Current Strategies and Novel Therapeutic Approaches for Metastatic Urothelial Carcinoma
Abstract
:1. Introduction
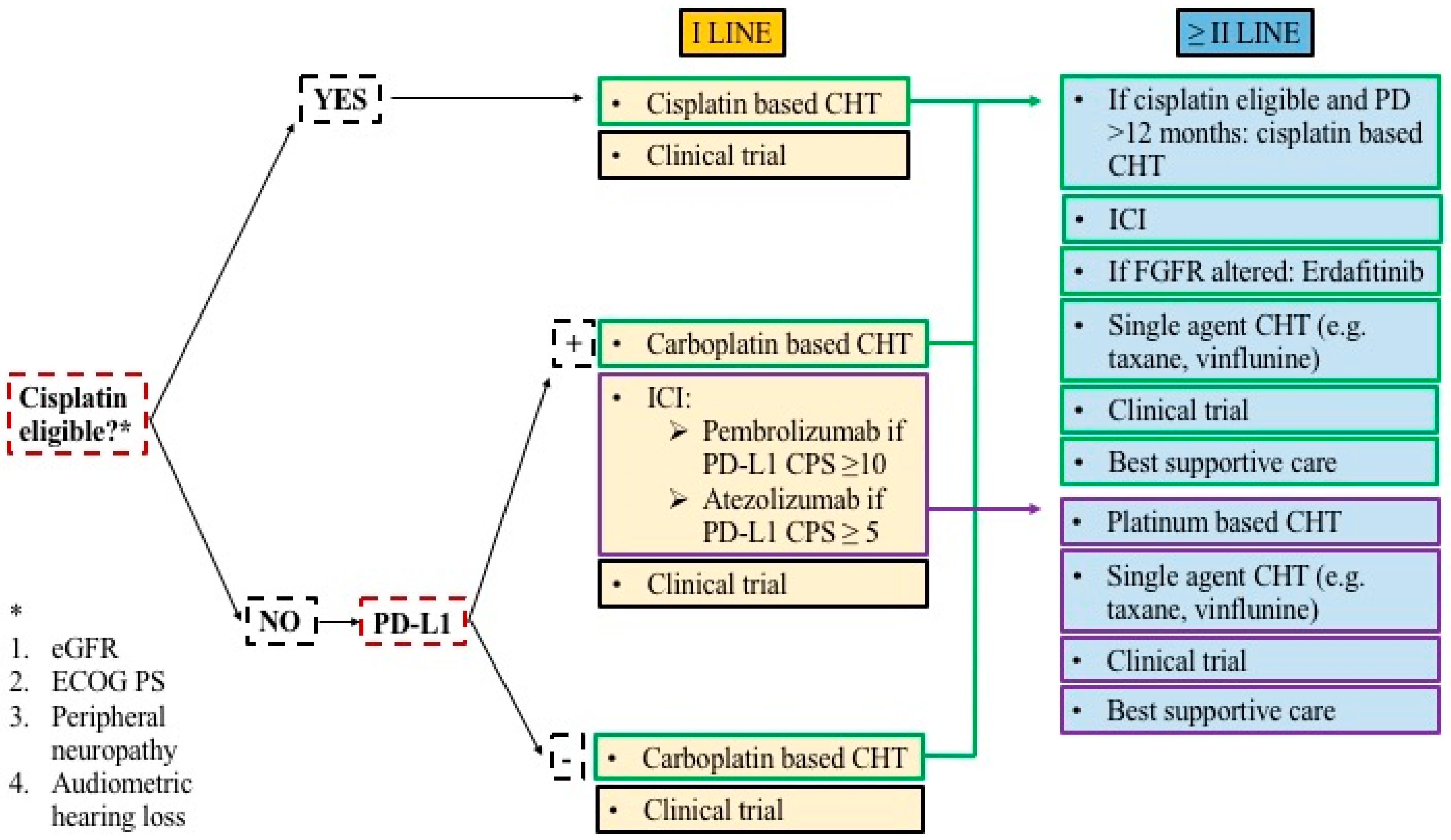
2. Treatment Strategies: State-of-the-Art
2.1. Immune Checkpoint Inhibitors
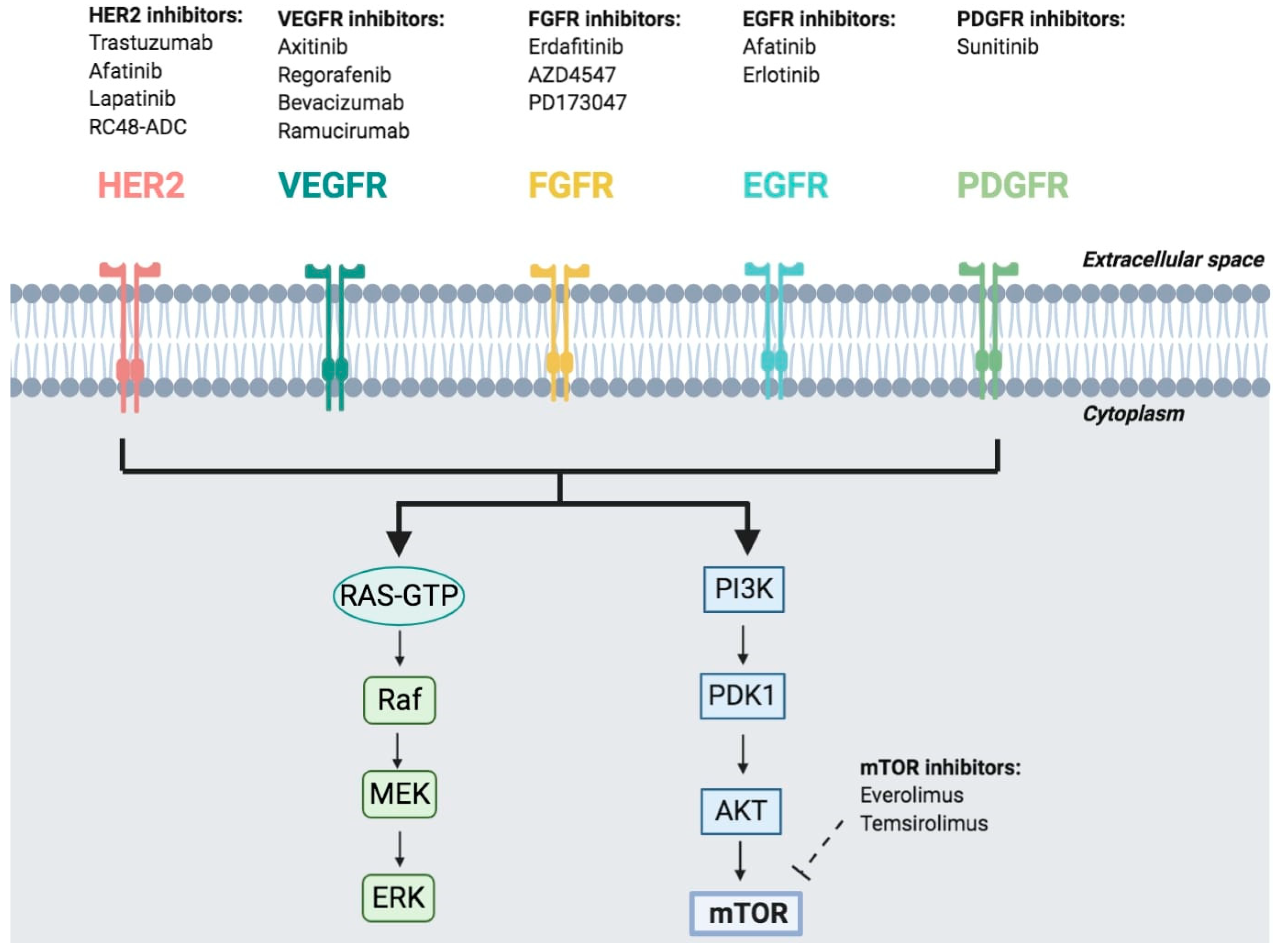
2.2. Target Therapies
2.3. Antibody-Drug Conjugates
3. Therapeutic Approaches UNDER Evaluation
3.1. Ongoing Trials Evaluating ICIS
3.1.1. Combination of ICIs with Cytotoxic Chemotherapy
3.1.2. Combination of ICIs with Other ICIs
3.1.3. Combination of ICIs with Antiangiogenic Agents
3.1.4. ICI Monotherapy
3.1.5. Novel Immunotherapy Approaches
3.2. PARP Inhibitors
3.3. Target Therapy
4. Conclusions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.B.; Deal, A.M.; Woods, M.E.; Wallen, E.M.; Pruthi, R.S.; Chen, R.C.; Milowsky, M.I.; Nielsen, M.E. Muscle-invasive bladder cancer: Evaluating treatment and survival in the National Cancer Data Base. BJU Int. 2014, 114, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Pond, G.R.; Raggi, D.; Giannatempo, P.; Vogelzang, N.J.; Grivas, P.; Galsky, M.D.; Bellmunt, J.; Sonpavde, G. Efficacy and safety of gemcitabine plus either taxane or carboplatin in the first- line setting of metastatic urothelial carcinoma: A systematic review and meta- analysis. Clin. Genitourin. Cancer 2016, 15, 331–340. [Google Scholar] [CrossRef]
- Sternberg, C.N.; de Mulder, P.; Schornagel, J.H.; Theodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, J.A.; Spina, M.; van Groeningen, C.J.; Duclos, B.; et al. Seven years update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur. J. Cancer 2006, 42, 50–54. [Google Scholar] [CrossRef]
- Galsky, M.D.; Chen, G.J.; Oh, W.K.; Bellmunt, J.; Roth, B.J.; Petrioli, R.; Dogliotti, L.; Dreicer, R.; Sonpavde, G. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann. Oncol. 2012, 23, 406–410. [Google Scholar] [CrossRef]
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.; Sternberg, C.N.; Bajorin, D.F.; et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J. Clin. Oncol. 2011, 29, 2432–2438. [Google Scholar] [CrossRef] [Green Version]
- Raggi, D.; Miceli, R.; Sonpavde, G.; Giannatempo, P.; Mariani, L.; Galsky, M.D.; Bellmunt, J.; Necchi, A. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 49–61. [Google Scholar] [CrossRef]
- Lattanzi, M.; Balar, A.V. Current status and future direction of immunotherapy in urothelial carcinoma. Curr. Oncol. Rep. 2019, 21, 24. [Google Scholar] [CrossRef]
- Godwin, J.L.; Hoffman-Censits, J.; Plimack, E. Recent developments in the treatment of advanced bladder cancer. Urol. Oncol. 2018, 36, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Rouanne, M.; Roumiguié, M.; Houédé, N.; Masson-Lecomte, A.; Colin, P.; Pignot, G.; Larré, S.; Xylinas, E.; Roupret, M.; Neuzillet, Y. Development of immunotherapy in bladder cancer: Present and future on targeting PD(L)1 and CTLA-4 pathways. World J. Urol. 2018, 36, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Hanna, K.S. Updates and novel treatments in urothelial carcinoma. J. Oncol. Pharm. Pract. 2019, 25, 648–656. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA- 4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [Green Version]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Castellano, D.; et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803e13. [Google Scholar] [CrossRef]
- Massari, F.; Di Nunno, V.; Mollica, V.; Montironi, R.; Cheng, L.; Cimadamore, A.; Blanca, A.; Lopez-Beltran, A. Immunotherapy in renal cell carcinoma from poverty to the spoiled of choice. Immunotherapy 2019, 11, 1507–1521. [Google Scholar] [CrossRef]
- Powles, T.; Smith, K.; Stenzl, A.; Bedke, J. Immune checkpoint inhibition in metastatic urothelial cancer. Eur. Urol. 2017, 72, 477–481. [Google Scholar] [CrossRef]
- Massari, F.; Di Nunno, V. Atezolizumab for platinum-treated metastatic urothelial carcinoma. Lancet 2018, 391, 716–718. [Google Scholar] [CrossRef]
- Ciccarese, C.; Iacovelli, R.; Bria, E.; Mosillo, C.; Bimbatti, D.; Fantinel, E.; Bisogno, I.; Brunelli, M.; Tortora, G. Second-line therapy for metastatic urothelial carcinoma: Defining the best treatment option among immunotherapy, chemotherapy, and antiangiogenic targeted therapies. A systematic review and meta-analysis. Semin. Oncol. 2019, 46, 65–72. [Google Scholar] [CrossRef]
- Rijnders, M.; de Wit, R.; Boormans, J.L.; Lolkema, M.P.J.; van der Veldt, A.A.M. Systematic review of immune checkpoint inhibition in urological cancers. Eur. Urol. 2017, 72, 411–423. [Google Scholar] [CrossRef]
- Di Nunno, V.; De Luca, E.; Buttigliero, C.; Tucci, M.; Vignani, F.; Gatto, L.; Zichi, C.; Ardizzoni, A.; Di Maio, M.; Massari, F. Immune-checkpoint inhibitors in previously treated patients with advanced or metastatic urothelial carcinoma: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 129, 124–132. [Google Scholar] [CrossRef]
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.; Sternberg, C.; Bajorin, D.F.; et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol. 2011, 12, 211–214. [Google Scholar] [CrossRef]
- Sonpavde, G.; Galsky, M.D.; Latini, D.; Chen, G.J. Cisplatin-ineligible and chemotherapy- ineligible patients should be the focus of new drug development in patients with advanced bladder cancer. Clin. Genitourin. Cancer 2014, 12, 71–73. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Bellmunt, J.; Mead, G.; Kerst, J.M.; Leahy, M.; Maroto, P.; Gil, T.; Marreaud, S.; Daugaard, G.; Skoneczna, I.; et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 2012, 30, 191–199. [Google Scholar] [CrossRef] [PubMed]
- von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; de Mulder, P.H.; Schornagel, J.H.; Théodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, F.; Spina, M.; van Groeningen, C.J.; de Balincourt, C.; et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European organization for research and treatment of cancer protocol no. 30924. J. Clin. Oncol. 2001, 19, 2638–2646. [Google Scholar] [PubMed]
- Massari, F.; Di Nunno, V.; Cubelli, M.; Santoni, M.; Fiorentino, M.; Montironi, R.; Cheng, L.; Lopez-Beltran, A.; Battelli, N.; Ardizzoni, A. Immune checkpoint inhibitors for metastatic bladder cancer. Cancer Treat. Rev. 2018, 64, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 7, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Nadal, R.; Bellmunt, J. Management of metastatic bladder cancer. Cancer Treat. Rev. 2019, 76, 10–21. [Google Scholar] [CrossRef]
- Zarrabi, K.; Paroya, A.; Wu, S. Emerging therapeutic agents for genitourinary cancers. J. Hematol. Oncol. 2019, 12, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorusso, V.; Pollera, C.F.; Antimi, M.; Luporini, G.; Gridelli, C.; Frassineti, G.L.; Oliva, C.; Pacini, M.; De Lena, M. A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Italian Co-operative Group on Bladder Cancer. Eur. J. Cancer 1998, 34, 1208–1212. [Google Scholar] [CrossRef]
- Bellmunt, J.; Théodore, C.; Demkov, T.; Komyakov, B.; Sengelov, L.; Daugaard, G.; Caty, A.; Carles, J.; Jagiello-Gruszfeld, A.; Karyakin, O.P.; et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J. Clin. Oncol. 2009, 27, 4454–4461. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.J.; Broome, C.M.; Hussain, M.; Gutheil, J.C.; Markowitz, A. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J. Clin. Oncol. 2002, 20, 937–940. [Google Scholar] [CrossRef]
- Al Harthy, M.; Redman, J.; Madan, R.A. Novel immunotherapy combinations for genitourinary cancers. Expert Opin. Biol. Ther. 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Pierantoni, F.; Maruzzo, M.; Gardi, M.; Bezzon, E.; Gardiman, M.P.; Porreca, A.; Basso, U.; Zagonel, V. Immunotherapy and urothelial carcinoma: An overview and future perspectives. Crit. Rev. Oncol. Hematol. 2019, 143, 46–55. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 16, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 7, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Powles, T.; Duran, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Sharma, P.; Callahan, M.K.; Bono, P.; Kim, J.; Spiliopoulou, P.; Calvo, E.; Pillai, R.N.; Ott, P.A.; De Braud, F.; Morse, M.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Patel, M.R.; Ellerton, J.; Infante, J.R.; Agawal, M.; Gordon, M.; Aljumaily, R.; Britten, C.D.; Dirix, L.; Lee, K.W.; Taylor, M.; et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018, 19, 51–64. [Google Scholar] [CrossRef]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Crist, M.; Iyer, G.; Hsu, M.; Huang, W.C.; Balar, A.V. Pembrolizumab in the treatment of locally advanced or metastatic urothelial carcinoma: Clinical trial evidence and experience. Ther. Adv. Urol. 2019, 11, 1756287219839285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, C.; Buzzatti, G.; Dellepiane, C.; Cavo, A.; Tolomeo, F.; Cattrini, C.; Boccardo, F. Genitourinary tumours in the targeted therapies era: New advances in clinical practice and future perspectives. Anticancer Drugs 2016, 27, 917. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjodahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Alifrangis, C.; McGovern, U.; Freeman, A.; Powles, T.; Linch, M. Molecular and histopathology directed therapy for advanced bladder cancer. Nat. Rev. Urol. 2019, 16, 465–483. [Google Scholar] [CrossRef]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef]
- Billerey, C.; Chopin, D.; Aubriot-Lorton, M.H.; Ricol, D.; Gil Diez de Medina, S.; Van Rhijn, B.; Bralet, M.P.; Lefrere-Belda, M.A.; Lahaye, J.B.; Abbou, C.C.; et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am. J. Pathol. 2001, 158, 1955–1959. [Google Scholar] [CrossRef] [Green Version]
- van Rhijn, B.W.; Lurkin, I.; Radvanyi, F.; Kirkels, W.J.; van der Kwast, T.H.; Zwarthoff, E.C. The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res. 2001, 61, 1265–1268. [Google Scholar] [PubMed]
- Tomlinson, D.C.; Baxter, E.W.; Loadman, P.M.; Hull, M.A.; Knowles, M.A. FGFR1-induced epithelial to mesenchymal transition through MAPK/PLCgamma/COX-2-mediated mechanisms. PLoS ONE 2012, 7, e38972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, T.; Roth, B.; Choi, W.; Black, P.C.; Dinney, C.D.; McConkey, D.J. Fibroblast growth factor receptors-1 and -3 play distinct roles in the regulation of bladder cancer growth and metastasis: Implications for therapeutic targeting. PLoS ONE 2013, 8, e57284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappellen, D.; De Oliveira, C.; Ricol, D.; de Medina, S.; Bourdin, J.; Sastre-Garau, X.; Chopin, D.; Thiery, J.P.; Radvanyi, F. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat. Genet. 1999, 23, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kuzrock, R. The FGFR landscape in cancer: Analysis of 4,853 tumors by next-generation sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, D.C.; Knowles, M.A. Altered splicing of FGFR1 is associated with high tumor grade and stage and leads to increased sensitivity to FGF1 in bladder cancer. Am. J. Pathol. 2010, 177, 2379–2386. [Google Scholar] [CrossRef]
- Sweis, R.F.; Spranger, S.; Bao, R.; Paner, G.P.; Stadler, W.M.; Steinberg, G.; Gajewski, T.F. Molecular Drivers of the Non-T-cell-Inflamed Tumor Microenvironment in Urothelial Bladder Cancer. Cancer Immunol. Res. 2016, 4, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Casadei, C.; Dizman, N.; Schepisi, G.; Cursano, M.C.; Basso, U.; Santini, D.; Pal, S.K.; De Giorgi, U. Targeted therapies for advanced bladder cancer: New strategies with FGFR inhibitors. Ther. Adv. Med. Oncol. 2019, 11, 1758835919890285. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazedeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Mazzola, C.R.; Chin, J. Targeting the VEGF pathway in metastatic bladder cancer. Expert Opin. Investig. Drugs 2015, 24, 913–927. [Google Scholar] [CrossRef]
- Aldebasi, Y.H.; Rahmani, A.H.; Khan, A.A.; Aly, S.M. The effect of vascular endothelial growth factor in the progression of bladder cancer and diabetic retinopathy. Int. J. Clin. Exp. Med. 2013, 6, 239–251. [Google Scholar] [PubMed]
- Dickinson, A.J.; Fox, S.B.; Persad, R.A.; Hollyer, J.; Sibley, G.N.; Harris, A.L. Quantification of angiogenesis as an independent predictor of prognosis in invasive bladder carcinomas. Br. J. Urol. 1994, 74, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Bochner, B.; Cote, R.J.; Weidner, N.; Groshen, S.; Chen, S.C.; Skinner, D.G.; Nichols, P.W. Angiogenesis in bladder cancer: Relationship between microvessel density and tumour prognosis. J. Natl. Cancer Inst. 1995, 87, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, S.; Fauconnet, S.; Chabannes, E.; Henry, P.C.; Adessi, G.; Bittard, H. Serum levels of vascular endothelial growth factor as a prognostic factor in bladder cancer. J. Urol. 2001, 166, 1275–1279. [Google Scholar] [CrossRef]
- Dreicer, R.; Li, H.; Stein, M.; DiPaola, R.; Eleff, M.; Roth, B.J.; Wilding, G. Phase 2 trial of sorafenib in patients with advanced urothelial cancer: A trial of the Eastern Cooperative Oncology Group. Cancer 2009, 115, 4090–4095. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Gonzalez-Larriba, J.L.; Prior, C.; Maroto, P.; Carles, J.; Castellano, D.; Mellado, B.; Gallardo, E.; Perez-Gracia, J.L.; Aguilar, G.; et al. Phase II study of sunitinib as first-line treatment of urothelial cancer patients ineligible to receive cisplatin-based chemotherapy: Baseline interleukin-8 and tumor contrast enhancement as potential predictive factors of activity. Ann. Oncol. 2011, 22, 2646–2653. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Mariani, L.; Zaffaroni, N.; Schwartz, L.H.; Giannatempo, P.; Crippa, F.; Morosi, C.; Lanocita, R.; Sava, T.; Ortega, C.; et al. Pazopanib in advanced and platinum-resistant urothelial cancer: An open-label, single group, phase 2 trial. Lancet Oncol. 2012, 13, 810–816. [Google Scholar] [CrossRef]
- Apolo, A.B.; Parnes, H.L.; Francis, D.C.; Cordes, L.M.; Berninger, M.; Lamping, E.; Costello, R.; Trepel, J.B.; Merino, M.J.; Folio, L.; et al. A phase II study of cabozantinib in patients (pts) with relapsed or refractory metastatic urothelial carcinoma (mUC). J. Clin. Oncol. 2016, 34 (Suppl. 4534), abstr 4534. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Ross, R.W.; Jacobus, S.; Vaishampayan, U.; Yu, E.Y.; Quinn, D.I.; Hahn, N.M.; Hutson, T.E.; Sonpavde, G.; Morrissey, S.C.; et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J. Clin. Oncol. 2012, 30, 507–512. [Google Scholar] [CrossRef]
- Galsky, M.D.; Hahn, N.M.; Powles, T.; Hellerstedt, B.A.; Lerner, S.P.; Gardner, T.A.; Yu, M.; O’Rourke, M.; Vogelzang, N.J.; Kocs, D.; et al. Gemcitabine, Cisplatin, and sunitinib for metastatic urothelial carcinoma and as preoperative therapy for muscle-invasive bladder cancer. Clin. Genitourin. Cancer 2013, 11, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Hahn, N.M.; Stadler, W.M.; Zon, R.T.; Waterhouse, D.; Picus, J.; Nattam, S.; Johnson, C.S.; Perkins, S.M.; Waddell, M.J.; Sweeney, C.J.; et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J. Clin. Oncol. 2011, 29, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Ballman, K.V.; Halabi, S.; Watt, C.; Hahn, O.M.; Steen, P.D.; Dreicer, R.; Flaig, T.W.; Stadler, W.M.; Sweeney, C.; et al. CALGB 90601 (Alliance): Randomized, double-blind, placebo-controlled phase III trial comparing gemcitabine and cisplatin with bevacizumab or placebo in patients with metastatic urothelial carcinoma. J. Clin. Oncol. 2019, 37 (Suppl. 15), 4503. [Google Scholar] [CrossRef]
- Petrylak, D.P.; de Wit, R.; Chi, K.N.; Drakaki, A.; Sternberg, C.N.; Nishiyama, H.; Castellano, D.; Hussain, S.; Flechon, A.; Bamias, A.; et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): A randomised, double-blind, phase 3 trial. Lancet 2017, 390, 2266–2277. [Google Scholar] [CrossRef] [Green Version]
- Petrylak, D.P.; de Wit, R.; Chi, K.N.; Drakaki, A.; Sternberg, C.N.; Nishiyama, H.; Castellano, D.; Hussain, S.; Flechon, A.; Bamias, A.; et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): Overall survival and updated results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2020, 21, 105–120. [Google Scholar] [CrossRef]
- Sarfaty, M.; Rosenberg, J.E. Antibody-Drug Conjugates in Urothelial Carcinomas. Curr. Oncol. Rep. 2020, 22, 13. [Google Scholar] [CrossRef]
- Rosenberg, J.; Sridhar, S.S.; Zhang, J.; Smith, D.; Ruether, D.; Flaig, T.W.; Baranda, J.; Lang, J.; Plimack, E.R.; Sangha, R.; et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients With Nectin-4-Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. J. Clin. Oncol. 2020, 38, 1041–1049. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Balar, A.V.; O’Donnell, P.H.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.; et al. EV-201: Results of enfortumab vedotin monotherapy for locally advanced or metastatic urothelial cancer previously treated with platinum and immune checkpoint inhibitors. J. Clin. Oncol. 2019, 37 (Suppl. 18), 4505. [Google Scholar] [CrossRef]
- Hoimes, C.J.; Rosenberg, J.E.; Srinivas, S.; Petrylak, D.P.; Milowsky, M.; Merchan, J.R.; Bilen, M.A.; Gupta, S.; Carret, A.S.; Yuan, N.; et al. EV-103: Initial results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. Ann. Oncol. 2019, 30 (Suppl. 5), v356–v402. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Flaig, T.W.; Friedlander, T.W.; Milowsky, M.I.; Srinivas, S.; Petrylak, D.P.; Merchan, J.R.; Bilen, M.A.; Carret, A.S.; Yuan, N.; et al. Study EV-103: Preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 2020, 38 (Suppl. 6), 441. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Morris Faltas, B.; Tat Lam, E.; Saylor, P.J.; Bardia, A.; Hajdenberg, J.; Morgans, A.K.; Lim, E.A.; Kalinsky, K.; Simpson, P.S.; et al. Sacituzumab govitecan (IMMU-132) in patients with previously treated metastatic urothelial cancer (mUC): Results from a phase I/II study. J. Clin. Oncol. 2019, 37 (Suppl. 354), 2019. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Balar, A.; Petrylak, D.P.; Grivas, P.; Agarwal, N.; Sternberg, C.N.; Hong, Q.; Gladden, A.; Kanwal, C.; Siemon-Hryczyk, P.; et al. Initial Results From Trophy-U-01: A Phase 2 Open-Label Study Of Sacituzumab Govitecan In Patients (Pts) With Metastatic Urothelial Cancer (Muc) After Failure Of Platinum-Based Regimens (Plt) Or Immunotherapy. Ann. Oncol. 2019, 30 (Suppl. 5), v851–v934. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tagawa, S.T.; Jain, R.K.; Bupathi, M.; Balar, A.V.; Rezazadeh, A.; George, S.; Palmbos, P.L.; Nordquist, L.T.; Davis, N.B.; et al. Early results of TROPHY-U-01 Cohort 2: Sacituzumab govitecan (SG) in platinum-ineligible patients (pts) with metastatic urothelial cancer (mUC) who progressed after prior checkpoint inhibitor (CPI) therapy. J. Clin. Oncol. 2020, 38 (Suppl. 15), 5027. [Google Scholar] [CrossRef]
- Powles, T.; Gschwend, J.E.; Loriot, Y.; Bellmunt, J.; Geczi, L.; Vulsteke, C.; Abdelslam, M.; Gafanov, R.; Bae, W.K.; Revesz, J.; et al. Phase 3 KEYNOTE-361 trial: Pembrolizumab (pembro) with or without chemotherapy versus chemotherapy alone in advanced urothelial cancer. J. Clin. Oncol. 2017, 35, e15. [Google Scholar] [CrossRef]
- Galsky, M.D.; Grande, E.; Davis, I.D.; De Santis, M.; Arranz Arija, J.A.; Kikuchi, E.; Mecke, A.; Thastrom, A.C.; Bamias, A. IMvigor130: A randomized, phase III study evaluating first-line (1L) atezolizumab (atezo) as monotherapy and in combination with platinum-based chemotherapy (chemo) in patients (pts) with locally advanced or metastatic urothelial carcinoma (mUC). J. Clin. Oncol. 2018, 36, e15. [Google Scholar] [CrossRef]
- Qin, S.; Finn, R.S.; Kudo, M.; Meyer, T.; Vogel, A.; Ducreux, M.; Macarulla, T.M.; Tomasello, G.; Boisserie, F.; Hou, J.; et al. RATIONALE 301 study: Tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019, 15, 1811–1822. [Google Scholar] [CrossRef] [Green Version]
- Mollica, V.; Di Nunno, V.; Gatto, L.; Santoni, M.; Cimadamore, A.; Cheng, L.; Lopez-Beltran, A.; Montironi, R.; Pisconti, S.; Battelli, N.; et al. Novel Therapeutic Approaches and Targets Currently Under Evaluation for Renal Cell Carcinoma: Waiting for the Revolution. Clin. Drug Investig. 2019, 39, 503–519. [Google Scholar] [CrossRef]
- Hassel, J.C.; Heinzerling, L.; Aberle, J.; Bahr, O.; Eigentler, T.K.; Grimm, M.O.; Grunwald, V.; Leipe, J.; Reinmuth, N.; Tietze, J.K.; et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat. Rev. 2017, 57, 36–49. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Siefker-Radtke, A.; De Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J Clin. Oncol. 2019, 37, 1608–1616. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, J.E.; Sharma, P.; De Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab(N) alone or in combination with ipilimumab (I) in patients (pts) with platinum-pretreated metastatic urothelial carcinoma (mUC), including the nivolumab 1 mg/kg + ipilimumab 3 mg/kg expansion from CheckMate 032. Ann. Oncol. 2018, 29, e32. [Google Scholar] [CrossRef]
- Galsky, M.D.; Powles, T.; Li, S.; Hennicken, D.; Sonpavde, G. A phase 3, open-label, randomized study of nivolumab plus ipilimumab or standard of care (SoC) vs. SoC alone in patients (pts) with previously untreated unresectable or metastatic urothelial carcinoma (mUC.; CheckMate 901). J. Clin. Oncol. 2018, 36, TPS4588. [Google Scholar] [CrossRef]
- Powles, T.; Galsky, M.D.; Castellano, D.; Van Der Heijden, M.S.; Petrylak, D.P.; Armstrong, J.; Belli, R.; Ferro, S.; Ben, Y.; Bellmunt, J. A phase 3 study of first-line durvalumab (MEDI4736) ± tremelimumab versus standard of care (SoC) chemotherapy(CT) in patients (pts) with unresectable stage IV urothelial bladder cancer (UBC): DANUBE. J. Clin. Oncol. 2016, 34, TPS4574. [Google Scholar] [CrossRef]
- Galsky, M.D.; Necchi, A.; Sridhar, S.S.; Ogawa, O.; Angra, N.; Hois, S.; He, P.; Ghiorghiu, D.C.; Bellmunt, J. A phase III, randomized, open label, multicenter, global study of first-line (1L) durvalumab in combination with standard of care (SOC) chemotherapy and durvalumab in combination with tremelimumab and SOC chemotherapy versus SOC chemotherapy alone in patients with unresectable locally advanced or metastatic urothelial cancer (UC). J. Clin. Oncol. 2019, 37, e15. [Google Scholar]
- Geldart, T.; Chester, J.; Casbard, A.; Crabb, S.; Elliott, T.; Protheroe, A.; Huddart, R.A.; Mead, G.; Barber, J.; Jones, R.J.; et al. SUCCINCT: An open-label, single-arm, non-randomised, phase 2 trial of gemcitabine and cisplatin chemotherapy in combination with sunitinib as first-line treatment for patients with advanced urothelial carcinoma. Eur. Urol. 2015, 67, 599–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrylak, D.P.; Arkenau, H.-T.; Perez-Gracia, J.L.; Krebs, M.; Santana- Davila, R.; Yang, J.; Rege, J.; Mi, G.; Ferry, D.; Herbst, R.S. A multicohort phase I study of ramucirumab (R) plus pembrolizumab (P): Interim safety and clinical activity in patients with urothelial carcinoma. J. Clin. Oncol. 2017, 35, e349. [Google Scholar] [CrossRef]
- Baxter, M.A.; Glen, H.; Evans, T.R. Lenvatinib and its use in the treatment of unresectable hepatocellular carcinoma. Future Oncol. 2018, 14, 2021–2029. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, A.; Vaishampayan, U. Cabozantinib for the treatment of kidney cancer. Expert Rev. Anticancer Ther. 2017, 17, 577–584. [Google Scholar] [CrossRef]
- Di Nunno, V.; Massari, F.; Mollica, V.; Cimadamore, A.; Santoni, M.; Cheng, L.; Lopez-Beltran, A.; Scarpelli, M.; Montironi, R. Another one in the chamber: Cabozantinib for patients with metastatic non clear cell renal cell carcinoma. Ann. Transl. Med. 2019, 7 (Suppl. 3), S137. [Google Scholar] [CrossRef] [Green Version]
- Apolo, A.B.; Mortazavi, A.; Stein, M.N.; Davarpanah, N.N.; Nadal, R.M.; Parnes, H.L.; Ning, Y.M.; Francis, D.C.; Cordes, L.M.; Berniger, M.A.; et al. A phase I study of cabozantinib plus nivolumab (CaboNivo) and cabonivo plus ipilimumab (CaboNivoIpi) in patients (pts) with refractory metastatic (m) urothelial carcinoma (UC) and other genitourinary (GU) tumors. J. Clin. Oncol. 2017, 35, e4562. [Google Scholar] [CrossRef]
- Dolan, M.; Mastri, M.; Tracz, A.; Christensen, J.G.; Chatta, G.; Ebos, J.M.L. Enhanced efficacy of sitravatinib in metastatic models of antiangiogenic therapy resistance. PLoS ONE 2019, 14, e0220101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sternberg, C.N.; Loriot, Y.; James, N.; Choy, E.; Castellano, D.; Lopez-Rios, F.; Banna, G.L.; De Giorgi, U.; Masini, C.; Bamias, A.; et al. Primary Results from SAUL, a Multinational Single-arm Safety Study of Atezolizumab Therapy for Locally Advanced or Metastatic Urothelial or Nonurothelial Carcinoma of the Urinary Tract. Eur. Urol. 2019, 76, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, L.; Ladomersky, E.; Lenzen, A.; Nguyen, B.; Patel, R.; Lauing, K.L.; Wu, M.; Wainwright, D.A. IDO1 in cancer: A Gemini of immune checkpoints. Cell Mol. Immunol. 2018, 15, 447–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theate, I.; van Baren, N.; Pilotte, L.; Moulin, P.; Larrieu, P.; Renauld, J.C.; Hervé, C.; Gutierrez-Roelens, I.; Marbaix, E.; Sempoux, C.; et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol. Res. 2015, 3, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meireson, A.; Chevolet, I.; Hulstaert, E.; Ferdinande, L.; Ost, P.; Geboes, K.; De Man, M.; Van de Putte, D.; Verset, L.; Kruse, V.; et al. Peritumoral endothelial indoleamine 2, 3-dioxygenase expression is an early independent marker of disease relapse in colorectal cancer and is influenced by DNA mismatch repair profile. Oncotarget 2018, 9, 25216–25224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dill, E.A.; Dillon, P.M.; Bullock, T.N.; Mills, A.M. IDO expression in breast cancer: An assessment of 281 primary and metastatic cases with comparison to PD-L1. Mod. Pathol. 2018, 31, 1513–1522. [Google Scholar] [CrossRef]
- Wing, J.B.; Tay, C.; Sakaguchi, S. Control of Regulatory T Cells by Co-signal Molecules. Adv. Exp. Med. Biol. 2019, 1189, 179–210. [Google Scholar]
- So, T.; Ishii, N. The TNF-TNFR Family of Co-signal Molecules. Adv. Exp. Med. Biol. 2019, 1189, 53–84. [Google Scholar]
- Kashima, J.; Okuma, Y.; Hosomi, Y.; Hishima, T. High Serum OX40 and OX40 Ligand (OX40L) Levels Correlate with Reduced Survival in Patients with Advanced Lung Adenocarcinoma. Oncology 2020, 25, 1–8. [Google Scholar] [CrossRef]
- Piconese, S.; Valzasina, B.; Colombo, M.P. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J. Exp. Med. 2008, 205, 825–839. [Google Scholar] [CrossRef] [Green Version]
- Redmond, W.L.; Linch, S.N.; Kasiewicz, M.J. Combined targeting of co-stimulatory (OX40) and co-inhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust anti-tumor immunity. Cancer Immunol. Res. 2014, 2, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Bentebibel, S.E.; Hurwitz, M.E.; Bernatchez, C.; Haymaker, C.; Hudgens, C.W.; Kluger, H.M.; Tetzlaff, M.T.; Tagliaferri, M.A.; Zalevsky, J.; Hoch, U.; et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL-2-receptor beta/gamma (betagamma)-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 2019, 9, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Khong, H.; Fa’ak, F.; Bentebible, S.E.; Janssen, L.M.E.; Chesson, B.C.; Creasy, C.A.; Forget, M.A.; Kahn, L.M.S.; Pazdrak, B.; et al. Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell-mediated cancer therapy. Nat. Commun. 2020, 11, 661. [Google Scholar] [CrossRef]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer 2018, 9, 176–189. [Google Scholar]
- Lichtenegger, F.S.; Rothe, M.; Schnorfeil, F.M.; Deiser, K.; Krupka, C.; Ausberger, C.; Schulter, M.; Neitz, J.; Subklewe, M. Targeting LAG-3 and PD-1 to Enhance T Cell Activation by Antigen-Presenting Cells. Front. Immunol. 2018, 9, 385. [Google Scholar] [CrossRef] [Green Version]
- Fujio, K.; Yamamoto, K.; Okamura, T. Overview of LAG-3-Expressing, IL-10-Producing Regulatory T Cells. Curr. Top. Microbiol. Immunol. 2017, 410, 29–45. [Google Scholar]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and its role in regulating antitumor immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Piao, Y.R.; Piao, L.Z.; Zhu, L.H.; Jin, Z.H.; Dong, X.Z. Prognostic value of T cell immunoglobulin mucin-3 in prostate cancer. Asian Pac. J. Cancer Prev. 2013, 14, 3897–3901. [Google Scholar] [CrossRef] [Green Version]
- Zhou, E.; Huang, Q.; Wang, J.; Fang, C.; Yang, L.; Zhu, M.; Chen, J.; Chen, L.; Dong, M. Up-regulation of Tim-3 is associated with poor prognosis of patients with colon cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 8018–8027. [Google Scholar]
- Yang, M.; Yu, Q.; Liu, J.; Fu, W.; Cao, Y.; Yu, L.; Shao, S.; Wang, X.; Niu, H.; Wang, Y. T-cell immunoglobulin mucin-3 expression in bladder urothelial carcinoma: Clinicopathologic correlations and association with survival. J. Surg. Oncol. 2015, 112, 430–435. [Google Scholar] [CrossRef]
- Knee, D.A.; Hewes, B.; Brogdon, J.L. Rationale for anti-GITR cancer immunotherapy. Eur. J. Cancer 2016, 67, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zappasodi, R.; Sirard, C.; Li, Y.; Budhu, S.; Abu-Akeel, M.; Liu, C.; Yang, X.; Zhong, H.; Newman, W.; Qi, J.; et al. Rational design of anti-GITR-based combination immunotherapy. Nat. Med. 2019, 25, 759–766. [Google Scholar] [CrossRef]
- Riccardi, C.; Ronchetti, S.; Nocentini, G. Glucocorticoid-induced TNFR-related gene (GITR) as a therapeutic target for immunotherapy. Expert Opin. Ther. Targets. 2018, 22, 783–797. [Google Scholar] [CrossRef]
- Sermer, D.; Brentjens, R. CAR T-cell therapy: Full speed ahead. Hematol. Oncol. 2019, 37 (Suppl. 1), 95–100. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wu, Z.; Liu, Y.; Han, W. New development in CAR-T cell therapy. J. Hematol. Oncol. 2017, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.E.; Mackall, C.L. CAR T cell therapy: Inroads to response and resistance. Nat. Rev. Immunol. 2019, 19, 73–74. [Google Scholar] [CrossRef]
- Aldous, A.R.; Dong, J.Z. Personalized neoantigen vaccines: A new approach to cancer immunotherapy. Bioorg. Med. Chem. 2018, 26, 2842–2849. [Google Scholar] [CrossRef]
- Criscitiello, C.; Viale, G.; Curigliano, G. Peptide vaccines in early breast cancer. Breast 2019, 44, 128–134. [Google Scholar] [CrossRef]
- Turner, N.; Tutt, A.; Ashworth, A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat. Rev. Cancer 2004, 4, 814–819. [Google Scholar] [CrossRef]
- Mateo, J.; Lord, C.J.; Tutt, A.; Balmana, J.; Castroviejo-Bermejo, M.; Cruz, C.; Oaknin, A.; Kaye, S.B.; de Bono, J.S. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. 2019, 30, 1437–1447. [Google Scholar] [CrossRef] [Green Version]
- Yap, K.L.; Kiyotani, K.; Tamura, K.; Antic, T.; Jang, M.; Montoya, M.; Campanile, A.; Yew, P.Y.; Ganshert, C.; Fujioka, T.; et al. Whole-exome sequencing of muscle-invasive bladder cancer identifies recurrent mutations of UNC5C and prognostic importance of DNA repair gene mutations on survival. Clin. Cancer Res. 2014, 20, 6605–6617. [Google Scholar] [CrossRef] [Green Version]
- Teo, M.Y.; Bambury, R.M.; Zabor, E.C.; Jordan, E.; Al-Ahmadie, H.; Boyd, M.E.; Bouvier, N.; Mullane, S.A.; Cha, E.K.; Roper, N.; et al. DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin. Cancer Res. 2017, 23, 3610–3618. [Google Scholar] [CrossRef] [Green Version]
- Fong, P.C.; Yap, T.A.; Boss, D.S.; Carden, C.P.; Mergui-Roelvink, M.; Gourley, C.; De Greve, J.; Lubinski, J.; Shanley, S.; Messiou, C.; et al. Poly(ADP)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 2010, 28, 2512–2519. [Google Scholar] [CrossRef]
- Rimar, K.J.; Tran, P.T.; Matulewicz, R.S.; Hussain, M.; Meeks, J.J. The emerging role of homologous recombination repair and PARP inhibitors in genitourinary malignancies. Cancer 2017, 123, 1912–1924. [Google Scholar] [CrossRef]
- Criscuolo, D.; Morra, F.; Giannella, R.; Visconti, R.; Cerrato, A.; Celetti, A. New combinatorial strategies to improve the PARP inhibitors efficacy in the urothelial bladder Cancer treatment. J. Exp. Clin. Cancer Res. 2019, 38, 91. [Google Scholar] [CrossRef]
- Garje, R.; Vaddepally, R.K.; Zakharia, Y. PARP Inhibitors in Prostate and Urothelial Cancers. Front. Oncol. 2020, 10, 114. [Google Scholar] [CrossRef]
- Grivas, P.; Loriot, Y.; Feyerabend, S.; Morales-Barrera, R.; Teo, M.Y.; Vogelzang, N.J.; Grande, E.; Zakharia, Y.; Adra, N.; Alva, A.S.; et al. Rucaparib for recurrent, locally advanced or metastatic urothelial carcinoma (mUC): Results from ATLAS, a phase 2 open-label trial. J. Clin. Oncol. 2020, 38 (Suppl. 6), 440. [Google Scholar] [CrossRef]
- Galsky, M.D.; Uzilov, A.V.; McBride, R.B.; Wang, H.; Patel, V.G.; Sfakianos, J.; Wang, L.; Akers, N.; Iyer, G.; Solit, D.B.; et al. DNA damage response (DDR) gene mutations (mut), mut load, and sensitivity to chemotherapy plus immune checkpoint blockade in urothelial cancer (UC). J. Clin. Oncol. 2017, 35 (Suppl. 6), 300. [Google Scholar] [CrossRef]
- Teo, M.Y.; Seier, K.; Ostrovnaya, I.; Regazzi, A.M.; Kania, B.E.; Moran, M.M.; Cipolla, C.K.; Bluth, M.J.; Chaim, J.; Al-Ahmadie, H.; et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. J. Clin. Oncol. 2018, 36, 1685–1694. [Google Scholar] [CrossRef]
- Powles, T.B.; Balar, A.; Gravis, G.; Jones, R.; Ravaud, A.; Florence, J.; Grivas, P.; Petrylak, D.P.; Galsky, M.; Carles, J.; et al. An Adaptive, Biomarker Directed Platform Study In Metastatic Urothelial Cancer (Biscay) With Durvalumab In Combination With Targeted Therapies. Ann. Oncol. 2019, 30 (Suppl. 5), v356–v402. [Google Scholar]
- Siefker-Radtke, A.O.; Lugowska, I.; Tupikowski, K.; Andric, Z.G.; Rezazadeh Kalebasty, A.; Curigliano, G.; Vaena, D.; Vogl, F.D.; Currie, G.; Abella, S.; et al. Clinical activity of vofatamab (V), an FGFR3 selective antibody in combination with pembrolizumab (P) in metastatic urothelial carcinoma (mUC), updated interim analysis of FIERCE-22. Ann. Oncol. 2019, 30 (Suppl. 5), v356–v402. [Google Scholar] [CrossRef]


| NCT (Clinicaltrials.gov) | Phase | Setting | Cisplatin Fit/Unfit | Arm A | Arm B | Arm C | Compounds Description | Number of Patients | Primary Outcome | Status | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03409458 (PAVE-1) | Ib/IIa | First- or later-line | All | Avelumab + PT-112 | PT-112: platinum- based agent belonging to the phosphaplatin family | 52 | Recommended dose of PT-112 to be used with avelumab | Recruiting | May 2020 | ||
| NCT02437370 | I | Second- or third-line | All | Pembrolizumab + docetaxel | Pembrolizumab + gemcitabine | Pembrolizumab: anti-PD-1 | 38 | Safety | Recruiting | December 2020 | |
| NCT03582475 | I | First- or later-line | All | Pembrolizumab + etoposide + cisplatin (or carboplatin) | Pembrolizumab: anti-PD-1 | 30 | Durable response rate ORR Duration of response PFS OS Safety | Recruiting | September 2021 | ||
| NCT02853305 (KEYNOTE-361) | III | First-line | All | Pembrolizumab | Pembrolizumab + gemcitabine + cisplatin (or carboplatin) | Placebo + gemcitabine + cisplatin (or carboplatin) | Pembrolizumab: anti-PD-1 | 990 | PFS OS | Active, not recruiting | May 2020 |
| NCT02807636 (IMvigor130 trial) | III | First-line | All | Atezolizumab | Atezolizumab + gemcitabine + cisplatin (or carboplatin) | Placebo + gemcitabine + cisplatin (or carboplatin) | Atezolizumab: anti-PD-L1 | 1200 | PFS OS Safety | Active, not recruiting | November 2020 |
| NCT03093922 | II | First-line | All | Atezolizumab + gemcitabine + cisplatin | Atezolizumab + gemcitabine + cisplatin (modified schedule) | Atezolizumab + gemcitabine + cisplatin (modified schedule) | Atezolizumab: anti-PD-L1 | 74 | ORR | Recruiting | March 2021 |
| NCT03967977 | III | First-line | All | Tislelizumab + gemcitabine + cisplatin (or carboplatin) | Placebo + gemcitabine + cisplatin (or carboplatin) | Tislelizumab: humanized monoclonal PD-1 antibody | 420 | OS | Recruiting | July 2022 | |
| NCT03737123 | II | Second-line (no prior platinum chemotherapy) | Cisplatin ineligible | Atezolizumab + chemotherapy (docetaxel or gemcitabine + carboplatin) | Atezolizumab: anti-PD-L1 | 33 | PFS | Recruiting | January 2022 | ||
| NCT03390595 | II | First-line | Cisplatin ineligible | Avelumab + gemcitabine + carboplatin | Gemcitabine + carboplatin | Avelumab: anti-PD-L1 | 85 | ORR | Active, not recruiting | August 2020 | |
| NCT03324282 | II | First-line | All | Avelumab + gemcitabine + cisplatin | Gemcitabine + cisplatin | Avelumab: anti-PD-L1 | 90 | ORR Safety | Recruiting | December 2022 | |
| NCT02581982 | II | Second- or later-line | All | Pembrolizumab + paclitaxel | Pembrolizumab: anti-PD-1 | 27 | ORR | Recruiting | April 2020 | ||
| NCT03744793 | II | Second-line or third-line | All | Avelumab + pemetrexed | Avelumab: anti-PD-L1 | 25 | ORR | Recruiting | January 2021 | ||
| NCT03575013 | I | Second- or later-line | All | Avelumab + paclitaxel | Avelumab: anti-PD-L1 | 21 | Safety | Recruiting | May 2020 |
| NCT (Clinicaltrials.gov) | Phase | Setting | Cisplatin Fit/Unfit | Arm A | Arm B | Arm C | Compounds Description | Number of Patients | Primary Outcome | Status | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03682068 (NILE) | III | First-line | All | Durvalumab + gemcitabine + cisplatin (or carboplatin) | Durvalumab + tremelimumab + gemcitabine + cisplatin (or carboplatin) | Gemcitabine + cisplatin (or carboplatin) | Durvalumab: anti-PD-L1. Tremelimumab: anti-CTLA-4. | 885 | PFS OS | Recruiting | April 2022 |
| NCT03036098 (CheckMate-901) | III | First-line | All | Nivolumab + ipilimumab | Gemcitabine + cisplatin (or carboplatin) | Nivolumab + gemcitabine + cisplatin (or carboplatin) | Nivolumab: anti-PD-1. Ipilimumab: anti-CTLA-4 | 990 | OS in cisplatin ineligible OS in PD-L1 ≥ 1% | Recruiting | December 2022 |
| NCT03219775, (TITAN-TCC) | II | First- or later-line | All | Nivolumab + ipilimumab | Nivolumab: anti-PD-1. Ipilimumab: anti-CTLA-4. | 80 | ORR | Recruiting | December 2020 | ||
| NCT02516241 (DANUBE) | III | First-line | All | Durvalumab + tremelimumab | Durvalumab | Gemcitabine + cisplatin (or carboplatin) | Durvalumab: anti-PD-L1. Tremelimumab: anti-CTLA-4. | 1126 | OS | Active, not recruiting | May 2020 |
| NCT03430895 | II | First- or later-line | All | Durvalumab + tremelimuamb | Durvalumab: anti-PD-L1. Tremelimumab: anti-CTLA-4. | 15 | ORR | Active, not recruiting | January 2021 |
| NCT (Clinicaltrials.gov) | Phase | Setting | Cisplatin Fit/Unfit | Arm A | Arm B | Arm C | Compounds Description | Number of Patients | Primary Outcome | Status | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT02496208 | I | Second- or later-line | All | Nivolumab + cabozantinib | Nivolumab + ipilimumab + cabozantinib | Nivolumab: anti-PD-1. Ipilimumab: anti-CTLA-4. Cabozantinib: tyrosine kinase inhibitor | 152 | Safety | Recruiting | September 2020 | |
| NCT02443324 | I | First- or later-line | All | Pembrolizumab + ramucirumab | Ramucirumab: anti-VEGF. Pembrolizumab: anti-PD-1. | 155 | Safety | Active, not recruiting | November 2020 | ||
| NCT03170960 | I/II | First- or later-line | All | Atezolizumab + cabozantinib | Atezolizumab: anti-PD-L1 Cabozantinib: tyrosine kinase inhibitor | 1723 | Safety ORR | Recruiting | December 2021 | ||
| NCT01552434 | I | Second- or later-line | All | Temsirolimus + bevacizumab + cetuximab | Temsirolimus + bevacizumab + valproic acid | Temsirolimus + bevacizumab | Temsirolimus: mTOR inhibitor. Bevacizumab: anti-VEGF. Cetuximab: anti-EGFR. | 216 | Safety | Recruiting | March 2021 |
| NCT03407976 (APPEASE) | I/IIa | Second- or later-line | All | Pembrolizumab + apatinib | Apatinib: tyrosine kinase inhibitor. Pembrolizumab: anti-PD-1. | 119 | Safety ORR | Active, not recruiting | June 2023 | ||
| NCT03170960 | I/II | Second- or later-line (with prior ICI) | All | Expansion Cohort 2, 3, 4, 5: atezolizumab plus cabozantinib | Expansion cohort 19: cabozantinib | Atezolizumab: anti-PD-L1. Cabozantinib: tyrosine kinase inhibitor. | 1732 | Safety ORR | Recruiting | December 2021 | |
| NCT03272217 | II | First-line | Cisplatin ineligible | Atezolizumab + bevacizumab | Bevacizumab: anti-VEGF | 70 | OS | Recruiting | June 2021 | ||
| NCT03472560 (JAVELIN Medley VEGF) | II | Second- or later-line | Cisplatin ineligible | Avelumab + axitinib | Avelumab: anti-PD-L1. Axitinib: tyrosine kinase inhibitor. | 61 | OR | Active, not recruiting | September 2020 | ||
| NCT03898180 (LEAP-011 trial) | III | First-line, PD-L1 ≥ 10% | Cisplatin ineligible | Pembrolizumab + lenvatinib | Pembrolizumab + placebo | Lenvatinib: tyrosine kinase inhibitor | 694 | PFS OS | Recruiting | December 2022 | |
| NCT03534804 (PemCab) | II | First-line | Cisplatin ineligible | Pembrolizumab + cabozantinib | Cabozantinib: tyrosine kinase inhibitor | 39 | ORR | Recruiting | September 2023 | ||
| NCT03824691 (ARCADIA) | II | Second-line or third-line | All | Durvalumab + cabozantinib | Durvalumab: anti-PD-L1. Cabozantinib: tyrosine kinase inhibitor. | 122 | OS | Recruiting | February 2023 | ||
| NCT03866382 | II | First- or later-line | All | Nivolumab + ipilimumab + cabozantinib | Nivolumab: anti-PD-1. Ipilimumab: anti-CTLA-4. Cabozantinib: tyrosine kinase inhibitor. | 186 | ORR | Recruiting | February 2023 | ||
| NCT04066595 (CabUC) | II | Second-line or third-line | All | Cabozantinib | Cabozantinib: tyrosine kinase inhibitor | 88 | ORR 6-month | Recruiting | September 2024 | ||
| NCT02717156 | II | Second- or later-line | All | Pembrolizumab + EphB4-HSA | EphB4-HSA: A recombinant fusion protein composed of the full-length extracellular domain (soluble) of human receptor tyrosine kinase ephrin type-B receptor 4 and fused to full-length human serum albumin, with potential anti-angiogenic and antineoplastic activities. Pembrolizumab: anti-PD-1. | 60 | Safety | Recruiting | November 2021 | ||
| NCT03606174 | II | First- or later-line | All | Nivolumab + sitravatinib | Sitravatinib: tyrosine kinase inhibitor able to inhibit VEGFR, PDGFR, KIT, RET and MET. Nivolumab: anti-PD-1. | 330 | ORR | Recruiting | September 2021 |
| NCT (Clinicaltrials.gov) | Phase | Setting | Cisplatin Fit/Unfit | Arm A | Arm B | Arm C | Compounds Description | Number of Patients | Primary Outcome | Status | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT02500121 | II | Maintenance after SD, RP or RC to first-line chemotherapy | All | Pembrolizumab | Placebo | Pembrolizumab: anti-PD-1 | 108 | 6-month PFS | Active, not recruiting | January 2020 | |
| NCT02603432 (JAVELIN Bladder 100) | III | Maintenance after SD, RP or RC to first-line chemotherapy | All | Avelumab | Best supportive care | Avelumab: anti-PD-L1 | 700 | OS | Active, not recruiting | June 2021 | |
| NCT02928406 (SAUL) | III | Second-, third or fourth- line | All | Atezolizumab | Atezolizumab: anti-PD-L1 | 1004 | Safety | Active, not recruiting | March 2022 | ||
| NCT03113266 | II | Second- or later-line | All | Toripalimab (JS001) | Toripalimab: anti-PD-1 | 370 | ORR | Recruiting | February 2022 | ||
| NCT03557918 | II | Second- or later-line | All | Tremelimumab | Tremelimumab: anti-CTLA-4 | 28 | ORR | Recruiting | July 2021 | ||
| NCT03212404 | I | Second- or later-line (no prior ICI) | All | Cosibelimab (CK-301) | Cosibelimab: anti-PD-L1 | 500 | Safety ORR | Recruiting | December 2021 | ||
| NCT03053466 | I | First- or later-line, PD-L1 ≥ 1% | All | APL-501 | APL-501: anti-PD-1 | 114 | Safety | Active, not recruiting | December 2021 |
| NCT (Clinicaltrials.gov) | Phase | Setting | Cisplatin Fit/Unfit | Arm A | Arm B | Arm C | Compounds Description | Number of Patients | Primary Outcome | Status | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03361865 (KEYNOTE-672/ECHO-307) | III | First-line | Cisplatin ineligible | Pembrolizumab + epacadostat | Pembrolizumab + placebo | Epacadostat: IDO1 inhibitor. Pembrolizumab: anti-PD-1. | 93 | ORR | Active, not recruiting | September 2020 | |
| NCT03374488 | III | Second- or later-line | All | Pembrolizumab + epacadostat | Pembrolizumab + placebo | Epacadostat: IDO1 inhibitor. Pembrolizumab: anti-PD-1. | 84 | ORR | Active, not recruiting | August 2020 | |
| NCT02554812 (JAVELIN Medley) | II | Second- or third-line | All | 6 cohorts, different combinations with avelumab, PF-04518600 and utomilumab (PF-05082566) | PF-04518600: OX40 agonist. Utomilumab: 4-1BB agonist. Avelumab: anti-PD-L1. | 620 | Safety OR | Recruiting | December 2022 | ||
| NCT03785925 (PIVOT-10) | II | First-line | Cisplatin ineligible | Nivolumab + Bempegaldesleukin (NKTR-214) | Bempegaldesleukin (NKTR-214): IL-2 pathway agonist designed to target CD122. Nivolumab: anti-PD-1. | 205 | ORR | Recruiting | March 2022 | ||
| NCT03513952 | II | Second- or later-line (prior platinum chemotherapy) | All | Atezolizumab + CYT-107 | Atezolizumab | CYT-107: glycosylated recombinant human interleukin-7. Atezolizumab: anti-PD-L1. | 54 | ORR | Recruiting | December 2020 | |
| NCT03915405 | I | Second- or later-line (no prior ICI, prior platinum chemotherapy) | All | Avelumab + KHK2455 | KHK2455: IDO1 inhibitor. Avelumab: anti-PD-L1. | 44 | Safety | Recruiting | February 2022 | ||
| NCT03217747 | I/II | First- or later-line | All | 6 cohorts, different combinations with utomilumab (PF-05082566), PF-04518600, and radiation therapy | PF-04518600: OX40 agonist Utomilumab: 4-1BB agonist | 184 | Safety CD8 immune markers | Recruiting | September 2023 | ||
| NCT04198766 | I | Second- or later-line | All | Pembrolizumab ± INBRX-106 | INBRX-106: OX40 agonist. Pembrolizumab: anti-PD-1. | 150 | Safety | Recruiting | March 2023 | ||
| NCT02983045 (PIVOT-02) | I/II | First- or later-line | All | Nivolumab + Bempegaldesleukin (NKTR-214) | Nivolumab + ipilimumab + NKTR-214 | NKTR-214: IL-2 pathway agonist designed to target CD122. Nivolumab: anti-PD-1. Ipilimumab: anti-CTLA-4. | 780 | ORR | Recruiting | December 2021 | |
| NCT03138889 (PROPEL) | I/II | First- or later-line | All | Pembrolizumab + Bempegaldesleukin (NKTR-214) | Bempegaldesleukin (NKTR-214): an interleukin-2 pathway agonist that targets CD122. Pembrolizumab: anti-PD-1. | 135 | Safety ORR | Recruiting | June 2023 | ||
| NCT03809624 | I | Second- or later-line | All | INBRX-105 | INBRX-105: next generation bispecific antibody targeting PD-L1 and 4-1BB, blocks inhibitory PD-1/PD-L1 axis and simultaneously activates essential co-stimulatory activity via 4-1BB | 90 | Safety | Recruiting | December 2021 | ||
| NCT03538028 | I | Second- or later-line | All | INCAGN02385 | INCAGN02385: LAG-3 inhibitor | 40 | Safety | Recruiting | September 2020 | ||
| NCT03652077 | I | First- or later-line | All | INCAGN02390 | INCAGN02390: TIM-3 inhibitor | 41 | Safety | Recruiting | January 2021 | ||
| NCT03126110 | I/II | First- or later-line | All | Nivolumab + INCAGN01876 | Ipilimumab + INCAGN01876 | Nivolumab + ipilimumab + INCAGN01876 | INCAGN01876: GITR agonist. Nivolumab: anti-PD-1. Ipilimumab: anti-CTLA-4. | 285 | Safety ORR | Recruiting | October 2021 |
| NCT03185468 | I/II | First- or later-line | All | CAR-T | 20 | OS Safety | Recruiting | December 2020 | |||
| NCT03639714 | I/II | Second-line | All | Nivolumab + ipilimumab + GRT-C901 + GRT-R902 | GRT-C901, GRT-R902: tumor vaccines. Nivolumab: anti-PD-1. Ipilimumab: anti-CTLA-4. | 214 | Safety ORR | Recruiting | March 2023 | ||
| NCT03228667 (QUILT-3.055) | II | Second- or later-line | All | ALT-803 + ICI (nivolumab or pembrolizumab or avelumab or atezolizumab) | ALT-803: ALT-803: Superagonist Interleukin-15. Pembrolizumab: anti-PD-1. Nivolumab: anti-PD-1. Avelumab: anti-PD-L1. Atezolizumab: anti-PD-L1. | 611 | ORR | Recruiting | August 2020 | ||
| NCT03639714 | I | Second- or later-line | All | ARG1-18, 19, 20 | ARG1-18, 19, 20: Arginase-1 Peptide Vaccine | 10 | Safety | Recruiting | June 2021 | ||
| NCT03917381 | I/II | Second- or later-line | All | GEN1046 | GEN1046: bispecific antibody targeting PD-L1 and 4-1BB | 192 | Safety | Recruiting | February 2022 | ||
| NCT03517488 (DUET-2) | I | Second- or later-line | All | XmAb20717 | XmAb20717: A Fc-engineered bispecific antibody directed against the human negative immunoregulatory checkpoint receptors PD-1 and CTLA-4 | 154 | Safety | Recruiting | March 2021 | ||
| NCT04044859 | I | Second- or later-line | All | Autologous genetically modified ADP-A2M4CD8 cells | Autologous genetically modified ADP-A2M4CD8 cells, directed to MAGE-A4, a member of the MAGE family expressed in a number of solid tumor types. | 30 | Safety | Recruiting | January 2021 | ||
| NCT03894618 | I | First-line (in platinum unfit) or second- or later-line | All | SL-279252 | SL-279252: agonist redirected checkpoint fusion protein consisting of the extracellular domains of PD- 1 and OX40L, linked by a central Fc domain (PD1-Fc-OX40L). | 87 | Safety | Recruiting | April 2022 | ||
| NCT03849469 (DUET-4) | I | Second- or later-line | All | XmAb®22841 | Pembrolizumab + XmAb®22841 | XmAb®22841: a Fc-engineered bispecific antibody directed against CTLA-4 and LAG-3. Pembrolizumab: anti-PD-1 | 242 | Safety | Recruiting | March 2027 | |
| NCT03752398 (DUET-3) | I | Second- or later-line | All | XmAb®23104 | XmAb23104: bispecific monoclonal antibody directed against PD-1 and inducible T-cell co-stimulator CD278 | 144 | Safety | Recruiting | March 2025 | ||
| NCT03758781 | I | First- or later-line | All | IRX-2 Regimen plus Nivolumab | IRX-2 Regimen: cyclophosphamide and subcutaneous IRX-2, a cell-free mixture comprising a variety of naturally derived cytokines obtained from normal, unrelated donor lymphocytes with potential immunostimulatory activity. The cytokines in IRX-2 include interleukin-1, -2, -6, -8, -10, -12, tumor necrosis factor alpha, interferon-gamma and colony stimulating factors. Nivolumab: anti-PD-1. | 100 | Safety | Recruiting | February 2022 | ||
| NCT03841110 | I | First- or later-line | All | FT500 | FT500 + nivolumab or pembrolizumab or atezolizumab | FT500: natural killer cell product that can bridge innate and adaptive immunity. Pembrolizumab: anti-PD-1. Nivolumab: anti-PD-1. Atezolizumab: anti-PD-L1. | 76 | Safety | Recruiting | June 2022 | |
| NCT03329950 | I | Second- or later-line | All | CDX-1140 | CDX-1140 + CDX-301 | CDX-1140 + pembrolizumab | CDX-1140: anti-CD40, a key activator of immune response which is found on dendritic cells, macrophages and B cells and is also expressed on many cancer cells. CDX-301: recombinant human FMS-like tyrosine kinase-3 ligand that acts by uniquely binding FMS-like tyrosine kinase-3 (CD135). Pembrolizumab: anti-PD-1. | 220 | Safety | Recruiting | November 2021 |
| NCT03674567 | I/II | First-line (in platinum unfit) or second- or later-line | All | FLX475 | FLX475 + pembrolizumab | FLX475: antagonist of C-C chemokine receptor type 4 with potential immunomodulatory and antineoplastic activities. FLX475 inhibits the binding of CCR4 to its signaling molecules, thereby blocking the recruitment of regulatory T cells to the tumor microenvironment. Pembrolizumab: anti-PD-1. | 375 | Safety ORR | Recruiting | August 2021 | |
| NCT03970382 | I | Second- or later-line | All | NeoTCR-P1 | NeoTCR-P1 + nivolumab | NeoTCR-P1: A preparation of autologous CD4- and CD8-positive T lymphocytes that have been engineered with site-specific nucleases to suppress the expression of most endogenous forms of the T-cell receptor and promote expression of a single, native T-cell receptor targeting a neoepitope presented on the surface of a patient’s tumor cells, with potential immunostimulating and antineoplastic activities. Nivolumab: anti-PD-1. | 148 | Safety | Recruiting | December 2023 | |
| NCT03277352 | I/II | Second- or later-line | All | INCAGN01876 + Pembrolizumab + Epacadostat | Epacadostat: IDO1-inhibitor.INCAGN01876: anti-human glucocorticoid-induced tumor necrosis factor receptor agonistic humanized monoclonal antibody, with potential immune checkpoint modulating activity. Pembrolizumab: anti-PD-1. | 10 | Phase 1: safety. Phase 2: ORR and complete response rate | Active, not recruiting | May 2020 | ||
| NCT03250832 (CITRINO) | I | Second- or later-line | All | TSR-033 | TSR-033 + an anti-PD-1 agent | TSR-033: anti-LAG-3 monoclonal antibody | 200 | Safety | Recruiting | May 2021 | |
| NCT03693612 | I/II | Second- or later-line | All | GSK3359609 plus tremelimumab | GSK3359609: agonistic antibody for the inducible T-cell co-stimulator (ICOS; CD278), with potential immune checkpoint inhibitory and antineoplastic activities. Tremelimumab: anti-CTLA-4. | 114 | Safety | Recruiting | April 2023 | ||
| NCT03739931 | I | First line in cisplatin ineligible and PD-L1 negative patients; second-line after platinum-containing chemotherapy | All | mRNA-2752 | mRNA-2752 plus durvalumab | mRNA-2752: lipid nanoparticle encapsulating mRNAs encoding human OX40L, IL-23, and IL-36γ. Durvalumab: anti-PD-L1. | 126 | Safety | Recruiting | July 2021 |
| NCT (Clinicaltrials.gov) | Phase | Setting | Cisplatin Fit/Unfit | Arm A | Arm B | Arm C | Compounds Description | Number of Patients | Primary Outcome | Status | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03682289 | II | Third- or later-line | All | AZD6738 | AZD6738 plus olaparib | AZD6738: an orally available morpholino-pyrimidine-based inhibitor of ataxia telangiectasia and rad3 related kinase. Olaparib: PARP inhibitor. | 68 | ORR | Recruiting | March 2023 | |
| NCT03945084 | II | Maintenance after first-line treatment | All | Niraparib + best supportive care | Best supportive care | Niraparib: PARP inhibitor | 77 | PFS | Recruiting | June 2024 | |
| NCT03375307 | II | Second- or third-line | All | Olaparib | Olaparib: PARP inhibitor | 150 | ORR | Recruiting | August 2023 | ||
| NCT03448718 | II | Second- or later-lines; cisplatin ineligible; chemotherapy naïve | All | Olaparib | Olaparib: PARP inhibitor | 30 | ORR | Recruiting | March 2023 | ||
| NCT03459846(BAYOU) | II | First-line | Cisplatin ineligible | Durvalumab + olaparib | Durvalumab + placebo | Durvalumab: anti-PD-L1. Olaparib: PARP inhibitor. | 154 | PFS | Active, not recruiting | September 2021 | |
| NCT03992131 | I/II | Second- or later-line | All | Rucaparib and Lucitanib (phase Ib) | Rucaparib and Sacituzumab govitecan (phase Ib and II) | Sacituzumab govitecan: humanized anti-Trop-2 monoclonal antibody linked with SN-38, the active metabolite of irinotecan. Lucitanib: VEGFR 1, 2 and 3, FGFR 1 and 2, and PDGFR alpha and beta inhibitor. Rucaparib: PARP inhibitor. | 329 | Safety and tolerability, dose limiting toxicities (phase Ib), ORR (phase II) | Recruiting | March 2024 | |
| NCT02546661 (BISCAY) | I | Second- or third-line | All | 8 cohorts: AZD4547; AZD4547 + durvalumab; durvalumab + olaparib; durvalumab + AZD1775; durvalumab; durvalumab + vistusertib; durvalumab + AZD9150; durvalumab + Selumetinib | AZD4547: FGFR-1, 2 and 3 inhibitor. AZD1775 (adavosertinib): inhibitor of the tyrosine kinase WEE1. Durvalumab: anti-PD-L1. Vistusertib: mTOR inhibitor. AZD9150 (danvatirsen): an antisense oligonucleotide targeting signal transducer and activator of transcription 3 (STAT3). Selumetinib: MEK or MAPK/ERK kinase 1 and 2 inhibitor. | 156 | Safety | Active, not recruiting | March 2020 |
| NCT (Clinicaltrials.gov) | Phase | Setting | Cisplatin Fit/Unfit | Arm A | Arm B | Arm C | Compounds Description | Number of Patients | Primary Outcome | Status | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03980041 (MARIO-275) | II | First- or later-line | All | Nivolumab + IPI-549 | Nivolumab + placebo | IPI-549: PI3K inhibitor. Nivolumab: anti-PD-1. | 160 | ORR | Recruiting | November 2022 | |
| NCT03745911 | II | Second- or later-line | All | Paclitaxel + TAK-228 | TAK-228: PI3K/AKT/mTOR inhibitor | 52 | ORR | Recruiting | November 2020 | ||
| NCT00805129 | II | Second-, third or fourth- line | All | Everolimus | Everolimus: mTOR inhibitor | 46 | PFS Safety | Active, not recruiting | December 2020 | ||
| NCT02567409 | II | First-line or second-line (only with prior ICI) | All | Gemcitabine + cisplatin + berzosertib (M6620) | Gemcitabine + cisplatin | Berzosertib (M6620): ATR kinase inhibitor | 90 | PFS | Active, not recruiting | August 2020 | |
| NCT03047213 | II | Second- or later-line | All | Sapanisertib | Sapanisertib: mTORC1 and mTORC2 inhibitor | 209 | ORR | Recruiting | June 2020 | ||
| NCT02535650 | II | Second- or later-line | All | Tipifarnib | Tipifarnib: farnesyltransferase inhibitor | 18 | 6-month PFS | Recruiting | March 2020 | ||
| NCT01551030 | II | Second- or later-line | All | Buparlisib | Buparlisib: PI3K inhibitor | 35 | PFS | Active, not recruiting | March 2020 | ||
| NCT04073602 | II | Second- or later-line; HER-2 negative | All | RC48-ADC | RC48-ADC: anti-HER2 monoclonal antibody | 20 | ORR | Recruiting | October 2020 | ||
| NCT03809013 | II | Second- or later-line; HER2 overexpressed | All | RC48-ADC | RC48-ADC: anti-HER2 monoclonal antibody | 60 | ORR | Recruiting | December 2021 | ||
| NCT02795156 | II | Second-line | All | Afatinib or Regorafenib or Cabozantinib (based on specific genomic alterations on next-generation sequencing) | Afatinib, Regorafenib, Cabozantinib: tyrosine kinase inhibitor | 160 | ORR | Recruiting | December 2020 | ||
| NCT02872714 (FIGHT-201) | II | Second- or later-line | All | Pemigatinib | Pemigatinib: FGFR inhibitor | 240 | ORR | Recruiting | August 2020 | ||
| NCT03410693 | II/III | Second- or later-line | All | Rogaratinib (BAY1163877) | Chemotherapy | Rogaratinib: FGFR inhibitor | 171 | OS | Active, not recruiting | November 2020 | |
| (FORT-1) | |||||||||||
| NCT04003610 (FIGHT-205) | II | First-line | Cisplatin ineligible | Pemigatinib + Pembrolizumab | Pemigatinib | Chemotherapy or pembrolizumab | Pemigatinib: FGFR inhibitor. Pembrolizumab: anti-PD-1. | 372 | PFS | Recruiting | June 2024 |
| NCT03190174 | I/II | Second- or later-line | All | Nivolumab + ABI-009 (Nab-rapamycin) | ABI-009 (Nab-rapamycin): mTOR inhibitor. Nivolumab: anti-PD-1. | 40 | Safety | Recruiting | April 2021 | ||
| NCT03523572 | I/II | Second- or later-line | All | Trastuzumab Deruxtecan (DS-8201a) + Nivolumab | Trastuzumab Deruxtecan (DS-8201a): antibody drug conjugated composed of trastuzumab, a monoclonal antibody targeting HER2 conjugated to deruxtecan, a derivative of the camptothecin analog exatecan, a DNA topoisomerase 1 inhibitor. Nivolumab: anti-PD-1. | 99 | Safety, ORR | Recruiting | September 2020 | ||
| NCT03330561 | I | Third- or later-line | All | PRS-343 | PRS-343: anti-HER2 monoclonal antibody linked to a CD137-targeting anticalin | 78 | Safety | Recruiting | September 2020 | ||
| NCT02546661 (BISCAY) | I | Second- or third-line | All | 8 cohorts: AZD4547; AZD4547 + durvalumab; durvalumab + olaparib; durvalumab + AZD1775; durvalumab;durvalumab + vistusertib; durvalumab + AZD9150; durvalumab + Selumetinib | AZD4547: FGFR-1, 2 and 3 inhibitor. AZD1775 (adavosertinib): inhibitor of the tyrosine kinase WEE1. Durvalumab: anti-PD-L1. Vistusertib: mTOR inhibitor. AZD9150 (danvatirsen): an antisense oligonucleotide targeting signal transducer and activator of transcription 3 (STAT3). Selumetinib: MEK or MAPK/ERK kinase 1 and 2 inhibitor. | 156 | Safety | Active, not recruiting | March 2020 | ||
| NCT02608125 | I | Third- or later-line | All | PRN1371 | PRN1371: FGFR inhibitor | 50 | Safety | Recruiting | February 2021 | ||
| NCT03473756 | I/II | First-line | Cisplatin ineligible | Rogaratinib + Atezolizumab | Placebo + Atezolizumab | Rogaratinib: FGFR inhibitor | 210 | Safety, PFS | Recruiting | September 2024 | |
| (FORT-2) | |||||||||||
| NCT04045613 | I/II | First-line; later-lines after prior FGFR inhibitor | Cisplatin ineligible | Derazantinib | Derazantinib + Atezolizumab | Derazantinib: FGFR inhibitor. Atezolizumab: anti-PD-L1. | 303 | ORR; recommended phase 2 dose | Recruiting | May 2022 | |
| NCT03473743 | I/II | Phase 1b: second- or later-line. Phase 2: first-line | Phase 2: cisplatin ineligible | Erdafitinib + Cetrelimab | Erdafitinib: FGFR inhibitor. Cetrelimab: anti-PD-1. | 150 | Safety, ORR | Recruiting | September 2021 |
| NCT (Clinicaltrials.gov) | Phase | Setting | Cisplatin Fit/Unfit | Arm A | Arm B | Arm C | Compounds Description | Number of Patients | Primary Outcome | Status | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03854474 | I/II | Second- or later-line | All | Pembrolizumab + tazemetostat | Tazemetostat: Enhancer of zeste homolog 2 (EZH2) methyltransferase inhibitor. EZH2 is a histone-lysine N-methyltransferase enzyme participating in histone methylation and, ultimately, transcriptional repression. Pembrolizumab: anti-PD-1. | 30 | ORR | Recruiting | June 2020 | ||
| NCT04200963 | I | Second- or later-line | All | KYN-175 | Aryl Hydrocarbon Receptor antagonist | 53 | Safety | Recruiting | September 2022 | ||
| NCT04007744 | I | First-line (in platinum unfit) or second- or later-line | All | Pembrolizumab + sonidegib | Sonidegib: Hedgehog signaling pathway inhibitor. Pembrolizumab: anti-PD-1. | 45 | Safety | Recruiting | June 2021 | ||
| NCT03829436 | I | First- or later-line | All | TPST-1120; TPST-1120 + nivolumab; TPST-1120 + docetaxel; TPST-1120 + cetuximab | TPST-1120: selective antagonist of peroxisome proliferator activated receptor alpha. Nivolumab: anti-PD-1. Cetuximab: anti-EGFR. | 338 | Safety | Recruiting | June 2024 | ||
| NCT02420847 | I/II | Second- or later-line | All | Ixazomib + Gemcitabine + Doxorubicin | Ixazomib: second generation proteasome inhibitor with potential antineoplastic activity | 50 | Safety | Active, not recruiting | July 2022 | ||
| NCT00365157 | I/II | Second- and third-line | All | Eribulin | Eribulin: microtubule-targeting agent | 132 | Maximum tolerated dose and recommended phase II dose, ORR | Active, not recruiting | December 2020 | ||
| NCT03179943 | II | First-line (in platinum unfit) or second- or later-line (with prior platinum-based chemotherapy or ICI) | All | Atezolizumab + guadecitabine | Guadecitabine: DNA methyltransferase (DNMT) inhibitor. Atezolizumab: anti- PD-L1. | 53 | Safety ORR | Recruiting | July 2022 | ||
| NCT03547973 | II | First-line (in platinum unfit) or second- or later-line (with prior platinum-based chemotherapy or ICI) | All | Sacituzumab govitecan (IMMU-132) | Sacituzumab govitecan (IMMU-132): Anti-Trop-2/SN-38 Antibody-Drug Conjugate | 201 | ORR | Recruiting | September 2021 | ||
| NCT04223856 (EV-302) | III | First-line | Cisplatin or carboplatin eligible | Enfortumab vedotin + pembrolizumab | Enfortumab vedotin + pembrolizumab + cisplatin or carboplatin | Gemcitabine + cisplatin or carboplatin | Enfortumab vedotin: anti nectin- 4 monoclonal antibody liked to a micro- tubule-disrupting agent (monomethyl auristatin E). Pembrolizumab: anti-PD-1. | 1095 | PFS, OS | Recruiting | November 2023 |
| NCT03474107 (EV-301) | III | Third- or later-line | All | Enfortumab vedotin | Chemotherapy (docetaxel, vinflunine, paclitaxel) | Enfortumab vedotin: anti nectin- 4 monoclonal antibody liked to a micro- tubule-disrupting agent (monomethyl auristatin E) | 608 | OS | Active, not recruiting | September 2021 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollica, V.; Rizzo, A.; Montironi, R.; Cheng, L.; Giunchi, F.; Schiavina, R.; Santoni, M.; Fiorentino, M.; Lopez-Beltran, A.; Brunocilla, E.; et al. Current Strategies and Novel Therapeutic Approaches for Metastatic Urothelial Carcinoma. Cancers 2020, 12, 1449. https://doi.org/10.3390/cancers12061449
Mollica V, Rizzo A, Montironi R, Cheng L, Giunchi F, Schiavina R, Santoni M, Fiorentino M, Lopez-Beltran A, Brunocilla E, et al. Current Strategies and Novel Therapeutic Approaches for Metastatic Urothelial Carcinoma. Cancers. 2020; 12(6):1449. https://doi.org/10.3390/cancers12061449
Chicago/Turabian StyleMollica, Veronica, Alessandro Rizzo, Rodolfo Montironi, Liang Cheng, Francesca Giunchi, Riccardo Schiavina, Matteo Santoni, Michelangelo Fiorentino, Antonio Lopez-Beltran, Eugenio Brunocilla, and et al. 2020. "Current Strategies and Novel Therapeutic Approaches for Metastatic Urothelial Carcinoma" Cancers 12, no. 6: 1449. https://doi.org/10.3390/cancers12061449
APA StyleMollica, V., Rizzo, A., Montironi, R., Cheng, L., Giunchi, F., Schiavina, R., Santoni, M., Fiorentino, M., Lopez-Beltran, A., Brunocilla, E., Brandi, G., & Massari, F. (2020). Current Strategies and Novel Therapeutic Approaches for Metastatic Urothelial Carcinoma. Cancers, 12(6), 1449. https://doi.org/10.3390/cancers12061449










