Monoclonal Gammopathy of Undetermined Significance (MGUS)—Not So Asymptomatic after All
Abstract
:1. Introduction
2. Osteoporosis and Bone Fractures
3. Monoclonal Gammopathy of Renal Significance (MGRS)
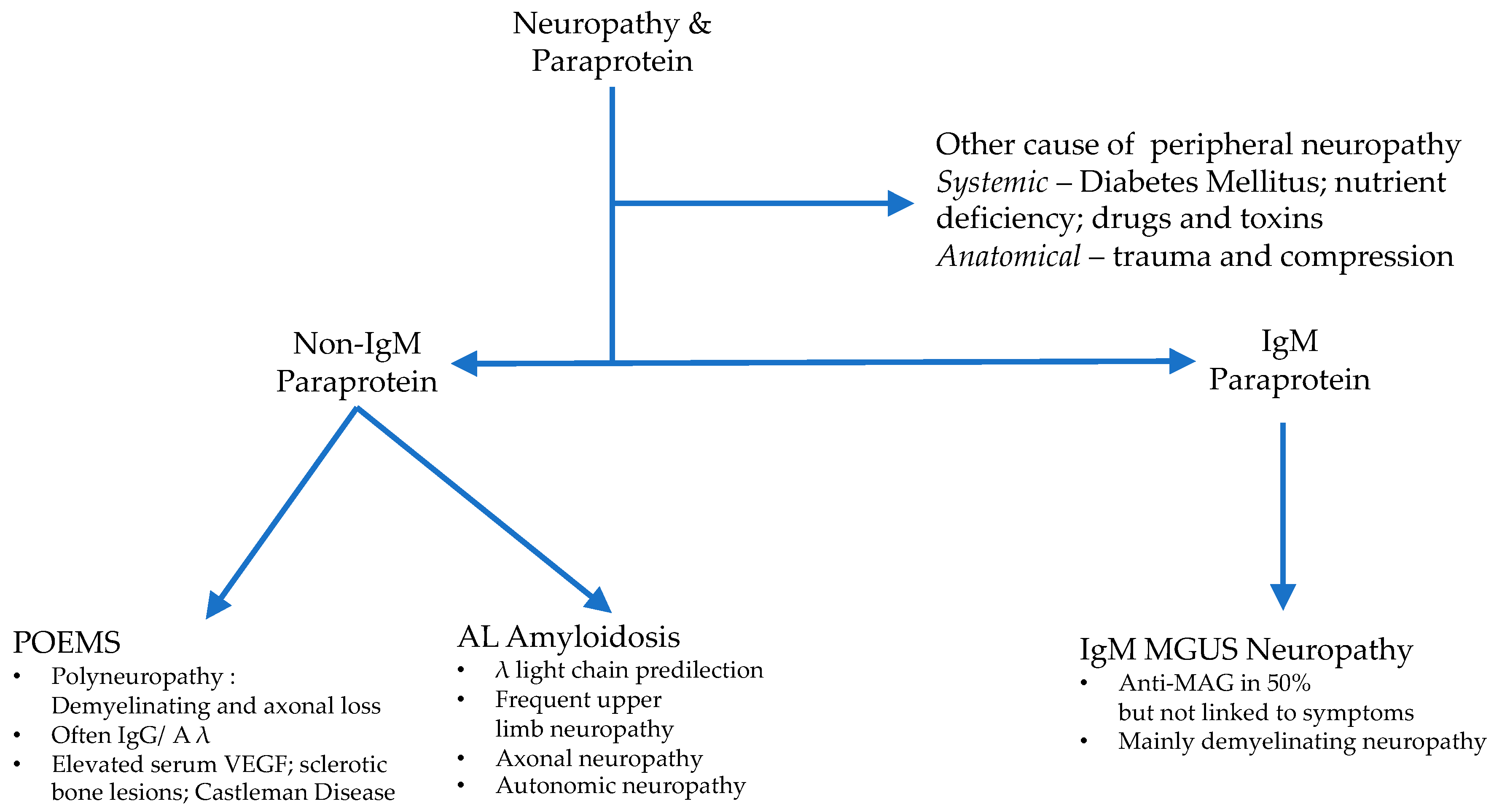
4. Peripheral Neuropathy
5. Immunodeficiency in MGUS
6. Cardiovascular Disease in MGUS
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kyle, R.A.; Benson, J.; Larson, D.; Therneau, T.; Dispenzieri, A.; Iii, L.J.M.; Rajkumar, S.V.; Melton, L.J. IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström’s macroglobulinemia. Clin. Lymphoma Myeloma 2009, 9, 17–18. [Google Scholar] [CrossRef] [Green Version]
- Kyle, R.A.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Cerhan, J.R.; Rajkumar, S.V. Long-term follow-up of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 2018, 378, 241–249. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Katzmann, J.A.; Kyle, R.A.; Larson, D.R.; Melton, L.J.; Colby, C.L.; Therneau, T.M.; Clark, R.; Kumar, S.K.; Bradwell, A.R.; et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: A retrospective population-based cohort study. Lancet 2010, 375, 1721–1728. [Google Scholar] [CrossRef] [Green Version]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Larson, D.R.; Plevak, M.F.; Offord, J.R.; Dispenzieri, A.; Katzmann, J.A.; Melton, L.J. Prevalence of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 2006, 354, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Marinac, C.R.; Ghobrial, I.M.; Birmann, B.M.; Soiffer, J.; Rebbeck, T.R. Dissecting racial disparities in multiple myeloma. Blood Cancer J. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef] [Green Version]
- Kyle, R.A. Monoclonal gammopathy of undetermined significance. Natural history in 241 cases. Am. J. Med. 1978, 64, 814–826. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Björkholm, M.; Andersson, T.M.-L.; Eloranta, S.; Dickman, P.W.; Goldin, L.R.; Blimark, C.; Mellqvist, U.-H.; Wahlin, A.; Turesson, I.; et al. Patterns of survival and causes of death following a diagnosis of monoclonal gammopathy of undetermined significance: A population-based study. Haematology 2009, 94, 1714–1720. [Google Scholar] [CrossRef] [Green Version]
- Lipsker, D. Monoclonal gammopathy of cutaneous significance: Review of a relevant concept. J. Eur. Acad. Dermatol. Venereol. 2016, 31, 45–52. [Google Scholar] [CrossRef]
- Bida, J.P.; Kyle, R.A.; Therneau, T.M.; Melton, L.J.; Plevak, M.F.; Larson, D.R.; Dispenzieri, A.; Katzmann, J.A.; Rajkumar, S.V. Disease associations with monoclonal gammopathy of undetermined significance: A population-based study of 17,398 patients. Mayo Clin. Proc. 2009, 84, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Bladé, J.; Merlini, G.; Mateos, M.-V.; Rajkumar, S.V.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Politou, M.; Terpos, E.; Anagnostopoulos, A.; Szydlo, R.; Laffan, M.; Layton, M.; Apperley, J.F.; Dimopoulos, M.-A.; Rahemtulla, A. Role of receptor activator of nuclear factor-kappa B ligand (RANKL), osteoprotegerin and macrophage protein 1-alpha (MIP-1a) in monoclonal gammopathy of undetermined significance (MGUS). Br. J. Haematol. 2004, 126, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Tang, M.; Pfeiffer, R.M.; Björkholm, M.; Blimark, C.; Mellqvist, U.-H.; Wahlin, A.; Turesson, I.; Landgren, O. Monoclonal gammopathy of undetermined significance and risk of skeletal fractures: A population-based study. Blood 2010, 116, 2651–2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregersen, H.; Ibsen, J.S.; Mellemkjoer, L.; Dahlerup, J.F.; Olsen, J.H.; Soerensen, H.T. Mortality and causes of death in patients with monoclonal gammopathy of undetermined significance. Br. J. Haematol. 2001, 112, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Luchini, C.; Solmi, M.; Sergi, G.; Manzato, E.; Stubbs, B. Monoclonal gammopathy of undetermined significance and bone health outcomes: A systematic review and exploratory meta-analysis. J. Bone Miner. Metab. 2017, 36, 128–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahamsen, B.; Andersen, I.; Christensen, S.S.; Madsen, J.S.; Brixen, K. Utility of testing for monoclonal bands in serum of patients with suspected osteoporosis: Retrospective, cross sectional study. BMJ 2005, 330, 818. [Google Scholar] [CrossRef] [Green Version]
- Piot, J.M.; Royer, M.; Schmidt-Tanguy, A.; Hoppé, E.; Gardembas, M.; Bourrée, T.; Hunault, M.; François, S.; Boyer, F.; Ifrah, N.; et al. Factors associated with an increased risk of vertebral fracture in monoclonal gammopathies of undetermined significance. Blood Cancer J. 2015, 5, e345. [Google Scholar] [CrossRef]
- Melton, L.J.; Rajkumar, S.V.; Khosla, S.; Achenbach, S.J.; Oberg, A.L.; Kyle, R.A. Fracture Risk in Monoclonal Gammopathy of Undetermined Significance. J. Bone Miner. Res. 2003, 19, 25–30. [Google Scholar] [CrossRef]
- Terpos, E.; Morgan, G.; Dimopoulos, M.A.; Drake, M.T.; Lentzsch, S.; Raje, N.; Sezer, O.; García-Sanz, R.; Shimizu, K.; Turesson, I.; et al. International myeloma working group recommendations for the treatment of multiple myeloma-related bone disease. J. Clin. Oncol. 2013, 31, 2347–2357. [Google Scholar] [CrossRef] [Green Version]
- Pepe, J.; Petrucci, M.T.; Nofroni, I.; Fassino, V.; Diacinti, D.; Romagnoli, E.; Minisola, S. Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br. J. Haematol. 2006, 134, 485–490. [Google Scholar] [CrossRef]
- Pepe, J.; Petrucci, M.T.; Mascia, M.L.; Piemonte, S.; Fassino, V.; Romagnoli, E.; Minisola, S. The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif. Tissue Int. 2008, 82, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Van De Donk, N.W.; Palumbo, A.; Johnsen, H.E.; Engelhardt, M.; Gay, F.; Gregersen, H.; Hájek, R.; Kleber, M.; Ludwig, H.; Morgan, G.; et al. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: Recommendations from the European Myeloma Network. Haematology 2014, 99, 984–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenson, J.R.; Yellin, O.; Boccia, R.V.; Flam, M.; Wong, S.-F.; Batuman, O.; Moezi, M.M.; Woytowitz, D.; Duvivier, H.; Nassir, Y.; et al. Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin. Cancer Res. 2008, 14, 6289–6295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorsteinsdottir, S.; Lund, S.H.; Lindqvist, E.K.; Thordardottir, M.; Sigurdsson, G.; Costello, R.; Burton, D.; Steingrímsdóttir, H.; Gudnason, V.; Eiriksdottir, G.; et al. Bone disease in monoclonal gammopathy of undetermined significance: Results from a screened population-based study. Blood Adv. 2017, 1, 2790–2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fermand, J.-P.; Bridoux, F.; Kyle, R.A.; Kastritis, E.; Weiss, B.M.; Cook, M.; Drayson, M.T.; Dispenzieri, A.; Leung, N. The International Kidney and Monoclonal Gammopathy Research Group. How I treat monoclonal gammopathy of renal significance (MGRS). Blood 2013, 122, 3583–3590. [Google Scholar] [CrossRef]
- Leung, N.; Bridoux, F.; Hutchison, C.A.; Nasr, S.H.; Cockwell, P.; Fermand, J.-P.; Dispenzieri, A.; Song, K.W.; Kyle, R.A. The International Kidney and Monoclonal Gammopathy Research Group. Monoclonal gammopathy of renal significance: When MGUS is no longer undetermined or insignificant. Blood 2012, 120, 4292–4295. [Google Scholar] [CrossRef] [Green Version]
- Zand, L.; Nasr, S.H.; A Gertz, M.; Dispenzieri, A.; Lacy, M.Q.; Buadi, F.K.; Rajkumar, S.V.; Kyle, R.A.; Fervenza, F.C.; Sethi, S.; et al. Clinical and prognostic differences among patients with light chain deposition disease, myeloma cast nephropathy and both. Leuk. Lymphoma 2015, 56, 3357–3364. [Google Scholar] [CrossRef]
- Bridoux, F.; The International Kidney and Monoclonal Gammopathy Research Group; Leung, N.; Hutchison, C.A.; Touchard, G.; Sethi, S.; Fermand, J.-P.; Picken, M.M.; Herrera, G.A.; Kastritis, E.; et al. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015, 87, 698–711. [Google Scholar] [CrossRef] [Green Version]
- Leung, N.; Bridoux, F.; Batuman, V.; Chaidos, A.; Cockwell, P.; D’Agati, V.D.; Dispenzieri, A.; Fervenza, F.C.; Fermand, J.-P.; Gibbs, S.; et al. Publisher correction: The evaluation of monoclonal gammopathy of renal significance: A consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat. Rev. Nephrol. 2019, 15, 121. [Google Scholar] [CrossRef] [Green Version]
- Bridoux, F.; Desport, E.; Frémeaux-Bacchi, V.; Chong, C.F.; Gombert, J.-M.; Lacombe, C.; Quellard, N.; Touchard, G. Glomerulonephritis with isolated C3 deposits and monoclonal gammopathy: A fortuitous association? Clin. J. Am. Soc. Nephrol. 2011, 6, 2165–2174. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Sukov, W.R.; Zhang, Y.; Fervenza, F.C.; Lager, N.J.; Miller, D.V.; Cornell, L.D.; Krishnan, S.G.S.; Smith, R.J.H. Dense deposit disease associated with monoclonal gammopathy of undetermined significance. Am. J. Kidney Dis. 2010, 56, 977–982. [Google Scholar] [CrossRef] [Green Version]
- Zand, L.; Kattah, A.; Fervenza, F.C.; Smith, R.J.H.; Nasr, S.H.; Zhang, Y.; Vrana, J.A.; Leung, N.; Cornell, L.D.; Sethi, S. C3 glomerulonephritis associated with monoclonal gammopathy: A case series. Am. J. Kidney Dis. 2013, 62, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauvet, S.; Roumenina, L.T.; Aucouturier, P.; Marinozzi, M.-C.; Dragon-Durey, M.-A.; Karras, A.; Delmas, Y.; Le Quintrec, M.; Guerrot, D.; Jourde-Chiche, N.; et al. Both monoclonal and polyclonal immunoglobulin contingents mediate complement activation in monoclonal gammopathy associated-C3 glomerulopathy. Front. Immunol. 2018, 9, 2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindran, A.; Fervenza, F.C.; Smith, R.J.H.; Sethi, S. C3 glomerulopathy associated with monoclonal Ig is a distinct subtype. Kidney Int. 2018, 94, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, I.E.; Gallan, A.; Huston, H.K.; Raphael, K.L.; Miller, D.V.; Revelo, M.P.; Khalighi, M.A. C3 glomerulopathy in adults: A distinct patient subset showing frequent association with monoclonal gammopathy and poor renal outcome. Clin. Kidney J. 2016, 9, 794–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yui, J.C.; Garceau, D.; Jhaveri, K.D.; Wanchoo, R.; Bijol, V.; Glezerman, I.; Hassoun, H.; Dispenzieri, A.; Russell, S.J.; Leung, N. Monoclonal gammopathy-associated thrombotic microangiopathy. Am. J. Hematol. 2019, 94, E250–E253. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, A.; Go, R.S.; Fervenza, F.C.; Sethi, S. Thrombotic microangiopathy associated with monoclonal gammopathy. Kidney Int. 2017, 91, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Blanc, C.; Togarsimalemath, S.K.; Chauvet, S.; Le Quintrec, M.; Moulin’, B.; Buchler, M.; Jokiranta, T.S.; Roumenina, L.T.; Fremeaux-Bacchi, V.; Dragon-Durey, M.-A. Anti–Factor H Autoantibodies in C3 Glomerulopathies and in atypical hemolytic uremic syndrome: One target, two diseases. J. Immunol. 2015, 194, 5129–5138. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Wang, C.; Cai, Q.-Q.; Cai, H.; Duan, M.; Li, J.; Cao, X.-X.; Zhou, D.; Li, J. Renal impairment in patients with polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy and skin changes syndrome: Incidence, treatment and outcome. Nephrol. Dial. Transplant. 2015, 31, 275–283. [Google Scholar] [CrossRef]
- Nasr, S.; Satoskar, A.; Markowitz, G.S.; Valeri, A.M.; Appel, G.B.; Stokes, M.B.; Nadasdy, T.; D’Agati, V.D. Proliferative glomerulonephritis with monoclonal IgG deposits. J. Am. Soc. Nephrol. 2009, 20, 2055–2064. [Google Scholar] [CrossRef] [Green Version]
- Heilman, R.L.; Velosa, J.A.; Holley, K.E.; Offord, K.P.; Kyle, R.A. Long-term follow-up and response to chemotherapy in patients with light-chain deposition disease. Am. J. Kidney Dis. 1992, 20, 34–41. [Google Scholar] [CrossRef]
- Dingli, D.; Kyle, R.A.; Rajkumar, S.V.; Nowakowski, G.S.; Larson, D.R.; Bida, J.P.; Gertz, M.A.; Therneau, T.M.; Melton, L.J.; Dispenzieri, A.; et al. Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood 2006, 108, 1979–1983. [Google Scholar] [CrossRef] [PubMed]
- Nambirajan, A.; Bhowmik, D.M.; Singh, G.; Agarwal, S.K.; Dinda, A. Monoclonal gammopathy of renal significance with light-chain deposition disease diagnosed postrenal transplant: A diagnostic and therapeutic challenge. Transpl. Int. 2014, 28, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.H.; Valeri, A.M.; Cornell, L.D.; Fidler, M.E.; Sethi, S.; D’Agati, V.D.; Leung, N. Renal Monoclonal Immunoglobulin Deposition Disease: A Report of 64 Patients from a Single Institution. Clin. J. Am. Soc. Nephrol. 2011, 7, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Kaposztas, Z.; Kahan, B.; Katz, S.; Van Buren, C.; Cherem, L. Bortezomib successfully reverses early recurrence of light-chain deposition disease in a renal allograft: A case report. Transplant. Proc. 2009, 41, 4407–4410. [Google Scholar] [CrossRef]
- Moiz, A.; Javed, T.; Garces, J.; Staffeld-Coit, C.; Paueksakon, P. Late recurrence of light chain deposition disease after kidney transplantation treated with bortezomib: A case report. Ochsner J. 2014, 14, 445–449. [Google Scholar]
- Pampa-Saico, S.; Rodríguez-Mendiola, N.; Valles-Carboneras, A.; Gomis-Couto, A.; Saiz, A.; A Martínez-González, M.; Díaz-Domínguez, M.; Liaño, F. Treatment with bortezomib in dense deposit disease associated with monoclonal gammopathy of undetermined significance. Br. J. Haematol. 2018, 184, 302–304. [Google Scholar] [CrossRef] [Green Version]
- Ronco, P.; Alyanakian, M.-A.; Mougenot, B.; Aucouturier, P. Light chain deposition disease: A model of glomerulosclerosis defined at the molecular level. J. Am. Soc. Nephrol. 2001, 12, 1558–1565. [Google Scholar]
- Batalini, F.; Econimo, L.; Quillen, K.; Sloan, J.M.; Sarosiek, S.; Brauneis, D.; Havasi, A.; Stern, L.; Dember, L.M.; Sanchorawala, V. High-dose melphalan and stem cell transplantation in patients on dialysis due to immunoglobulin light-chain amyloidosis and monoclonal immunoglobulin deposition disease. Boil. Blood Marrow Transplant. 2018, 24, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Badros, A.Z.; Barlogie, B.; Siegel, E.; Roberts, J.; Langmaid, C.; Zangari, M.; Desikan, R.; Shaver, M.J.; Fassas, A.B.-T.; McConnell, S.; et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br. J. Haematol. 2001, 114, 822–829. [Google Scholar] [CrossRef]
- Delanaye, P.; Glassock, R.J.; Pottel, H.; Rule, A.D. An age-calibrated definition of chronic kidney disease: Rationale and benefits. Clin. Biochem. Rev. 2016, 37, 17–26. [Google Scholar] [PubMed]
- Rögnvaldsson, S.; Steingrímsson, V.; Turesson, I.; Björkholm, M.; Landgren, O.; Kristinsson, S.Y. Peripheral neuropathy and monoclonal gammopathy of undetermined significance: A population-based study including 15,351 cases and 58,619 matched controls. Haematology 2020. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Osserman, E.F. Pathologic conditions associated with plasma cell dyscrasias: A study of 806 cases. Ann. N. Y. Acad. Sci. 1971, 190, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.B.; Thomas, P.K.; King, R.H.; Waddy, H.; Will, R.G.; A Hughes, R.; Gregson, N.A.; Leibowitz, S. The clinical spectrum of peripheral neuropathies associated with benign monoclonal IgM, IgG and IgA paraproteinaemia. Comparative clinical, immunological and nerve biopsy findings. J. Neurol. 1991, 238, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Stork, A.C.; Jacobs, B.C.; Tio-Gillen, A.P.; Eurelings, M.; Jansen, M.; Berg, L.H.V.D.; Notermans, N.C.; Van Der Pol, W.-L. Prevalence, specificity and functionality of anti-ganglioside antibodies in neuropathy associated with IgM monoclonal gammopathy. J. Neuroimmunol. 2014, 268, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Nobile-Orazio, E.; Marmiroli, P.; Baldini, L.; Spagnol, G.; Barbieri, S.; Moggio, M.; Polli, N.; Polli, E.; Scarlato, G. Peripheral neuropathy in macroglobulinemia: Incidence and antigen-specificity of M proteins. Neurology 1987, 37, 1506. [Google Scholar] [CrossRef]
- Meucci, N.; Baldini, L.; Cappellari, A.; Di Troia, A.; Allaria, S.; Scarlato, G.; Nobile-Orazio, E. Anti-myelin-associated glycoprotein antibodies predict the development of neuropathy in asymptomatic patients with IgM monoclonal gammopathy. Ann. Neurol. 1999, 46, 119–122. [Google Scholar] [CrossRef]
- Benedetti, L.; Briani, C.; Franciotta, D.; Carpo, M.; Padua, L.; Zara, G.; Zambello, R.; Sormani, M.P.; Mancardi, G.L.; Nobile-Orazio, E.; et al. Long-term effect of rituximab in anti-mag polyneuropathy. Neurology 2008, 71, 1742–1744. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Rakocevic, G.; Salajegheh, M.; Dambrosia, J.M.; Hahn, A.F.; Raju, R.; McElroy, B. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein antibody demyelinating neuropathy. Ann. Neurol. 2009, 65, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Levine, T.D.; Pestronk, A. IgM antibody-related polyneuropathies: B-cell depletion chemotherapy using Rituximab. Neurology 1999, 52, 1701. [Google Scholar] [CrossRef]
- Dispenzieri, A. POEMS syndrome: 2019 Update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2019, 94, 812–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karam, C.; Klein, C.J.; Dispenzieri, A.; Dyck, P.J.B.; Mandrekar, J.; D’Souza, A.; Mauermann, M.L. Polyneuropathy improvement following autologous stem cell transplantation for POEMS syndrome. Neurology 2015, 84, 1981–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kourelis, T.V.; Gertz, M. Immunoglobulin light chain amyloidosis. Handb. Hematol. Malig. 2018, 89, 1132–1140. [Google Scholar] [CrossRef]
- Nikolich-Žugich, J. Author Correction: The twilight of immunity: Emerging concepts in aging of the immune system. Nat. Immunol. 2018, 19, 1146. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Tang, M.; Pfeiffer, R.M.; Björkholm, M.; Goldin, L.R.; Blimark, C.; Mellqvist, U.-H.; Wahlin, A.; Turesson, I.; Landgren, O. Monoclonal gammopathy of undetermined significance and risk of infections: A population-based study. Haematologica 2011, 97, 854–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, R.A.; Remstein, E.D.; Therneau, T.M.; Dispenzieri, A.; Kurtin, P.J.; Hodnefield, J.M.; Larson, D.R.; Plevak, M.F.; Jelinek, D.F.; Fonseca, R.; et al. Clinical Course and Prognosis of Smoldering (Asymptomatic) Multiple Myeloma. N. Engl. J. Med. 2007, 356, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- Schütt, P.; Brandhorst, D.; Stellberg, W.; Poser, M.; Ebeling, P.; Müller, S.; Buttkereit, U.; Opalka, B.; Lindemann, M.; Grosse-Wilde, H.; et al. Immune parameters in multiple myeloma patients: Influence of treatment and correlation with opportunistic infections. Leuk. Lymphoma 2006, 47, 1570–1582. [Google Scholar] [CrossRef]
- Augustson, B.M.; Begum, G.; Dunn, J.A.; Barth, N.J.; Davies, F.; Morgan, G.; Behrens, J.; Smith, A.; Child, J.A.; Drayson, M.T. Early mortality after diagnosis of multiple myeloma: Analysis of patients entered onto the united kingdom medical research council trials between 1980 and 2002—Medical research council adult leukaemia working party. J. Clin. Oncol. 2005, 23, 9219–9226. [Google Scholar] [CrossRef]
- Zavidij, O.; Haradhvala, N.J.; Mouhieddine, T.H.; Sklavenitis-Pistofidis, R.; Cai, S.; Reidy, M.; Rahmat, M.; Flaifel, A.; Ferland, B.; Su, N.K.; et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat. Rev. Cancer 2020, 1, 493–506. [Google Scholar] [CrossRef]
- De Magalhães, R.J.P.; Vidriales, M.-B.; Paiva, B.; Fernandez-Gimenez, C.; García-Sanz, R.; Mateos, M.-V.; Gutierrez, N.C.; Lecrevisse, Q.; Blanco, J.F.; Hernández, J.; et al. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematology 2012, 98, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, J.; Andréasson, B.; Kondori, N.; Erman, E.; Riesbeck, K.; Hogevik, H.; Wennerås, C. Comparative study of immune status to infectious agents in elderly patients with multiple myeloma, waldenstrom’s macroglobulinemia, and monoclonal gammopathy of undetermined significance. Clin. Vaccine Immunol. 2011, 18, 969–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, E.; Kaye, D.; Abrutyn, E.; Gross, P.; Dorfman, M.; Murasko, D.M. Immune response to influenza vaccination in a large healthy elderly population. Vaccine 1999, 17, 82–94. [Google Scholar] [CrossRef]
- Tete, S.M.; Kipling, D.; Westra, J.; De Haan, A.; Bijl, M.; Dunn-Waters, D.K.; Sahota, S.S.; Bos, N.A. Monoclonal paraprotein influences baseline B-cell repertoire diversity and perturbates influenza vaccination-induced B-cell response. Exp. Hematol. 2015, 43, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Prabhala, R.H.; Efebera, Y.A.; Lee, S.; Han, A.J.; Pelluru, D.; Nanjappa, P.; Amin, S.; Pai, C.; Ghobrial, I.; Schlossman, R.L.; et al. Lack of response to vaccination in MGUS and stable myeloma. Blood 2009, 114, 1852. [Google Scholar] [CrossRef]
- Branagan, A.R.; Duffy, E.; Albrecht, R.A.; Cooper, D.L.; Seropian, S.; Parker, T.L.; Gan, G.; Li, F.; Zelterman, D.; Boddupalli, C.S.; et al. Clinical and serologic responses after a two-dose series of high-dose influenza vaccine in plasma cell disorders: A prospective, single-arm trial. Clin. Lymphoma Myeloma Leuk. 2017, 17, 296–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasiarski, M.; Sosnowska-Pasiarska, B.; Grywalska, E.; Stelmach-Gołdyś, A.; Kowalik, A.; Góźdź, S.; Roliński, J. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in patients with monoclonal gammopathy of undetermined significance—Relationship with selected immune and clinical parameters. Clin. Interv. Aging 2019, 14, 1741–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christenson, B.; Lundbergh, P.; Hedlund, J.; Örtqvist, Å. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in adults aged 65 years or older: A prospective study. Lancet 2001, 357, 1008–1011. [Google Scholar] [CrossRef]
- Renaud, L.; Schraen, S.; Fouquet, G.; Guidez, S.; Demarquette, H.; Nudel, M.; Cayssials, E.; Bories, C.; Herbaux, C.; Systchenko, T.; et al. Response to pneumococcal vaccination in multiple myeloma. Cancer Med. 2019, 8, 3822–3830. [Google Scholar] [CrossRef]
- Orange, J.S.; Ballow, M.; Stiehm, E.R.; Ballas, Z.K.; Chinen, J.; De La Morena, M.; Kumararatne, D.; Harville, T.O.; Hesterberg, P.; Koleilat, M.; et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2012, 130, S1–S24. [Google Scholar] [CrossRef]
- Karlsson, J.; Roalfe, L.; Hogevik, H.; Zancolli, M.; Andréasson, B.; Goldblatt, D.; Wennerås, C. Poor correlation between pneumococcal IgG and IgM titers and opsonophagocytic activity in vaccinated patients with multiple myeloma and waldenstrom’s macroglobulinemia. Clin. Vaccine Immunol. 2016, 23, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Ramón, S.; Dhalla, F.; Chapel, H. Challenges in the role of gammaglobulin replacement therapy and vaccination strategies for hematological malignancy. Front. Immunol. 2016, 7, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Whitaker, J.; Shanafelt, T.D.; Poland, G.A.; Kay, N.E. Room for improvement: Immunizations for patients with monoclonal B-cell lymphocytosis or chronic lymphocytic leukemia. Clin. Adv. Hematol. Oncol. 2014, 12, 440–450. [Google Scholar] [PubMed]
- Kotton, C.; Poznansky, M.C. Vaccination of oncology patients: An effective tool and an opportunity not to be missed. Oncology 2012, 17, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemu, A.; Singh, M.; Blumberg, C.; Richards, J.O.; Oaks, M.K.; Thompson, M.A. Multiple myeloma vaccination patterns in a large health system: A pilot study. J. Patient-Cent. Res. Rev. 2017, 4, 53–59. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Pfeiffer, R.M.; Björkholm, M.; Goldin, L.R.; Schulman, S.; Blimark, C.; Mellqvist, U.-H.; Wahlin, A.; Turesson, I.; Landgren, O. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: A population-based study. Blood 2010, 115, 4991–4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallah, S.; Husain, A.; Wan, J.; Vos, P.; Nguyen, N.P. The risk of venous thromboembolic disease in patients with monoclonal gammopathy of undetermined significance. Ann. Oncol. 2004, 15, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- Srkalovic, G.; Cameron, M.G.; Rybicki, L.; Deitcher, S.R.; Kattke-Marchant, K.; Hussein, M.A. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer 2004, 101, 558–566. [Google Scholar] [CrossRef]
- Cohen, A.L.; Sarid, R. The relationship between monoclonal gammopathy of undetermined significance and venous thromboembolic disease. Thromb. Res. 2010, 125, 216–219. [Google Scholar] [CrossRef]
- Gregersen, H.; Nørgaard, M.; Severinsen, M.T.; Engebjerg, M.C.; Jensen, P.; Soerensen, H.T. Monoclonal gammopathy of undetermined significance and risk of venous thromboembolism. Eur. J. Haematol. 2010, 86, 129–134. [Google Scholar] [CrossRef]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to prevent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2018, 380, 711–719. [Google Scholar] [CrossRef]
- Auwerda, J.J.; Sonneveld, P.; De Maat, M.P.; Leebeek, F.W. Prothrombotic coagulation abnormalities in patients with paraprotein-producing B-cell disorders. Clin. Lymphoma Myeloma 2007, 7, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuster, J.J.; MacLauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.-L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef] [Green Version]
- Haefliger, S.; Juskevicius, D.; Höller, S.; Buser, U.; Dirnhofer, S.; Tzankov, A. How to resolve a clinical and molecular puzzle: Concomitant monoclonal gammopathy of undetermined significance (MGUS) with neutrophilia and clonal hematopoiesis of indeterminate potential (CHIP). Ann. Hematol. 2019, 98, 2431–2432. [Google Scholar] [CrossRef]
- Mouhieddine, T.S.; Redd, R.A.; Park, J.; Leventhal, M.; Gibson, C.; Manier, S.; Nassar, A.; Capelletti, M.; Huynh, D.; Bustoros, M.W.; et al. Clonal hematopoiesis is associated with adverse outcomes in multiple myeloma patients undergoing transplant. Nat. Commun. 2020, in press. [Google Scholar]
- Go, R.S.; Swanson, K.M.; Sangaralingham, L.R.; Habermann, E.B.; Shah, N.D. Clinical prevalence (diagnosed cases) of monoclonal gammopathy of undetermined significance in the US: Estimating the burden on health care. Leukemia 2015, 30, 1443–1446. [Google Scholar] [CrossRef]
- Bianchi, G.; Kyle, R.A.; Colby, C.L.; Larson, D.R.; Kumar, S.; Katzmann, J.A.; Dispenzieri, A.; Therneau, T.M.; Cerhan, J.R.; Melton, L.J.; et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma-related complications. Blood 2010, 116, 2019–2025. [Google Scholar] [CrossRef]
- Landgren, O.; Hofmann, J.N.; McShane, C.M.; Santo, L.; Hultcrantz, M.; Korde, N.; Mailankody, S.; Kazandjian, D.; Murata, K.; Thoren, K.; et al. Association of immune marker changes with progression of monoclonal gammopathy of undetermined significance to multiple myeloma. JAMA Oncol. 2019, 5, 1293. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Identifier NCT03327597. Available online: https://clinicaltrials.gov/ct2/show/NCT03327597 (accessed on 13 April 2020).
- ClinicalTrials.gov. Identifier NCT03689595. Available online: https://clinicaltrials.gov/ct2/show/NCT03689595 (accessed on 13 April 2020).
| Condition | Definition |
|---|---|
| Non-IgM MGUS | 1. Serum monoclonal immunoglobulin ≤3 g/dL 2. Plasma cells in the bone marrow ≤10% 3. Absence of: lytic bone lesions, anemia, hypercalcemia, and renal impairment. |
| IgM MGUS | 1. Serum monoclonal immunoglobulin ≤3 g/dL 2. Lymphoplasmacytic cells in the bone marrow ≤10% 3. Absence of: constitutional symptoms or symptoms and signs of hyper-viscosity, anemia or lymphadenopathy |
| Light chain MGUS | 1. Abnormal free light chain ratio 2. Increased concentration of involved light chain 3. Complete loss of heavy chain immunoglobulin expression |
| Co-Morbidity | Presentation | Management |
|---|---|---|
| Increased fracture risk | Increased incidence of reduced bone mineral density and atraumatic fractures compared to controls | Careful evaluation of myeloma-defining event Calcium/Vitamin D with bisphosphonates |
| Renal impairment (Monoclonal Gammopathy of Renal significance—MGRS) | Regular monitoring for renal impairment | Careful evaluation of myeloma-defining event. Prompt referral to nephrology for consideration of biopsy, considering the wide differential diagnosis |
| Peripheral neuropathy | Usually polyneuropathy λ light chain—consider POEMS and AL Amyloid | Close collaboration with neurology for testing, including nerve conduction studies and biopsy. Anti-MAG, ganglioside or asialo-GM1 antibodies may be useful to monitor response to therapy |
| Secondary immunodeficiency | Recurrent bacterial infections, especially sinopulmonary | Measure antibody levels for IgG, IgA and IgM as well as specific responses to tetanus, pneumococcus and Hemophilus. Consider prophylactic antibiotics, immunoglobulin replacement and ensure vaccinations maintained |
| Cardiovascular disease | Increased risk of arterial and venous thrombotic events | Encourage adherence to good practice in primary prevention—lifestyle modification and blood pressure control. No indication for prophylactic antiplatelet or anticoagulant therapy |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomas, O.C.; Mouhieddine, T.H.; Tahri, S.; Ghobrial, I.M. Monoclonal Gammopathy of Undetermined Significance (MGUS)—Not So Asymptomatic after All. Cancers 2020, 12, 1554. https://doi.org/10.3390/cancers12061554
Lomas OC, Mouhieddine TH, Tahri S, Ghobrial IM. Monoclonal Gammopathy of Undetermined Significance (MGUS)—Not So Asymptomatic after All. Cancers. 2020; 12(6):1554. https://doi.org/10.3390/cancers12061554
Chicago/Turabian StyleLomas, Oliver C., Tarek H. Mouhieddine, Sabrin Tahri, and Irene M. Ghobrial. 2020. "Monoclonal Gammopathy of Undetermined Significance (MGUS)—Not So Asymptomatic after All" Cancers 12, no. 6: 1554. https://doi.org/10.3390/cancers12061554
APA StyleLomas, O. C., Mouhieddine, T. H., Tahri, S., & Ghobrial, I. M. (2020). Monoclonal Gammopathy of Undetermined Significance (MGUS)—Not So Asymptomatic after All. Cancers, 12(6), 1554. https://doi.org/10.3390/cancers12061554





