Targeting Pharmacokinetic Drug Resistance in Acute Myeloid Leukemia Cells with CDK4/6 Inhibitors
Abstract
:1. Introduction
2. Results
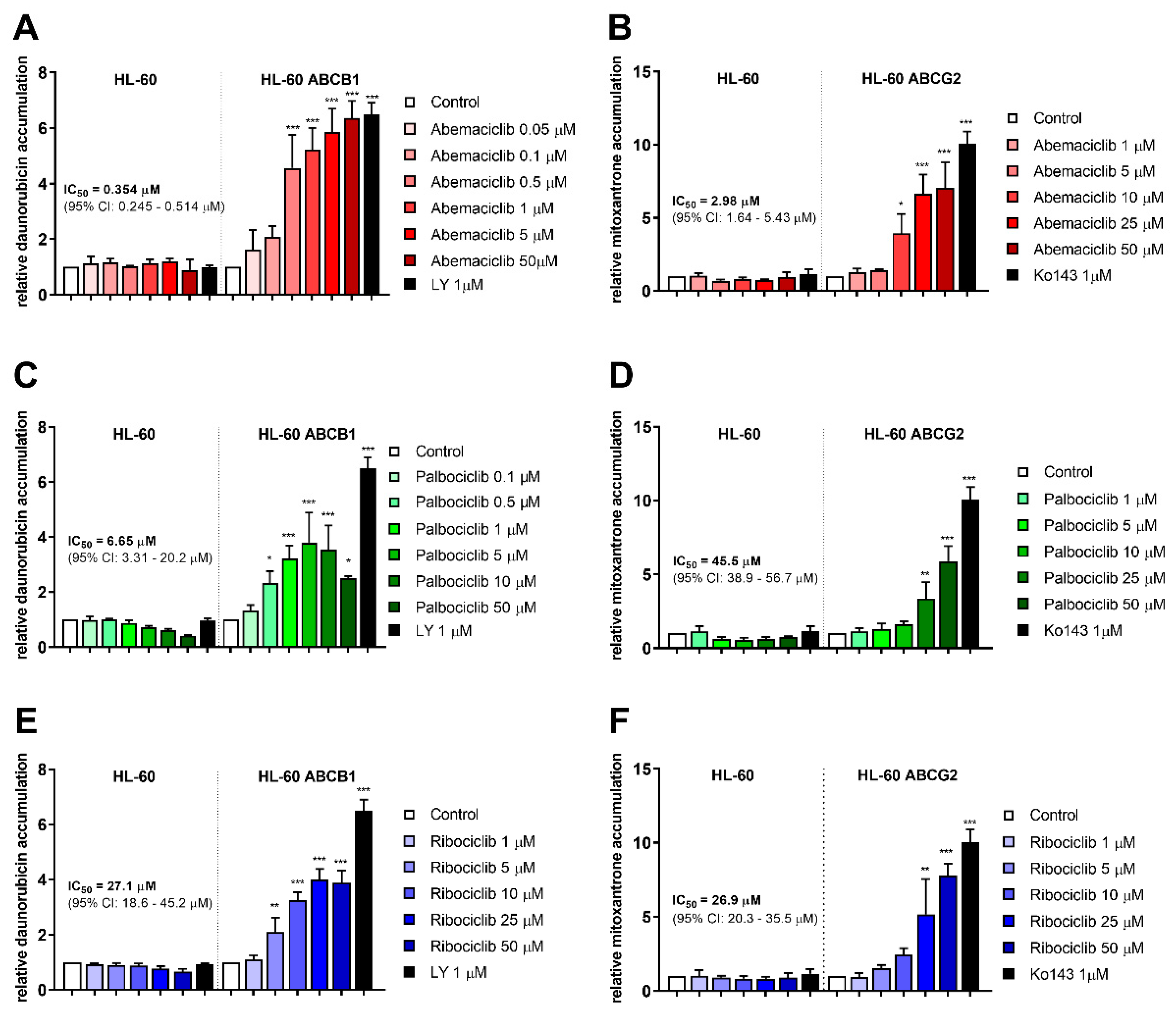
2.1. CDKI Enhance Daunorubicin and Mitoxantrone Accumulation in HL-60 Cells
2.2. CDKI Do Not Inhibit Carbonyl Reducing Enzymes
2.3. Daunorubicin- and Mitoxantrone-Induced Proapoptotic Behavior of Resistant HL-60 Cells after Exposure to CDKI
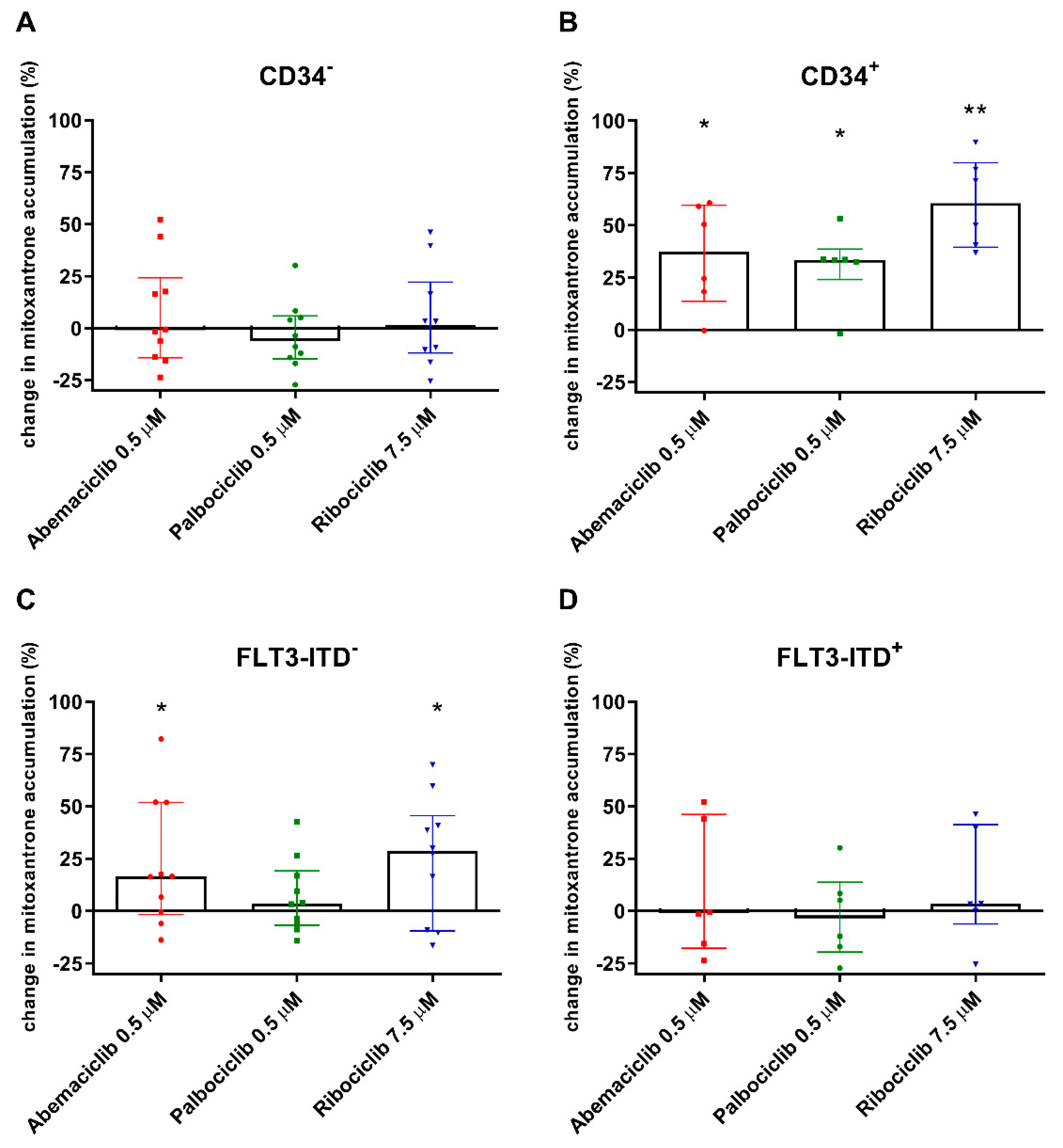
2.4. CDKI Affect Mitoxantrone Accumulation in CD34+ and FLT3-ITD− PBMC
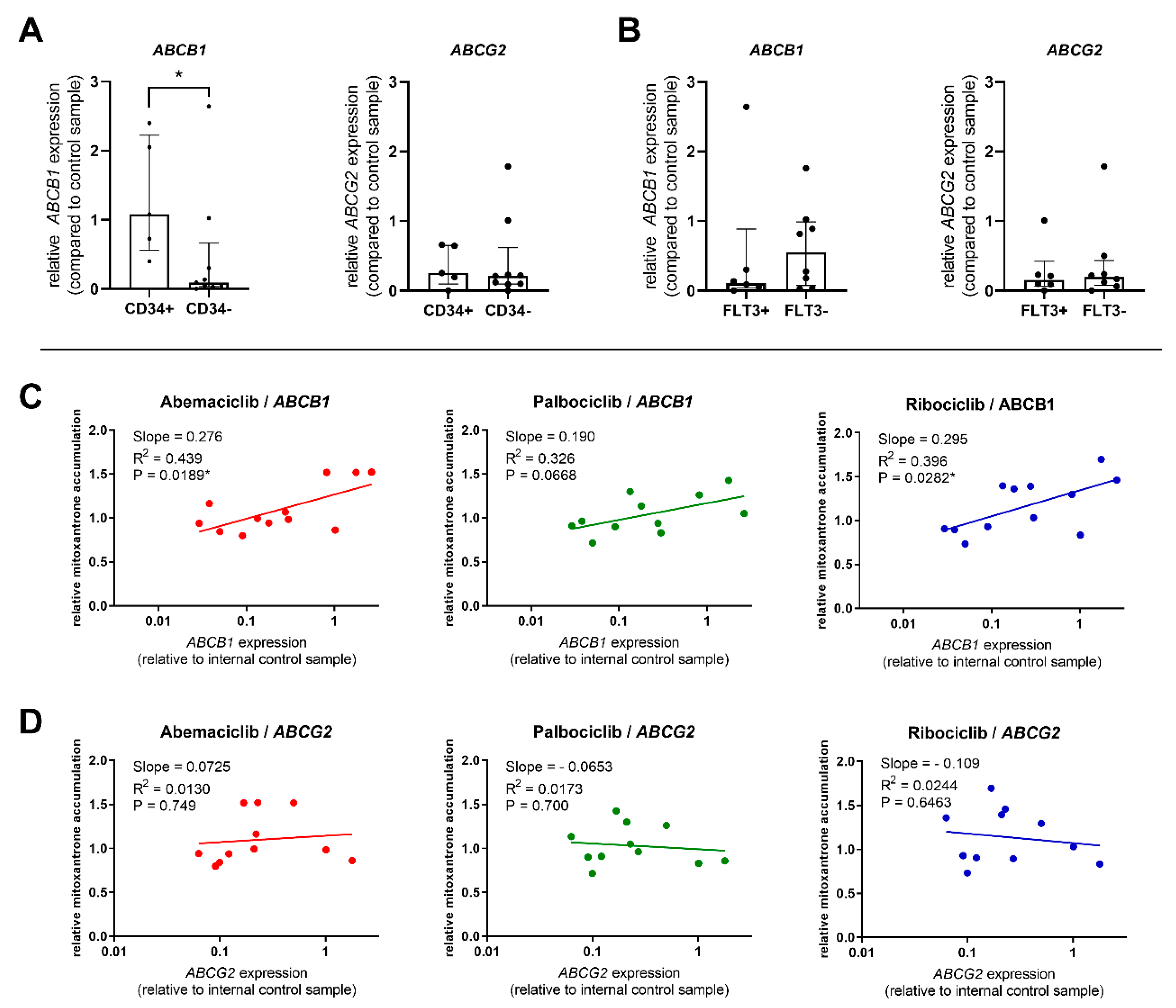
2.5. Correlations between Transporter Expression and Effect of CDKI on Mitoxantrone Accumulation in PBMC
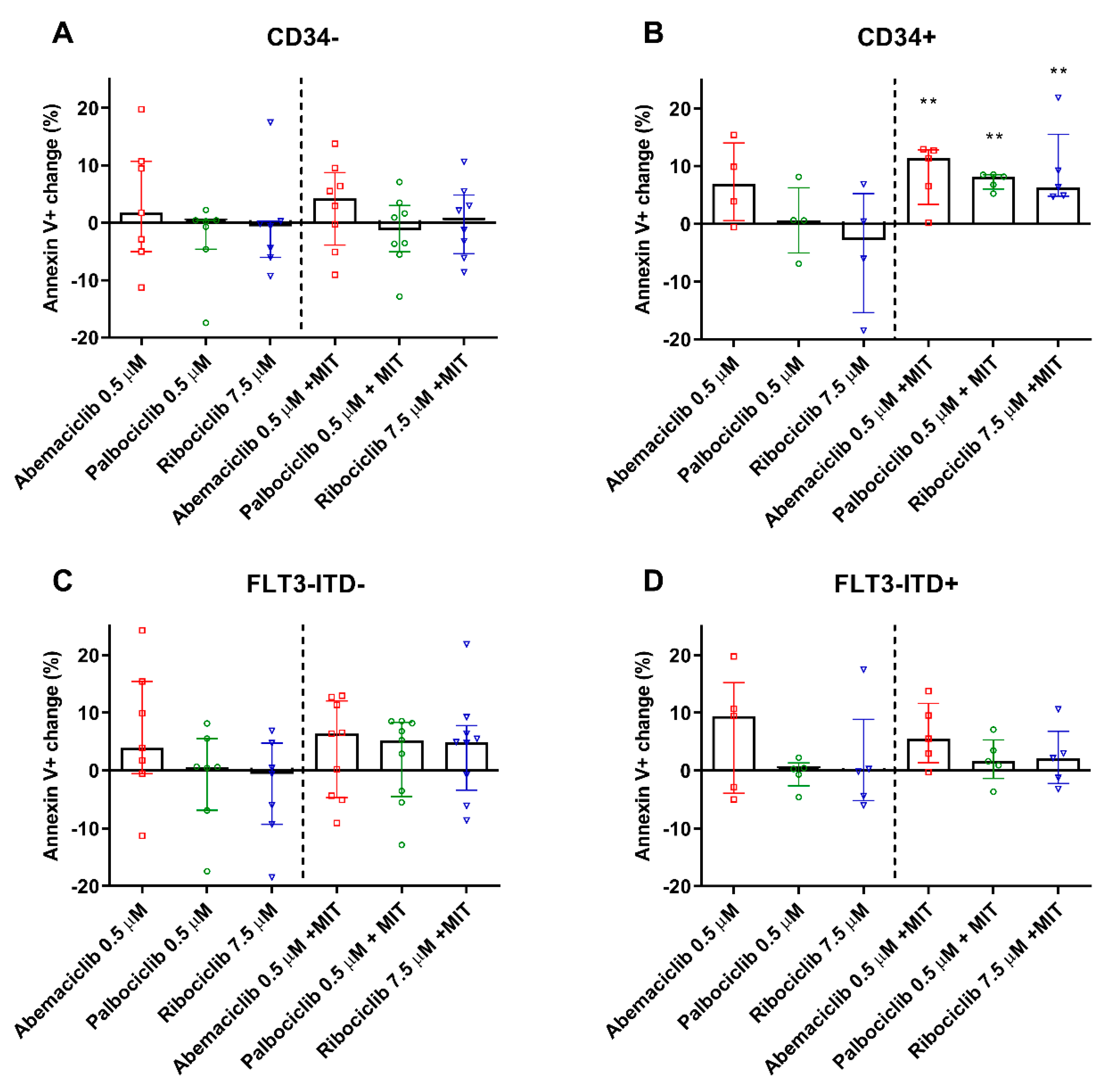
2.6. CDKI Enhance Apoptosis of PBMC from CD34+ Patients
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Cultures
4.3. Daunorubicin and Mitoxantrone Accumulation in HL-60 cells
4.4. Screening for Inhibitory Activity on Recombinant CREs
4.5. Inhibitory Assay on HCT116 Cells with Transient AKR1C3 Protein Expression
4.6. Annexin V/PI Staining of HL-60 Cells
4.7. Assessment of Sub-G1 Fraction of HL-60 Cells
4.8. Isolation of PBMC from AML Patients
4.9. Mitoxantrone Accumulation and Apoptosis Detection in Patients’ PBMC
4.10. Quantitative Reverse Transcription–Polymerase Chain Reaction Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalban-Bravo, G.; Garcia-Manero, G. Novel drugs for older patients with acute myeloid leukemia. Leukemia 2015, 29, 760–769. [Google Scholar] [CrossRef] [PubMed]
- NCI. Cancer Stat Facts: Leukemia—Acute Myeloid Leukemia (AML). Available online: https://seer.cancer.gov/statfacts/html/amyl.html (accessed on 23 August 2019).
- Pollyea, D.A.; Jordan, C.T. Therapeutic targeting of acute myeloid leukemia stem cells. Blood 2017, 129, 1627–1635. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Szakacs, G.; Varadi, A.; Ozvegy-Laczka, C.; Sarkadi, B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov. Today 2008, 13, 379–393. [Google Scholar] [CrossRef]
- Marzac, C.; Garrido, E.; Tang, R.; Fava, F.; Hirsch, P.; De Benedictis, C.; Corre, E.; Lapusan, S.; Lallemand, J.Y.; Marie, J.P.; et al. ATP Binding Cassette transporters associated with chemoresistance: Transcriptional profiling in extreme cohorts and their prognostic impact in a cohort of 281 acute myeloid leukemia patients. Haematologica 2011, 96, 1293–1301. [Google Scholar] [CrossRef] [Green Version]
- Ho, M.M.; Hogge, D.E.; Ling, V. MDR1 and BCRP1 expression in leukemic progenitors correlates with chemotherapy response in acute myeloid leukemia. Exp. Hematol. 2008, 36, 433–442. [Google Scholar] [CrossRef]
- Raaijmakers, M.H.; de Grouw, E.P.; Heuver, L.H.; van der Reijden, B.A.; Jansen, J.H.; Scheper, R.J.; Scheffer, G.L.; de Witte, T.J.; Raymakers, R.A. Breast cancer resistance protein in drug resistance of primitive CD34+38- cells in acute myeloid leukemia. Clin. Cancer Res. 2005, 11, 2436–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartholomae, S.; Gruhn, B.; Debatin, K.M.; Zimmermann, M.; Creutzig, U.; Reinhardt, D.; Steinbach, D. Coexpression of multiple ABC-transporters is strongly associated with treatment response in childhood acute myeloid leukemia. Pediatr. Blood Cancer 2016, 63, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Lian, S.; Schuetz, J.D. Leukemia and ABC transporters. Adv. Cancer Res. 2015, 125, 171–196. [Google Scholar] [CrossRef]
- Liu, B.; Li, L.J.; Gong, X.; Zhang, W.; Zhang, H.; Zhao, L. Co-expression of ATP binding cassette transporters is associated with poor prognosis in acute myeloid leukemia. Oncol. Lett. 2018, 15, 6671–6677. [Google Scholar] [CrossRef] [Green Version]
- Mordente, A.; Meucci, E.; Silvestrini, A.; Martorana, G.E.; Giardina, B. New developments in anthracycline-induced cardiotoxicity. Curr. Med. Chem. 2009, 16, 1656–1672. [Google Scholar] [CrossRef]
- Skarka, A.; Skarydova, L.; Stambergova, H.; Wsol, V. Anthracyclines and their metabolism in human liver microsomes and the participation of the new microsomal carbonyl reductase. Chem. Biol. Interact. 2011, 191, 66–74. [Google Scholar] [CrossRef]
- Piska, K.; Koczurkiewicz, P.; Bucki, A.; Wojcik-Pszczola, K.; Kolaczkowski, M.; Pekala, E. Metabolic carbonyl reduction of anthracyclines-role in cardiotoxicity and cancer resistance. Reducing enzymes as putative targets for novel cardioprotective and chemosensitizing agents. Investig. New Drugs 2017, 35, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Slupe, A.; Williams, B.; Larson, C.; Lee, L.M.; Primbs, T.; Bruesch, A.J.; Bjorklund, C.; Warner, D.L.; Peloquin, J.; Shadle, S.E.; et al. Reduction of 13-deoxydoxorubicin and daunorubicinol anthraquinones by human carbonyl reductase. Cardiovasc. Toxicol. 2005, 5, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Bains, O.S.; Grigliatti, T.A.; Reid, R.E.; Riggs, K.W. Naturally occurring variants of human aldo-keto reductases with reduced in vitro metabolism of daunorubicin and doxorubicin. J. Pharmacol. Exp. Ther. 2010, 335, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Kassner, N.; Huse, K.; Martin, H.J.; Godtel-Armbrust, U.; Metzger, A.; Meineke, I.; Brockmoller, J.; Klein, K.; Zanger, U.M.; Maser, E.; et al. Carbonyl reductase 1 is a predominant doxorubicin reductase in the human liver. Drug Metab. Dispos. 2008, 36, 2113–2120. [Google Scholar] [CrossRef] [Green Version]
- Malatkova, P.; Wsol, V. Carbonyl reduction pathways in drug metabolism. Drug Metab. Rev. 2014, 46, 96–123. [Google Scholar] [CrossRef] [PubMed]
- Bogason, A.; Masquelier, M.; Lafolie, P.; Skogastierna, C.; Paul, C.; Gruber, A.; Vitols, S. Daunorubicin metabolism in leukemic cells isolated from patients with acute myeloid leukemia. Drug Metab. Lett. 2010, 4, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Laffin, B.; Petrash, J.M. Expression of the aldo-ketoreductases AKR1B1 and AKR1B10 in human cancers. Front. Pharmacol. 2012, 3, 104. [Google Scholar] [CrossRef] [Green Version]
- Penning, T.M. AKR1C3 (type 5 17beta-hydroxysteroid dehydrogenase/prostaglandin F synthase): Roles in malignancy and endocrine disorders. Mol. Cell. Endocrinol. 2019, 489, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Varatharajan, S.; Panetta, J.C.; Abraham, A.; Karathedath, S.; Mohanan, E.; Lakshmi, K.M.; Arthur, N.; Srivastava, V.M.; Nemani, S.; George, B.; et al. Population pharmacokinetics of Daunorubicin in adult patients with acute myeloid leukemia. Cancer Chemother. Pharmacol. 2016, 78, 1051–1058. [Google Scholar] [CrossRef]
- Verma, K.; Zang, T.; Gupta, N.; Penning, T.M.; Trippier, P.C. Selective AKR1C3 inhibitors potentiate chemotherapeutic activity in multiple acute myeloid leukemia (AML) cell lines. ACS Med. Chem. Lett. 2016, 7, 774–779. [Google Scholar] [CrossRef] [Green Version]
- Verma, K.; Zang, T.; Penning, T.M.; Trippier, P.C. Potent and highly selective aldo-keto reductase 1C3 (AKR1C3) inhibitors act as chemotherapeutic potentiators in acute myeloid leukemia and T-Cell acute lymphoblastic leukemia. J. Med. Chem. 2019, 62, 3590–3616. [Google Scholar] [CrossRef]
- De Groot, A.F.; Kuijpers, C.J.; Kroep, J.R. CDK4/6 inhibition in early and metastatic breast cancer: A review. Cancer Treat. Rev. 2017, 60, 130–138. [Google Scholar] [CrossRef]
- Sorf, A.; Hofman, J.; Kucera, R.; Staud, F.; Ceckova, M. Ribociclib shows potential for pharmacokinetic drug-drug interactions being a substrate of ABCB1 and potent inhibitor of ABCB1, ABCG2 and CYP450 isoforms in vitro. Biochem. Pharmacol. 2018, 154, 10–17. [Google Scholar] [CrossRef]
- Wu, T.; Chen, Z.; To, K.K.W.; Fang, X.; Wang, F.; Cheng, B.; Fu, L. Effect of abemaciclib (LY2835219) on enhancement of chemotherapeutic agents in ABCB1 and ABCG2 overexpressing cells in vitro and in vivo. Biochem. Pharmacol. 2017, 124, 29–42. [Google Scholar] [CrossRef]
- Novotna, E.; Bukum, N.; Hofman, J.; Flaxova, M.; Kouklikova, E.; Louvarova, D.; Wsol, V. Roscovitine and purvalanol a effectively reverse anthracycline resistance mediated by the activity of aldo-keto reductase 1C3 (AKR1C3): A promising therapeutic target for cancer treatment. Biochem. Pharmacol. 2018, 156, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Novotna, E.; Bukum, N.; Hofman, J.; Flaxova, M.; Kouklikova, E.; Louvarova, D.; Wsol, V. Aldo-keto reductase 1C3 (AKR1C3): A missing piece of the puzzle in the dinaciclib interaction profile. Arch. Toxicol. 2018, 92, 2845–2857. [Google Scholar] [CrossRef] [PubMed]
- Sorf, A.; Novotna, E.; Hofman, J.; Morell, A.; Staud, F.; Wsol, V.; Ceckova, M. Cyclin-dependent kinase inhibitors AZD5438 and R547 show potential for enhancing efficacy of daunorubicin-based anticancer therapy: Interaction with carbonyl-reducing enzymes and ABC transporters. Biochem. Pharmacol. 2019, 163, 290–298. [Google Scholar] [CrossRef] [PubMed]
- International Transporter Consortium; Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef]
- FDA. In Vitro Metabolismand TransporterMediated Drug-Drug Interaction Studies Guidance for Industry. Available online: https://www.fda.gov/media/108130/download (accessed on 23 August 2019).
- Infante, J.R.; Cassier, P.A.; Gerecitano, J.F.; Witteveen, P.O.; Chugh, R.; Ribrag, V.; Chakraborty, A.; Matano, A.; Dobson, J.R.; Crystal, A.S.; et al. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 2016, 22, 5696–5705. [Google Scholar] [CrossRef] [Green Version]
- Patnaik, A.; Rosen, L.S.; Tolaney, S.M.; Tolcher, A.W.; Goldman, J.W.; Gandhi, L.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Hilton, J.F.; et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016, 6, 740–753. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Mukai, H.; Naito, Y.; Yonemori, K.; Kodaira, M.; Tanabe, Y.; Yamamoto, N.; Osera, S.; Sasaki, M.; Mori, Y.; et al. Phase I study of palbociclib, a cyclin-dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci. 2016, 107, 755–763. [Google Scholar] [CrossRef] [Green Version]
- Belhoussine, R.; Morjani, H.; Gillet, R.; Palissot, V.; Manfait, M. Two distinct modes of oncoprotein expression during apoptosis resistance in vincristine and daunorubicin multidrug-resistant HL60 cells. Adv. Exp. Med. Biol. 1999, 457, 365–381. [Google Scholar] [CrossRef]
- Masquelier, M.; Zhou, Q.F.; Gruber, A.; Vitols, S. Relationship between daunorubicin concentration and apoptosis induction in leukemic cells. Biochem. Pharmacol. 2004, 67, 1047–1056. [Google Scholar] [CrossRef]
- Efferth, T.; Fabry, U.; Osieka, R. Apoptosis and resistance to daunorubicin in human leukemic cells. Leukemia 1997, 11, 1180–1186. [Google Scholar] [CrossRef] [Green Version]
- Van den Heuvel-Eibrink, M.M.; van der Holt, B.; Burnett, A.K.; Knauf, W.U.; Fey, M.F.; Verhoef, G.E.; Vellenga, E.; Ossenkoppele, G.J.; Lowenberg, B.; Sonneveld, P. CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann. Hematol. 2007, 86, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shman, T.V.; Fedasenka, U.U.; Savitski, V.P.; Aleinikova, O.V. CD34+ leukemic subpopulation predominantly displays lower spontaneous apoptosis and has higher expression levels of Bcl-2 and MDR1 genes than CD34- cells in childhood AML. Ann. Hematol. 2008, 87, 353–360. [Google Scholar] [CrossRef]
- Marzac, C.; Teyssandier, I.; Calendini, O.; Perrot, J.Y.; Faussat, A.M.; Tang, R.; Casadevall, N.; Marie, J.P.; Legrand, O. Flt3 internal tandem duplication and P-glycoprotein functionality in 171 patients with acute myeloid leukemia. Clin. Cancer Res. 2006, 12, 7018–7024. [Google Scholar] [CrossRef] [Green Version]
- Boyer, T.; Gonzales, F.; Barthelemy, A.; Marceau-Renaut, A.; Peyrouze, P.; Guihard, S.; Lepelley, P.; Plesa, A.; Nibourel, O.; Delattre, C.; et al. Clinical significance of ABCB1 in acute myeloid leukemia: A comprehensive study. Cancers (Basel) 2019, 11, 1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, P.; Tang, R.; Marzac, C.; Perrot, J.Y.; Fava, F.; Bernard, C.; Jeziorowska, D.; Marie, J.P.; Legrand, O. Prognostic impact of high ABC transporter activity in 111 adult acute myeloid leukemia patients with normal cytogenetics when compared to FLT3, NPM1, CEBPA and BAALC. Haematologica 2012, 97, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Koehn, J.; Hogge, D.E. Identification of prognostic subgroups among acute myeloid leukemia patients with intermediate risk cytogenetics using a flow-cytometry-based assessment of ABC-transporter function. Leuk. Res. 2015, 39, 689–695. [Google Scholar] [CrossRef]
- Hollo, Z.; Homolya, L.; Hegedus, T.; Sarkadi, B. Transport properties of the multidrug resistance-associated protein (MRP) in human tumour cells. FEBS Lett. 1996, 383, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Ozvegy-Laczka, C.; Hegedus, T.; Varady, G.; Ujhelly, O.; Schuetz, J.D.; Varadi, A.; Keri, G.; Orfi, L.; Nemet, K.; Sarkadi, B. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol. Pharmacol. 2004, 65, 1485–1495. [Google Scholar] [CrossRef]
- Ujhelly, O.; Ozvegy, C.; Varady, G.; Cervenak, J.; Homolya, L.; Grez, M.; Scheffer, G.; Roos, D.; Bates, S.E.; Varadi, A.; et al. Application of a human multidrug transporter (ABCG2) variant as selectable marker in gene transfer to progenitor cells. Hum. Gene Ther. 2003, 14, 403–412. [Google Scholar] [CrossRef]
- Skarydova, L.; Nobilis, M.; Wsol, V. Role of carbonyl reducing enzymes in the phase I biotransformation of the non-steroidal anti-inflammatory drug nabumetone in vitro. Xenobiotica 2013, 43, 346–354. [Google Scholar] [CrossRef]
- Hofman, J.; Malcekova, B.; Skarka, A.; Novotna, E.; Wsol, V. Anthracycline resistance mediated by reductive metabolism in cancer cells: The role of aldo-keto reductase 1C3. Toxicol. Appl. Pharmacol. 2014, 278, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br. J. Haematol. 1976, 33, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 0034. [Google Scholar] [CrossRef] [Green Version]

| Inhibition (%) (50 μM Inhibitor Concentration) | |||||
|---|---|---|---|---|---|
| CBR1 | AKR1A1 | AKR1B1 | AKR1B10 | AKR1C3 | |
| Abemaciclib | 0 | 0 | 0 | 2.08 ± 0.40 | 26.34 ± 1.30 |
| Palbociclib | 0 | 0 | 5.54 ± 3.40 | 0 | 23.51 ± 6.67 |
| Ribociclib | 0 | 0 | 4.58 ± 2.41 | 7.41 ± 1.08 | 36.18 ± 2.83 |
| Sex | Age | FAB | Karyotype | CD34 | Mutations | ELN Risk |
|---|---|---|---|---|---|---|
| M | 61 | M4 | 46,XY | − | NPM1A, IDH2 | favorable |
| F | 64 | M4 | - | − | FLT3, NPM1A | N/A |
| F | 64 | M4 | 46,XX | + | FLT3, NPM1D | favorable |
| M | 67 | M2 | 44,XY,del(2)(q?21q?31), del(5)(q12q34),del(6)(q21q25),add(8)(q24),der(9) t(?8;9),dic(16)t(16;17) (?;q10),del(20q) [25] | + | TP53 | adverse |
| M | 69 | M5a | 46,XY | − | NPM1A | favorable |
| M | 71 | M2 | − | + | − | N/A |
| M | 54 | M4 | 46,XY | − | FLT3, NPM1A | intermediate |
| M | 65 | M1 | 46,XY | + | AML1-ETO | favorable |
| F | 74 | M2 | − | − | FLT3, NPM1A | favorable |
| M | 63 | M2 | 46,XY,t(1;3)(p36;q21), der(1)t(1;3)(p36;q21) [25] | − | − | intermediate |
| M | 54 | M1 | 46,XY | − | FLT3 | intermediate |
| F | 66 | M2 | 46,XX | − | FLT3, NPM1D | intermediate |
| F | 68 | M2 | 44,XX,-2,del(5)(q21q34), der(13)t(2;13;?),?del(17p)-18, del(20)(q12) [30] | + | TP53 | adverse |
| F | 70 | M4 | 46,XX | + | − | intermediate |
| M | 30 | M2 | 46,XY | − | NPM1A | adverse |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorf, A.; Sucha, S.; Morell, A.; Novotna, E.; Staud, F.; Zavrelova, A.; Visek, B.; Wsol, V.; Ceckova, M. Targeting Pharmacokinetic Drug Resistance in Acute Myeloid Leukemia Cells with CDK4/6 Inhibitors. Cancers 2020, 12, 1596. https://doi.org/10.3390/cancers12061596
Sorf A, Sucha S, Morell A, Novotna E, Staud F, Zavrelova A, Visek B, Wsol V, Ceckova M. Targeting Pharmacokinetic Drug Resistance in Acute Myeloid Leukemia Cells with CDK4/6 Inhibitors. Cancers. 2020; 12(6):1596. https://doi.org/10.3390/cancers12061596
Chicago/Turabian StyleSorf, Ales, Simona Sucha, Anselm Morell, Eva Novotna, Frantisek Staud, Alzbeta Zavrelova, Benjamin Visek, Vladimir Wsol, and Martina Ceckova. 2020. "Targeting Pharmacokinetic Drug Resistance in Acute Myeloid Leukemia Cells with CDK4/6 Inhibitors" Cancers 12, no. 6: 1596. https://doi.org/10.3390/cancers12061596





