET | Mesenchymal epithelial transition |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MUC1 | Mucin 1 |
| MUC5AC | Mucin 5AC |
| MUC6 | Mucin 6 |
| MUC16 | Mucin 16 |
| NF-IL6 | Nuclear factor for interleukin 6 |
| NWGR | Aspartate-tryptophan-glycine-arginine |
| OS | Overall survival |
| PBMC | Peripheral blood mononuclear cell |
| PFS | Progression free survival |
| PI3K | Phosphoinositol 3 kinase |
| shRNA | Small hairpin RNA |
| siRNA | Small interfering RNA |
| TG | Thomsen–Friedenreich (TF) |
| TLR | Toll-like receptor |
| TLR-3 | Toll-like receptor-3 |
| TLR-4 | Toll-like receptor-4 |
| TMA | Tissue microarray |
| Treg | T regulatory |
References
- Levi, G.; I Teichberg, V. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J. Boil. Chem. 1981, 256, 5735–5740. [Google Scholar]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; A Gitt, M.; Hirabayashi, J.; Hughes, C.; Kasai, K. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Teichberg, V.I.; Silman, I.; Beitsch, D.D.; Resheff, G. A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc. Natl. Acad. Sci. USA 1975, 72, 1383–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, C.A.; A Dunlap, K.; Burghardt, R.; Spencer, T.E. Galectin-15 in ovine uteroplacental tissues. Reproduction 2005, 130, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, D.; Littler, D.; Shahine, A.; Troy, S.; Johnson, M.; Rossjohn, J.; Piedrafita, D.; Beddoe, T. Cloning, expression, purification and crystallographic studies of galectin-11 from domestic sheep (Ovis aries). Acta Crystallogr. Sect. F Struct. Boil. Commun. 2015, 71, 993–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirabayashi, J.; Kasai, K. The family of metazoan metal-independent beta-galactoside-binding lectins: Structure, function and molecular evolution. Glycobiology 1993, 3, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.-L.; Liu, F.-T. The expression and function of galectins in skin physiology and pathology. Exp. Dermatol. 2018, 27, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T.; Patterson, R.J.; Wang, J.L. Intracellular functions of galectins. Biochim. Biophys. Acta (BBA) Bioenerg. 2002, 1572, 263–273. [Google Scholar] [CrossRef]
- Stowell, S.R.; Qian, Y.; Karmakar, S.; Koyama, N.S.; Dias-Baruffi, M.; Leffler, H.; McEver, R.P.; Cummings, R.D. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J. Immunol. 2008, 180, 3091–3102. [Google Scholar] [CrossRef] [Green Version]
- Rabinovich, G.A.; Toscano, M.A.; Jackson, S.S.; Vasta, G.R. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Boil. 2007, 17, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.-T.; Rabinovich, G.A. Galectins: Regulators of acute and chronic inflammation. Ann. N. Y. Acad. Sci. 2010, 1183, 158–182. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Norling, L.V.; Perretti, M. Novel insights into the inhibitory effects of Galectin-1 on neutrophil recruitment under flow. J. Leukoc. Boil. 2008, 83, 1459–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, E.A.; Montes, C.L.; Motrán, C.C.; Zuniga, E.I.; Liu, F.-T.; Rabinovich, G.A.; Gruppi, A. Galectin-3 mediates IL-4-induced survival and differentiation of B cells: Functional cross-talk and implications during Trypanosoma cruzi infection. J. Immunol. 2004, 172, 493–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilarregui, J.M.; O Croci, D.; A Bianco, G.; A Toscano, M.; Salatino, M.; E Vermeulen, M.; Geffner, J.R.; A Rabinovich, G. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat. Immunol. 2009, 10, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Toscano, M.A. Turning ’sweet’ on immunity: Galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, E.; Mizoguchi, A. Is the sugar always sweet in intestinal inflammation? Immunol. Res. 2007, 37, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.A.; Commodaro, A.G.; Ilarregui, J.M.; Bianco, G.A.; Liberman, A.; Serra, H.; Hirabayashi, J.; Rizzo, L.V.; Rabinovich, G.A. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J. Immunol. 2006, 176, 6323–6332. [Google Scholar] [CrossRef] [Green Version]
- Joo, H.G.; Goedegebuure, P.S.; Sadanaga, N.; Nagoshi, M.; Von Bernstorff, W.; Eberlein, T.J. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J. Leukoc. Boil. 2001, 69, 555–564. [Google Scholar]
- Matsuura, A.; Tsukada, J.; Mizobe, T.; Higashi, T.; Mouri, F.; Tanikawa, R.; Yamauchi, A.; Hirashima, M.; Tanaka, Y. Intracellular galectin-9 activates inflammatory cytokines in monocytes. Genes Cells 2009, 14, 511–521. [Google Scholar] [CrossRef]
- Forsman, H.; Islander, U.; Andréasson, E.; Andersson, A.; Önnheim, K.; Karlström, A.; Sävman, K.; Magnusson, M.; Brown, K.; Karlsson, A. Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis Rheum. 2011, 63, 445–454. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1855, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Saussez, S.; Lorfevre, F.; Lequeux, T.; Laurent, G.; Chantrain, G.; Vertongen, F.; Toubeau, G.; Decaestecker, C.; Kiss, R. The determination of the levels of circulating galectin-1 and -3 in HNSCC patients could be used to monitor tumor progression and/or responses to therapy. Oral Oncol. 2008, 44, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Hwang, J.-A.; Ro, J.Y.; Lee, Y.-S.; Chun, K.-H. Galectin-7 is epigenetically-regulated tumor suppressor in gastric cancer. Oncotarget 2013, 4, 1461–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Choi, I.; Cheong, T.; Lee, S.; Lotan, R.; Park, S.H.; Chun, K.-H. Galectin-3 Increases Gastric Cancer Cell Motility by Up-regulating Fascin-1 Expression. Gastroenterology 2010, 138, 1035–1045. [Google Scholar] [CrossRef]
- Nishimura, O.; Watanabe, M.; Takemasa, I.; Kaneko, N.; Yokoyama, Y.; Matsuo, E.-I.; Iwasa, S.; Mori, M.; Matsuura, N.; Monden, M. Clinical significance of circulating galectins as colorectal cancer markers. Oncol. Rep. 2011, 25, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zheng, M.; Qu, Y.; Li, J.; Ji, J.; Feng, B.; Lu, A.; Li, J.; Wang, M.; Liu, B. Different roles of galectin-9 isoforms in modulating E-selectin expression and adhesion function in LoVo colon carcinoma cells. Mol. Boil. Rep. 2008, 36, 823–830. [Google Scholar] [CrossRef]
- Sakaki, M.; Oka, N.; Nakanishi, R.; Yamaguchi, K.; Fukumori, T.; Kanayama, H.-O. Serum level of galectin-3 in human bladder cancer. J. Med. Investig. 2008, 55, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, N.; Bane, S.M.; Ahire, S.D.; Ingle, A.D.; Kalraiya, R.D. Poly N-acetyllactosamine substitutions on N- and not O-oligosaccharides or Thomsen–Friedenreich antigen facilitate lung specific metastasis of melanoma cells via galectin-3. Glycoconj. J. 2008, 26, 445–456. [Google Scholar] [CrossRef]
- Kageshita, T.; Kashio, Y.; Yamauchi, A.; Seki, M.; Abedin, M.J.; Nishi, N.; Shoji, H.; Nakamura, T.; Ono, T.; Hirashima, M. Possible role of galectin-9 in cell aggregation and apoptosis of human melanoma cell lines and its clinical significance. Int. J. Cancer 2002, 99, 809–816. [Google Scholar] [CrossRef]
- Kohrenhagen, N.; Volker, H.U.; Kapp, M.; Dietl, J.; Kammerer, U. Increased expression of galectin-1 during the progression of cervical neoplasia. Int. J. Gynecol. Cancer 2006, 16, 2018–2022. [Google Scholar] [CrossRef]
- Kim, M.K.; Sung, C.O.; Do, I.-G.; Jeon, H.-K.; Song, T.J.; Park, H.S.; Lee, Y.-Y.; Kim, B.-G.; Lee, J.-W.; Bae, D.-S. Overexpression of Galectin-3 and its clinical significance in ovarian carcinoma. Int. J. Clin. Oncol. 2011, 16, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Brustmann, H.; Riss, M.; Naudé, S. Galectin-3 Expression in Normal, Hyperplastic, and Neoplastic Endometrial Tissues. Pathol. Res. Pr. 2003, 199, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ellerhorst, J.; Nguyen, T.; Cooper, D.N.; Estrov, Y.; Lotan, D.; Lotan, R. Induction of differentiation and apoptosis in the prostate cancer cell line LNCaP by sodium butyrate and galectin-1. Int. J. Oncol. 1999, 14, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; He, B.; Kuchenbecker, K.M.; You, L.; Xu, Z.; Mikami, I.; Yagui-Beltran, A.; Clement, G.; Lin, Y.-C.; Okamoto, J.; et al. Inhibition ofWnt-2 and galectin-3 synergistically destabilizes β-catenin and induces apoptosis in human colorectal cancer cells. Int. J. Cancer 2007, 121, 1175–1181. [Google Scholar] [CrossRef]
- Matarrese, P.; Fusco, O.; Tinari, N.; Natoli, C.; Liu, F.T.; Semeraro, M.L.; Malorni, W.; Iacobelli, S. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int. J. Cancer 2000, 85, 545–554. [Google Scholar] [CrossRef]
- Demers, M.; Rose, A.; Grosset, A.-A.; Biron-Pain, K.; Gaboury, L.; Siegel, P.M.; St-Pierre, Y. Overexpression of Galectin-7, A Myoepithelial Cell Marker, Enhances Spontaneous Metastasis of Breast Cancer Cells. Am. J. Pathol. 2010, 176, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Ueda, S.; Watanabe, J.; Kuwabara, I.; Ogawa, O.; Nishiyama, H. Sensitizing Effect of Galectin-7 in Urothelial Cancer to Cisplatin through the Accumulation of Intracellular Reactive Oxygen Species. Cancer Res. 2007, 67, 1212–1220. [Google Scholar] [CrossRef] [Green Version]
- Wiersma, V.R.; De Bruyn, M.; Van Ginkel, R.J.; Sigar, E.; Hirashima, M.; Niki, T.; Nishi, N.; Samplonius, U.F.; Helfrich, W.; Bremer, E. The Glycan-Binding Protein Galectin-9 Has Direct Apoptotic Activity toward Melanoma Cells. J. Investig. Dermatol. 2012, 132, 2302–2305. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, N.; Arimoto, K.-I.; Mizutani, A.; Endo-Ichikawa, Y.; Nakada, H.; Taketani, S. Galectin-1 induces cell adhesion to the extracellular matrix and apoptosis of non-adherent human colon cancer Colo201 cells. J. Biochem. 2003, 134, 869–874. [Google Scholar] [CrossRef]
- Huang, E.-Y.; Chen, Y.-F.; Chen, Y.-M.; Lin, I.-H.; Wang, C.-C.; Su, W.-H.; Chuang, P.-C.; Yang, K.-D. A novel radioresistant mechanism of galectin-1 mediated by H-Ras-dependent pathways in cervical cancer cells. Cell Death Dis. 2012, 3, e251. [Google Scholar] [CrossRef] [Green Version]
- Chung, L.-Y.; Tang, S.-J.; Sun, G.-H.; Chou, T.-Y.; Yeh, T.-S.; Yu, S.-L.; Sun, K.-H. Galectin-1 Promotes Lung Cancer Progression and Chemoresistance by Upregulating p38 MAPK, ERK, and Cyclooxygenase-2. Clin. Cancer Res. 2012, 18, 4037–4047. [Google Scholar] [CrossRef] [Green Version]
- Moon, B.-K.; Lee, Y.J.; Battle, P.; Jessup, J.M.; Raz, A.; Kim, H.-R.C. Galectin-3 Protects Human Breast Carcinoma Cells against Nitric Oxide-Induced Apoptosis. Am. J. Pathol. 2001, 159, 1055–1060. [Google Scholar] [CrossRef]
- Dias-Baruffi, M.; Zhu, H.; Cho, M.; Karmakar, S.; McEver, R.P.; Cummings, R.D. Dimeric Galectin-1 Induces Surface Exposure of Phosphatidylserine and Phagocytic Recognition of Leukocytes without Inducing Apoptosis. J. Boil. Chem. 2003, 278, 41282–41293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, O.; Abe, M. Cell surface N-glycosylation and sialylation regulate galectin-3-induced apoptosis in human diffuse large B cell lymphoma. Oncol. Rep. 2008, 19, 743–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, S.; Kuwabara, I.; Liu, F.-T. Suppression of Tumor Growth by Galectin-7 Gene Transfer. Cancer Res. 2004, 64, 5672–5676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Califice, S.; Castronovo, V.; Bracke, M.; Brûle, F.V.D. Fr Dual activities of galectin-3 in human prostate cancer: Tumor suppression of nuclear galectin-3 vs tumor promotion of cytoplasmic galectin-3. Oncogene 2004, 23, 7527–7536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Cannistra, S.A. Cancer of the ovary. N. Engl. J. Med. 2004, 351, 2519–2529. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Prim. 2016, 2, 16061. [Google Scholar] [CrossRef]
- Chang, S.-J.; Hodeib, M.; Chang, J.; Bristow, R. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: A meta-analysis. Gynecol. Oncol. 2013, 130, 493–498. [Google Scholar] [CrossRef]
- Vergote, I.; Coens, C.; Nankivell, M.; Kristensen, G.B.; Parmar, M.K.B.; Ehlen, T.; Jayson, G.; Johnson, N.; Swart, A.M.; Verheijen, R.; et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: Pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018, 19, 1680–1687. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.K.; Brady, M.F.; Penson, R.T.; Huang, H.; Birrer, M.J.; Walker, J.L.; DiSilvestro, P.A.; Rubin, S.C.; Martin, L.P.; Davidson, S.A.; et al. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. N. Engl. J. Med. 2016, 374, 738–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A. Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R. Phase III Trial of Carboplatin and Paclitaxel Compared With Cisplatin and Paclitaxel in Patients With Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Mandika, C.; Saroj, T.; Hu, X.; Song, Y.; Zhang, J.; Zhu, H.; Zhu, X.; Chetry, M.; Thapa, S. The Role of Galectins in Tumor Progression, Treatment and Prognosis of Gynecological Cancers. J. Cancer 2018, 9, 4742–4755. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zheng, Y.; Zhang, H.; Liu, Y.; Sun, H.; Zhang, P. Galectin-1 induces metastasis and epithelial-mesenchymal transition (EMT) in human ovarian cancer cells via activation of the MAPK JNK/p38 signalling pathway. Am. J. Transl. Res. 2019, 11, 3862–3878. [Google Scholar]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A small protein with major functions. Glycobiology 2006, 16, 137R–157R. [Google Scholar] [CrossRef]
- Cho, M.; Cummings, R.D. Galectin-1, a β-Galactoside-binding Lectin in Chinese Hamster Ovary Cells. J. Boil. Chem. 1995, 270, 5198–5206. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, N.; Ilarregui, J.M.; Toscano, M.; Rabinovich, G. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens 2004, 64, 1–12. [Google Scholar] [CrossRef]
- Paz, A.; Haklai, R.; Elad-Sfadia, G.; Ballan, E.; Kloog, Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 2001, 20, 7486–7493. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001, 29, 3595–3602. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T. Galectins: Novel anti-inflammatory drug targets. Expert Opin. Ther. Targets 2002, 6, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Astorgues-Xerri, L.; Riveiro, M.E.; Tijeras-Raballand, A.; Serova, M.; Neuzillet, C.; Albert, S.; Raymond, E.; Faivre, S. Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer Treat. Rev. 2014, 40, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Brûle, F.A.V.D.; Waltregny, D.; Castronovo, V. Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. J. Pathol. 2001, 193, 80–87. [Google Scholar] [CrossRef]
- Camby, I.; Belot, N.; Rorive, S.; Lefranc, F.; Maurage, C.-A.; Lahm, H.; Kaltner, H.; Hadari, Y.; Ruchoux, M.-M.; Brotchi, J.; et al. Galectins Are Differentially Expressed in Supratentorial Pilocytic Astrocytomas, Astrocytomas, Anaplastic Astrocytomas and Glioblastomas, and Significantly Modulate Tumor Astrocyte Migration. Brain Pathol. 2006, 11, 12–26. [Google Scholar] [CrossRef]
- Chen, K.; Cai, Y.; Zhang, M.; Wu, Z.; Wu, Y. Both serum and tissue Galectin-1 levels are associated with adverse clinical features in neuroblastoma. Pediatr. Blood Cancer 2018, 65, e27229. [Google Scholar] [CrossRef]
- Shih, T.-C.; Liu, R.; Wu, C.-T.; Li, X.; Xiao, W.; Deng, X.; Kiss, S.; Wang, T.; Chen, X.; Carney, R.P.; et al. Targeting Galectin-1 Impairs Castration-Resistant Prostate Cancer Progression and Invasion. Clin. Cancer Res. 2018, 24, 4319–4331. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.L.; Wu, C.Y.; Hung, J.Y.; Lin, Y.S.; Huang, M.S.; Kuo, P.L. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin alpha6beta4 and Notch1/Jagged2 signaling pathway. Carcinogenesis 2013, 34, 1370–1381. [Google Scholar] [CrossRef] [Green Version]
- Brûle, F.V.D.; Califice, S.; Garnier, F.; Fernandez, P.L.; Berchuck, A.; Castronovo, V. Galectin-1 accumulation in the ovary carcinoma peritumoral stroma is induced by ovary carcinoma cells and affects both cancer cell proliferation and adhesion to laminin-1 and fibronectin. Lab. Investig. 2003, 83, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Jeon, H.-K.; Cho, Y.J.; Park, Y.A.; Choi, J.-J.; Do, I.-G.; Song, S.Y.; Lee, Y.-Y.; Choi, C.H.; Kim, T.-J.; et al. High galectin-1 expression correlates with poor prognosis and is involved in epithelial ovarian cancer proliferation and invasion. Eur. J. Cancer 2012, 48, 1914–1921. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, B.; Zhou, M.; Jiang, H.; Zhang, H.; Pan, X.; Gao, H.; Sun, H.; Li, Z. Galectin-1 overexpression promotes progression and chemoresistance to cisplatin in epithelial ovarian cancer. Cell Death Dis. 2014, 5, e991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Spencer-Smith, R.; O’Bryan, J.P. Direct inhibition of RAS: Quest for the Holy Grail? Semin. Cancer Boil. 2017, 54, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Pylayeva-Gupta, Y.; Grabocka, E.; Bar-Sagi, D. RAS oncogenes: Weaving a tumorigenic web. Nat. Rev. Cancer 2011, 11, 761–774. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.-F.; Wu, J.; Luo, J.-H.; Li, K.-S.; Wang, F.; Huang, W.; Wu, Y.; Gao, S.-P.; Zhang, X.-M.; Zhang, P.-N. SNHG22 overexpression indicates poor prognosis and induces chemotherapy resistance via the miR-2467/Gal-1 signaling pathway in epithelial ovarian carcinoma. Aging 2019, 11, 8204–8216. [Google Scholar] [CrossRef]
- Bin Park, G.; Chung, Y.H.; Jeong, J.-Y. Induction of galectin-1 by TLR-dependent PI3K activation enhances epithelial-mesenchymal transition of metastatic ovarian cancer cells. Oncol. Rep. 2017, 37, 3137–3145. [Google Scholar] [CrossRef]
- Shen, K.; Li, C.; Chien, L.; Huang, C.; Su, C.; Liao, A.C.; Wu, T.-F. Role of galectin-1 in urinary bladder urothelial carcinoma cell invasion through the JNK pathway. Cancer Sci. 2016, 107, 1390–1398. [Google Scholar] [CrossRef]
- Miao, J.-H.; Wang, S.-Q.; Zhang, M.-H.; Yu, F.; Zhang, L.; Yu, Z.-X.; Kuang, Y. Knockdown of galectin-1 suppresses the growth and invasion of osteosarcoma cells through inhibition of the MAPK/ERK pathway. Oncol. Rep. 2014, 32, 1497–1504. [Google Scholar] [CrossRef] [Green Version]
- Alcorn, J.F.; Guala, A.; Van Der Velden, J.; McElhinney, B.; Irvin, C.G.; Davis, R.J.; Janssen-Heininger, Y.M.W. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J. Cell Sci. 2008, 121, 1036–1045. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Kuiatse, I.; Lee, A.V.; Pan, J.; Giuliano, A.; Cui, X. Sustained c-Jun-NH2-kinase activity promotes epithelial-mesenchymal transition, invasion, and survival of breast cancer cells by regulating extracellular signal-regulated kinase activation. Mol. Cancer Res. 2010, 8, 266–277. [Google Scholar] [CrossRef] [Green Version]
- Ebelt, N.; Cantrell, M.A.; Berg, C.V.D. c-Jun N-Terminal Kinases Mediate a Wide Range of Targets in the Metastatic Cascade. Genes Cancer 2013, 4, 378–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongre, A.; A Weinberg, R. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Boil. 2018, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, L.; Zhao, L.; Zhou, R.; Ding, Y.; Li, G.; Zhao, L. LASP2 suppresses colorectal cancer progression through JNK/p38 MAPK pathway meditated epithelial-mesenchymal transition. Cell Commun. Signal. 2017, 15, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Yao, Y.; Sun, L.; Zhou, J.; Liu, J.; Wang, J.; Li, J.; Tang, J. Clinical implication of the serum galectin-1 expression in epithelial ovarian cancer patients. J. Ovarian Res. 2015, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Seelenmeyer, C.; Wegehingel, S.; Lechner, J.; Nickel, W. The cancer antigen CA125 represents a novel counter receptor for galectin-1. J. Cell Sci. 2003, 116, 1305–1318. [Google Scholar] [CrossRef] [Green Version]
- Schulz, H.; Schmoeckel, E.; Kuhn, C.; Hofmann, S.; Mayr, D.; Mahner, S.; Jeschke, U. Galectins-1, -3, and -7 Are Prognostic Markers for Survival of Ovarian Cancer Patients. Int. J. Mol. Sci. 2017, 18, 1230. [Google Scholar] [CrossRef] [Green Version]
- Labrie, M.; De Araujo, L.O.F.; Communal, L.; Mes-Masson, A.-M.; St-Pierre, Y. Tissue and plasma levels of galectins in patients with high grade serous ovarian carcinoma as new predictive biomarkers. Sci. Rep. 2017, 7, 13244. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.G.; Kim, D.-H.; Kim, S.-J.; Cho, Y.; Jung, J.; Jang, W.; Chun, K.-H. Galectin-3 supports stemness in ovarian cancer stem cells by activation of the Notch1 intracellular domain. Oncotarget 2016, 7, 68229–68241. [Google Scholar] [CrossRef] [Green Version]
- Oishi, T.; Itamochi, H.; Kigawa, J.; Kanamori, Y.; Shimada, M.; Takahashi, M.; Shimogai, R.; Kawaguchi, W.; Sato, S.; Terakawa, N. Galectin-3 may contribute to Cisplatin resistance in clear cell carcinoma of the ovary. Int. J. Gynecol. Cancer 2007, 17, 1040–1046. [Google Scholar] [CrossRef]
- Wang, D.; You, D.; Li, L. Galectin-3 regulates chemotherapy sensitivity in epithelial ovarian carcinoma via regulating mitochondrial function. J. Toxicol. Sci. 2019, 44, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Jeon, H.-K.; Lee, J.K.; Sung, C.O.; Do, I.-G.; Choi, C.H.; Kim, T.-J.; Kim, B.-G.; Bae, D.-S.; Lee, J.-W. Clinical significance of galectin-7 in epithelial ovarian cancer. Anticancer. Res. 2013, 33, 1555–1561. [Google Scholar] [PubMed]
- Labrie, M.; Vladoiu, M.C.; Grosset, A.-A.; Gaboury, L.; St-Pierre, Y. Expression and functions of galectin-7 in ovarian cancer. Oncotarget 2014, 5, 7705–7721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, H.; Kuhn, C.; Hofmann, S.; Mayr, D.; Mahner, S.; Jeschke, U.; Schmoeckel, E. Overall Survival of Ovarian Cancer Patients Is Determined by Expression of Galectins-8 and -9. Int. J. Mol. Sci. 2018, 19, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghaei, M.; Jafari, S.M.; Nazri, A.; Shabani, M.; Balajam, N.Z. Galectin-9 induces apoptosis in OVCAR-3 ovarian cancer cell through mitochondrial pathway. Res. Pharm. Sci. 2018, 13, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Califice, S.; Castronovo, V.; Brûle, F.V.D. Galectin-3 and cancer (Review). Int. J. Oncol. 2004, 25, 983–992. [Google Scholar]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavao, M.S. Extracellular Galectin-3 in Tumor Progression and Metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef] [Green Version]
- Boscher, C.; Dennis, J.W.; Nabi, I.R. Glycosylation, galectins and cellular signaling. Curr. Opin. Cell Boil. 2011, 23, 383–392. [Google Scholar] [CrossRef]
- Delacour, D.; Koch, A.; Jacob, R. The Role of Galectins in Protein Trafficking. Traffic 2009, 10, 1405–1413. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, Y.; Fukumori, T.; Raz, A. Galectin-3 and metastasis. Glycoconj. J. 2002, 19, 543–549. [Google Scholar] [CrossRef]
- Lepur, A.; Salomonsson, E.; Nilsson, U.J.; Leffler, H. Ligand Induced Galectin-3 Protein Self-association. J. Boil. Chem. 2012, 287, 21751–21756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harazono, Y.; Kho, D.H.; Balan, V.; Nakajima, K.; Zhang, T.; Hogan, V.; Raz, A. Galectin-3 leads to attenuation of apoptosis through Bax heterodimerization in human thyroid carcinoma cells. Oncotarget 2014, 5, 9992–10001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nangia-Makker, P.; Hogan, V.; Raz, A. Galectin-3 and cancer stemness. Glycobiology 2018, 28, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, N. Galectin-3 Precipitates as a Pentamer with Synthetic Multivalent Carbohydrates and Forms Heterogeneous Cross-linked Complexes. J. Boil. Chem. 2003, 279, 10841–10847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, C.-F.; Zhang, Y.; Sun, H.; Li, D.-F.; Wang, D.-C. Structural Basis for Distinct Binding Properties of the Human Galectins to Thomsen-Friedenreich Antigen. PLoS ONE 2011, 6, e25007. [Google Scholar] [CrossRef] [Green Version]
- Paron, I.; Scaloni, A.; Pines, A.; Bachi, A.; Liu, F.-T.; Puppin, C.; Pandolfi, M.; Ledda, L.; Di Loreto, C.; Damante, G.; et al. Nuclear localization of Galectin-3 in transformed thyroid cells: A role in transcriptional regulation. Biochem. Biophys. Res. Commun. 2003, 302, 545–553. [Google Scholar] [CrossRef]
- Yu, F.; Finley, R.; Raz, A.; Kim, H.-R.C. Galectin-3 Translocates to the Perinuclear Membranes and Inhibits CytochromecRelease from the Mitochondria. J. Boil. Chem. 2002, 277, 15819–15827. [Google Scholar] [CrossRef] [Green Version]
- Gilson, R.C.; Gunasinghe, S.D.; Johannes, L.; Gaus, K. Galectin-3 modulation of T-cell activation: Mechanisms of membrane remodelling. Prog. Lipid Res. 2019, 76, 101010. [Google Scholar] [CrossRef]
- Wu, K.-L.; Kuo, C.-M.; Huang, E.-Y.; Pan, H.-M.; Huang, C.-C.; Chen, Y.-F.; Hsiao, C.-C.; Yang, K.D. Extracellular galectin-3 facilitates colon cancer cell migration and is related to the epidermal growth factor receptor. Am. J. Transl. Res. 2018, 10, 2402–2412. [Google Scholar]
- Ochieng, J.; Furtak, V.; Lukyanov, P. Extracellular functions of galectin-3. Glycoconj. J. 2002, 19, 527–535. [Google Scholar] [CrossRef]
- Bänfer, S.; Schneider, M.; Dewes, J.; Strauss, M.T.; Freibert, S.A.; Heimerl, T.; Maier, U.G.; Elsasser, H.-P.; Jungmann, R.; Jacob, R. Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc. Natl. Acad. Sci. USA 2018, 115, E4396–E4405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Giobbie-Hurder, A.; Connolly, E.M.; Li, J.; Liao, X.; Severgnini, M.; Zhou, J.; Rodig, S.; Hodi, F.S. Anti-CTLA-4 based therapy elicits humoral immunity to galectin-3 in patients with metastatic melanoma. OncoImmunology 2018, 7, e1440930–e1441025. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.H.; Rode, J.; Howlader, A.; Eckermann, M.; Santos, J.T.; Armada, D.H.; Zheng, R.; Zou, C.; Cairo, C.W. Galectin-3 alters the lateral mobility and clustering of β1-integrin receptors. PLoS ONE 2017, 12, e0184378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boscher, C.; Zheng, Y.Z.; Lakshminarayan, R.; Johannes, L.; Dennis, J.W.; Foster, L.J.; Nabi, I.R. Galectin-3 Protein Regulates Mobility of N-cadherin and GM1 Ganglioside at Cell-Cell Junctions of Mammary Carcinoma Cells. J. Boil. Chem. 2012, 287, 32940–32952. [Google Scholar] [CrossRef] [Green Version]
- Newlaczyl, A.U.; Yu, L.-G. Galectin-3 – A jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef]
- Elmore, S.A. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Humlová, Z. Protooncogene bcl-2 in process of apoptosis. Review article. Sb. Lek. 2002, 103, 419–425. [Google Scholar]
- Al-Alem, L.; Baker, A.; Pandya, U.; Eisenhauer, E.; Rueda, B.R. Understanding and Targeting Apoptotic Pathways in Ovarian Cancer. Cancers 2019, 11, 1631. [Google Scholar] [CrossRef] [Green Version]
- Fukumori, T.; Oka, N.; Takenaka, Y.; Nangia-Makker, P.; Elsamman, E.; Kasai, T.; Shono, M.; Kanayama, H.-O.; Ellerhorst, J.; Lotan, R.; et al. Galectin-3 Regulates Mitochondrial Stability and Antiapoptotic Function in Response to Anticancer Drug in Prostate Cancer. Cancer Res. 2006, 66, 3114–3119. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, S.; Tian, Y.; Wang, Y.; Zhang, Q.; Zhou, X.; Meng, X.; Song, N. Prognostic role of galectin-3 expression in patients with solid tumors: A meta-analysis of 36 eligible studies. Cancer Cell Int. 2018, 18, 172. [Google Scholar] [CrossRef]
- Al-Alem, L.F.; Pandya, U.M.; Baker, A.T.; Bellio, C.; Zarrella, B.D.; Clark, J.; DiGloria, C.M.; Rueda, B.R. Ovarian cancer stem cells: What progress have we made? Int. J. Biochem. Cell Boil. 2019, 107, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Bellio, C.; DiGloria, C.; Foster, R.; James, K.; Konstantinopoulos, P.A.; Growdon, W.B.; Rueda, B.R. PARP Inhibition Induces Enrichment of DNA Repair–Proficient CD133 and CD117 Positive Ovarian Cancer Stem Cells. Mol. Cancer Res. 2018, 17, 431–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilmer, M.; Mazurek, N.; Byrd, J.C.; Ramirez, K.; Hafley, M.; Alt, E.; Vykoukal, J.; Bresalier, R.S. Cell surface galectin-3 defines a subset of chemoresistant gastrointestinal tumor-initiating cancer cells with heightened stem cell characteristics. Cell Death Dis. 2016, 7, e2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, R.S.; Fernandes, V.C.; Nepomuceno, T.C.; Rodrigues, D.C.; Woods, N.T.; Suarez-Kurtz, G.; Chammas, R.; Monteiro, A.N.; Carvalho, M.A. Characterization of LGALS3 (galectin-3) as a player in DNA damage response. Cancer Boil. Ther. 2014, 15, 840–850. [Google Scholar] [CrossRef] [Green Version]
- Diao, B.; Liu, Y.; Xu, G.; Zhang, Y.; Xie, J.; Gong, J. The role of galectin-3 in the tumorigenesis and progression of pituitary tumors. Oncol. Lett. 2018, 15, 4919–4925. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Glinsky, V.V.; Landon, L.A.; Matthews, L.; Deutscher, S. Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancer cell adhesion. Carcinogenesis 2004, 26, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Khaldoyanidi, S.K.; Glinsky, V.V.; Sikora, L.; Glinskii, A.B.; Mossine, V.V.; Quinn, T.P.; Glinsky, G.V.; Sriramarao, P. MDA-MB-435 Human Breast Carcinoma Cell Homo- and Heterotypic Adhesion under Flow Conditions Is Mediated in Part by Thomsen-Friedenreich Antigen-Galectin-3 Interactions. J. Boil. Chem. 2002, 278, 4127–4134. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.-G. The oncofetal Thomsen–Friedenreich carbohydrate antigen in cancer progression. Glycoconj. J. 2007, 24, 411–420. [Google Scholar] [CrossRef]
- Ghazizadeh, M.; Ogawa, H.; Sasaki, Y.; Araki, T.; Aihara, K. Mucin carbohydrate antigens (T, Tn, and sialyl-Tn) in human ovarian carcinomas: Relationship with histopathology and prognosis. Hum. Pathol. 1997, 28, 960–966. [Google Scholar] [CrossRef]
- Ricardo, S.; Da Silva, L.M.; David, L. Mucin carriers of TF in ovarian cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1867–1868. [Google Scholar] [CrossRef]
- Pinto, R.; Carvalho, A.S.; Conze, T.; Magalhães, A.; Picco, G.; Burchell, J.; Taylor-Papadimitriou, J.; Reis, C.A.; Almeida, R.; Mandel, U.; et al. Identification of new cancer biomarkers based on aberrant mucin glycoforms by in situ proximity ligation. J. Cell. Mol. Med. 2012, 16, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, S.; Da Silva, L.M.; Pereira, D.; Pinto, R.; Almeida, R.; Söderberg, O.; Mandel, U.; Clausen, H.; Félix, A.; Lunet, N.; et al. Detection of glyco-mucin profiles improves specificity of MUC16 and MUC1 biomarkers in ovarian serous tumours. Mol. Oncol. 2014, 9, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Akita, K.; Yashiro, M.; Sawada, T.; Hirakawa, K.; Murata, T.; Nakada, H. Binding of Galectin-3, a β-Galactoside-binding Lectin, to MUC1 Protein Enhances Phosphorylation of Extracellular Signal-regulated Kinase 1/2 (ERK1/2) and Akt, Promoting Tumor Cell Malignancy. J. Boil. Chem. 2015, 290, 26125–26140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Barclay, M.; Hilkens, J.; Guo, X.; Barrow, H.; Rhodes, J.M.; Yu, L.-G. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 2010, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Guo, X.; Nash, G.B.; Stone, P.C.; Hilkens, J.; Rhodes, J.M.; Yu, L.-G. Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 2009, 69, 6799–6806. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.-G.; Andrews, N.; Zhao, Q.; Mckean, D.; Williams, J.F.; Connor, L.J.; Gerasimenko, J.V.; Hilkens, J.; Hirabayashi, J.; Kasai, K.; et al. Galectin-3 Interaction with Thomsen-Friedenreich Disaccharide on Cancer-associated MUC1 Causes Increased Cancer Cell Endothelial Adhesion. J. Boil. Chem. 2006, 282, 773–781. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Kaur, T.; Kamboj, S.S.; Singh, J. Roles of Galectin-7 in Cancer. Asian Pac. J. Cancer Prev. 2016, 17, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Advedissian, T.; Deshayes, F.; Viguier, M. Galectin-7 in Epithelial Homeostasis and Carcinomas. Int. J. Mol. Sci. 2017, 18, 2760. [Google Scholar] [CrossRef] [Green Version]
- Advedissian, T.; Proux-Gillardeaux, V.; Nkosi, R.; Peyret, G.; Nguyen, T.; Poirier, F.; Viguier, M.; Deshayes, F. E-cadherin dynamics is regulated by galectin-7 at epithelial cell surface. Sci. Rep. 2017, 7, 17086. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Rabinovich, G.A.; Liu, F.-T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10. [Google Scholar] [CrossRef]
- Si, Y.; Wang, Y.; Gao, J.; Song, C.; Feng, S.; Zhou, Y.; Tai, G.; Su, J. Crystallization of Galectin-8 Linker Reveals Intricate Relationship between the N-terminal Tail and the Linker. Int. J. Mol. Sci. 2016, 17, 2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadari, Y.R.; Paz, K.; Dekel, R.; Mestrovic, T.; Accili, D.; Zick, Y. Galectin-8. J. Boil. Chem. 1995, 270, 3447–3453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, V.M.C.; Nugnes, L.G.; Colombo, L.L.; Troncoso, M.F.; Fernández, M.M.; Malchiodi, E.L.; Frahm, I.; Croci, D.O.; Compagno, D.; Rabinovich, G.A.; et al. Modulation of endothelial cell migration and angiogenesis: A novel function for the “tandem-repeat” lectin galectin-8. FASEB J. 2010, 25, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Norambuena, A.; Metz, C.; Vicuna, L.; Silva, A.; Pardo, E.; Oyanadel, C.; Massardo, L.; Gonzalez, A.; Soza, A. Galectin-8 induces apoptosis in Jurkat T cells by phosphatidic acid-mediated ERK1/2 activation supported by protein kinase A down-regulation. J. Biol. Chem. 2009, 284, 12670–12679. [Google Scholar] [CrossRef] [Green Version]
- Nishi, N.; Itoh, A.; Shoji, H.; Miyanaka, H.; Nakamura, T. Galectin-8 and galectin-9 are novel substrates for thrombin. Glycobiology 2006, 16, 15C–20C. [Google Scholar] [CrossRef] [Green Version]
- Heusschen, R.; Griffioen, A.W.; Thijssen, V.L. Galectin-9 in tumor biology: A jack of multiple trades. Biochim. Biophys. Acta (BBA) Rev. Cancer 2013, 1836, 177–185. [Google Scholar] [CrossRef]
- Kim, S.-N.; Lee, H.-J.; Jeon, M.-S.; Yi, T.; Song, S.U. Galectin-9 is Involved in Immunosuppression Mediated by Human Bone Marrow-derived Clonal Mesenchymal Stem Cells. Immune Netw. 2015, 15, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, S.; Mori, H.; Kobara, H.; Rafiq, K.; Niki, T.; Hirashima, M.; Masaki, T. Galectin-9 in Cancer Therapy. Recent Patents Endocr. Metab. Immune Drug Discov. 2013, 7, 130–137. [Google Scholar] [CrossRef]
- Fujita, K.; Iwama, H.; Oto, T.; Okura, R.; Kobayashi, K.; Takano, J.; Katsura, A.; Tatsuta, M.; Maeda, E.; Mimura, S.; et al. Galectin-9 suppresses the growth of hepatocellular carcinoma via apoptosis in vitro and in vivo. Int. J. Oncol. 2015, 46, 2419–2430. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Jin, M.-S.; Kong, F.; Cao, N.; Ma, H.-X.; Jia, Z.; Wang, Y.-P.; Suo, J.; Cao, X. Decreased Galectin-9 and Increased Tim-3 Expression Are Related to Poor Prognosis in Gastric Cancer. PLoS ONE 2013, 8, e81799. [Google Scholar] [CrossRef]
- He, Y.; Jia, K.; Dziadziuszko, R.; Zhao, S.; Zhang, X.; Deng, J.; Wang, H.; Hirsch, F.; Zhou, C. Galectin-9 in non-small cell lung cancer. Lung Cancer 2019, 136, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Irie, A.; Yamauchi, A.; Kontani, K.; Kihara, M.; Liu, D.; Shirato, Y.; Seki, M.; Nishi, N.; Nakamura, T.; Yokomise, H.; et al. Galectin-9 as a Prognostic Factor with Antimetastatic Potential in Breast Cancer. Clin. Cancer Res. 2005, 11, 2962–2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, J.; Yamamoto, M.; Nagoshi, H.; Kobayashi, T.; Sasaki, N.; Horiike, S.; Kimura, S.; Yamauchi, A.; Hirashima, M.; Taniwaki, M.; et al. Targeting Activating Transcription Factor 3 by Galectin-9 Induces Apoptosis and Overcomes Various Types of Treatment Resistance in Chronic Myelogenous Leukemia. Mol. Cancer Res. 2010, 8, 994–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiersma, V.R.; De Bruyn, M.; Helfrich, W.; Bremer, E. Therapeutic potential of Galectin-9 in human disease. Med. Res. Rev. 2011, 33, E102–E126. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kuroda, J.; Ashihara, E.; Oomizu, S.; Terui, Y.; Taniyama, A.; Adachi, S.; Takagi, T.; Yamamoto, M.; Sasaki, N.; et al. Galectin-9 exhibits anti-myeloma activity through JNK and p38 MAP kinase pathways. Leukemia 2010, 24, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Lahm, H.; André, S.; Hoeflich, A.; Fischer, J.R.; Sordat, B.; Kaltner, H.; Wolf, E.; Gabius, H.-J. Comprehensive galectin fingerprinting in a panel of 61 human tumor cell lines by RT-PCR and its implications for diagnostic and therapeutic procedures. J. Cancer Res. Clin. Oncol. 2001, 127, 375–386. [Google Scholar] [CrossRef]
- Aanhane, E.; Schulkens, I.A.; Heusschen, R.; Castricum, K.; Leffler, H.; Griffioen, A.W.; Thijssen, V.L. Different angioregulatory activity of monovalent galectin-9 isoforms. Angiogenesis 2018, 21, 545–555. [Google Scholar] [CrossRef] [Green Version]
- Lagana, A.; Goetz, J.G.; Cheung, P.; Raz, A.; Dennis, J.W.; Nabi, I.R. Galectin Binding to Mgat5-Modified N-Glycans Regulates Fibronectin Matrix Remodeling in Tumor Cells. Mol. Cell. Boil. 2006, 26, 3181–3193. [Google Scholar] [CrossRef] [Green Version]
- Zavareh, R.B.; Sukhai, M.A.; Hurren, R.; Gronda, M.; Wang, X.; Simpson, C.D.; MacLean, N.; Zih, F.; Ketela, T.; Swallow, C.J.; et al. Suppression of Cancer Progression by MGAT1 shRNA Knockdown. PLoS ONE 2012, 7, e43721. [Google Scholar] [CrossRef] [Green Version]
- Feldcamp, L.; Doucet, J.-S.; Pawling, J.; Fadel, M.P.; Fletcher, P.J.; Maunder, R.G.; Dennis, J.; Wong, A. Mgat5 modulates the effect of early life stress on adult behavior and physical health in mice. Behav. Brain Res. 2016, 312, 253–264. [Google Scholar] [CrossRef]
- Traber, P.G.; Chou, H.; Zomer, E.; Hong, F.; Klyosov, A.; Fiel, M.-I.; Friedman, S.L. Regression of Fibrosis and Reversal of Cirrhosis in Rats by Galectin Inhibitors in Thioacetamide-Induced Liver Disease. PLoS ONE 2013, 8, e75361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streetly, M.J.; Maharaj, L.; Joel, S.; Schey, S.A.; Gribben, J.G.; Cotter, F.E. GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood 2010, 115, 3939–3948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inohara, H.; Raz, A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj. J. 1994, 11, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Klyosov, A.A.; Dotsenko, G.S.; Hinz, S.W.; Sinitsyn, A.P. Structural features of beta-(1-->4)-D-galactomannans of plant origin as a probe for beta-(1-->4)-mannanase polymeric substrate specificity. Carbohydr. Res. 2012, 352, 65–69. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Abdelmalek, M.F.; Garcia-Tsao, G.; Vuppalanchi, R.; Alkhouri, N.; Rinella, M.; Noureddin, M.; Pyko, M.; Shiffman, M.; Sanyal, A.; et al. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients With Nonalcoholic Steatohepatitis With Cirrhosis and Portal Hypertension. Gastroenterology 2020, 158, 1334–1345. [Google Scholar] [CrossRef] [Green Version]
- Hirani, N.; Nicol, L.; MacKinnon, A.; Ford, P.; Schambye, H.; Nilsson, U.; Leffler, H.; Thomas, T.; Knott, O. TD139, A Novel Inhaled Galectin-3 Inhibitor for The Treatment of Idiopathic Pulmonary Fibrosis (IPF). Results from The First in (IPF) Patients Study. QJM Int. J. Med. 2016, 109, S16. [Google Scholar] [CrossRef] [Green Version]
- Astorgues-Xerri, L.; Riveiro, M.E.; Tijeras-Raballand, A.; Serova, M.; Rabinovich, G.A.; Bièche, I.; Vidaud, M.; De Gramont, A.; Martinet, M.; Cvitkovic, E.; et al. OTX008, a selective small-molecule inhibitor of galectin-1, downregulates cancer cell proliferation, invasion and tumour angiogenesis. Eur. J. Cancer 2014, 50, 2463–2477. [Google Scholar] [CrossRef]
- Zucchetti, M.; Bonezzi, K.; Frapolli, R.; Sala, F.; Borsotti, P.; Zangarini, M.; Cvitkovic, E.; Noel, K.; Ubezio, P.; Giavazzi, R.; et al. Pharmacokinetics and antineoplastic activity of galectin-1-targeting OTX008 in combination with sunitinib. Cancer Chemother. Pharmacol. 2013, 72, 879–887. [Google Scholar] [CrossRef]
- Dings, R.; Kumar, N.; Miller, M.C.; Loren, M.; Rangwala, H.; Hoye, T.R.; Mayo, K.H. Structure-based optimization of angiostatic agent 6DBF7, an allosteric antagonist of galectin-1. J. Pharmacol. Exp. Ther. 2012, 344, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Vuong, L.; Kouverianou, E.; Rooney, C.M.; McHugh, B.J.; Howie, S.E.; Gregory, C.D.; Forbes, S.J.; Henderson, N.C.; Zetterberg, F.R.; Nilsson, U.J.; et al. An Orally Active Galectin-3 Antagonist Inhibits Lung Adenocarcinoma Growth and Augments Response to PD-L1 Blockade. Cancer Res. 2019, 79, 1480–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Tang, X.; Lu, J.; Yuan, S. Therapeutic inhibition of galectin-3 improves cardiomyocyte apoptosis and survival during heart failure. Mol. Med. Rep. 2017, 17, 4106–4112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croci, D.O.; Salatino, M.; Rubinstein, N.; Cerliani, J.P.; Cavallin, L.E.; Leung, H.J.; Ouyang, J.; Ilarregui, J.M.; Toscano, M.A.; Domaica, C.I.; et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J. Exp. Med. 2012, 209, 1985–2000. [Google Scholar] [CrossRef] [PubMed]

| Galectin | Histological Subtypes | Distribution | Cellular Localization | Function in Ovarian Cancer | Prognosis |
|---|---|---|---|---|---|
| Gal-1 | Serous Endometrioid Mucinous Clear cell [70,84,86] | Tumor cell Tumor stroma [69] Stroma [70] | Nucleus, Cytoplasm [70] | Mediate EMT, cell proliferation, migration, invasion, and cell signaling [71,76] | Higher levels of Gal-1 in the peritumoral stroma associated with poor PFS [70] Cytoplasmic levels of Gal-1 were closely related with OS [86] Interstitial Gal-1 was an independent prognostic factor in ovarian cancer patients [86] |
| Gal-3 | Serous Endometrioid Mucinous Clear cell [87] | Cancer cell Stroma [86,87] | Cytoplasm [31,86,87] Nucleus [86] | Contributing to stem-like properties [88] Promoting chemoresistance [31,88,89,90] Promoting cell invasion and migration [88] | High Gal-3 cytoplasmic expression correlated with poor PFS [31] Low Gal-3 expression in nuclei associated with reduced OS [86] High Gal-3 expression correlated with reduced OS [90] |
| Gal-7 | Serous Endometrioid Mucinous [91] Serous Endometrioid Mucinous Clear cell [86] | Epithelial cell [92] | Nucleus, Cytoplasm [91] Cytoplasm [86] | Down-regulation of Gal-7 expression inhibited tumor cell proliferation [91] Expression increased the invasive properties of ovarian cancer cells and by killing immune cells [92] | Higher levels had a more inferior OS [91] High expression reduced OS and higher expression is an independent prognostic factor for OS [86] |
| Gal-8 | Serous Endometrioid Mucinous Clear cell [87,93] | Tumor cell [87,93] Stroma [87] | Nucleus [93] Cytoplasm [87,93] | None found | High expression in epithelial component correlated with chemoresistance [87] Positive nuclear staining correlated with lower stage [93] Low expression in cytoplasm correlated with lymph node metastasis and higher stage [93] Higher plasma levels correlated with lower 5-year DFS and 5-year OS [87] High expression in the cytoplasm correlated with better DFS and OS [93] |
| Gal-9 | Serous Endometrioid Mucinous clear cell [87,93] | Cancer cell [87,93] Stroma [87] | Cytoplasm [87,93] | Inhibiting cell proliferation and pushing cells towards apoptosis [94] | High expression presented more often with low tumor stage, lower grading, and younger age [93] Epithelial expression correlated with a lower 5-year OS [87] High plasma levels were associated with a lower 5-year DFS and 5-year OS [87] High expression showed the more favorable PFS and OS [93] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, C.; Xu, R.; Al-Alem, L.; Stasenko, M.; Spriggs, D.R.; Rueda, B.R. Galectins and Ovarian Cancer. Cancers 2020, 12, 1421. https://doi.org/10.3390/cancers12061421
Shimada C, Xu R, Al-Alem L, Stasenko M, Spriggs DR, Rueda BR. Galectins and Ovarian Cancer. Cancers. 2020; 12(6):1421. https://doi.org/10.3390/cancers12061421
Chicago/Turabian StyleShimada, Chisa, Rui Xu, Linah Al-Alem, Marina Stasenko, David R. Spriggs, and Bo R. Rueda. 2020. "Galectins and Ovarian Cancer" Cancers 12, no. 6: 1421. https://doi.org/10.3390/cancers12061421
APA StyleShimada, C., Xu, R., Al-Alem, L., Stasenko, M., Spriggs, D. R., & Rueda, B. R. (2020). Galectins and Ovarian Cancer. Cancers, 12(6), 1421. https://doi.org/10.3390/cancers12061421






