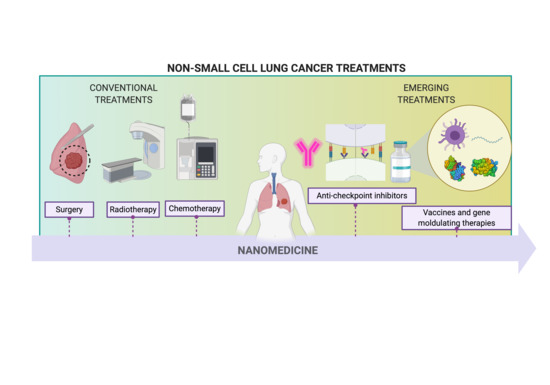
Nanomedicine in Non-Small Cell Lung Cancer: From Conventional Treatments to Immunotherapy
Abstract
:1. Introduction
2. Conventional Treatments
2.1. Surgery and Radiotherapy
2.2. Chemotherapy
2.2.1. Mitotic Inhibitors
2.2.2. Alkylating Agents
2.2.3. Antimetabolite Drugs
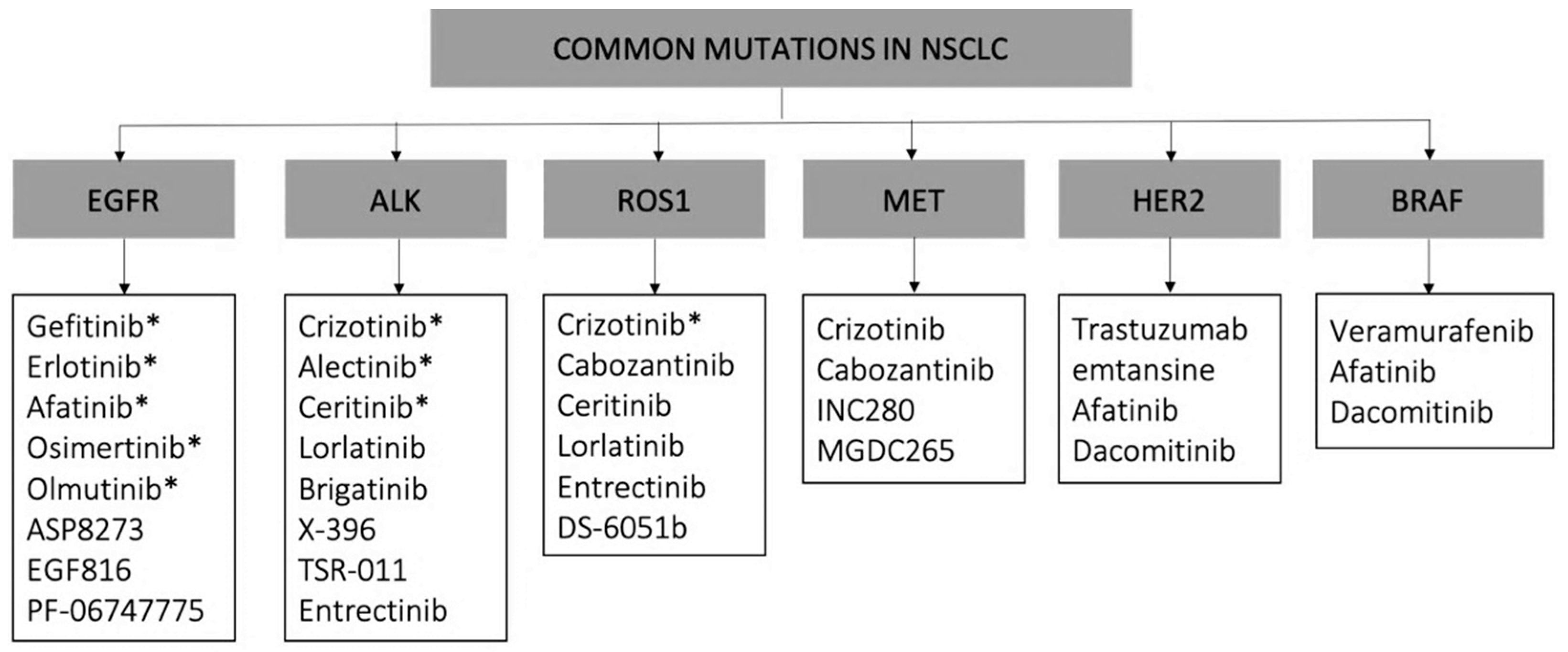
2.3. Molecular Targeted Therapies: Tyrosine Kinase Inhibitors (TKIs)
3. Emerging Treatments
3.1. Immunotherapies
3.1.1. Immune Checkpoint Inhibitors
3.1.2. Therapeutic Vaccines
Protein and Peptide-Based Vaccines
mRNA Vaccines
3.2. Modulating Gene Therapy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Positron Emission Tomography (PET). Detects pairs of gamma rays emitted by a radioligand previously introduced in the body after the combination of the introduced positrons with electrons from the patients’ body. Thus, it requires the use of contrast agents conjugated to a biologically relevant molecule, involved in the disease to be detected [15,35,152]. Computed Tomography (CT). Measures the attenuation of X rays emitted after interacting with tissues. It may require contrast agents if the difference of attenuation after interaction with different tissues of interest is not evident. After the acquisition, cross-sectional images are obtained using tomographic reconstruction [15,35,152]. Magnetic Resonance Imaging (MRI). Measures changes in the nuclear momentum of atoms while applying an external magnetic field. Hydrogen atoms are usually evaluated due to their simplicity and abundance in the human body. The relaxing times of H atoms in different tissues are measured to obtain images [15,35,152]. Does not require contrast agents. Bronchoscopy. Imaging technique consisting of the use of flexible bronchoscopy, including a camera, to visualize the inside of the airways for diagnostic and therapeutic purposes [22,35,153]. Mediastinoscopy. Small incision in the center of the thoracic cavity to section a small part of the tissue for biopsy purposes [22,35,153]. Bronchoalveolar lavage (BAL). Introduction of a measured volume of fluid in the lungs through the appropriate airways for further examination [22,35,153]. |
Appendix B
| Dendritic cells (DC). These are Antigen-Presenting Cells (APC), infiltrating in the tumor microenvironment and capturing antigens. They play an important role in both the adaptative and the innate immune responses, activating naïve T lymphocytes in secondary lymphoid organs [154,155]. Natural Killers (NK). They accumulate at the tumor site and are important secretors of IFN-. When activated, NK eliminate tumor cells independently of tumor antigen exposure. Their activity is regulated by activating and inhibitory signals released by normal and abnormal cells [156,157]. CD8+ T cells. Activated T cells recognizing antigens presented by MHC class I molecules found on all nucleated cells. The recognition of these antigens stimulates the release of cytokines and cytotoxic granules, destroying infected cells via Fas/FasL interactions [158]. CD4+ T cells. Activated T cells recognizing antigens presented by MHC class II molecules in APC cells. The recognition of these antigens stimulates the adaptative immune response through the maturation to T helper (Th)17, Th1, or Th2 cells [159]. Macrophages. Specialized cells involved in the detection and phagocytosis of bacteria and non-self-recognized elements. They are also APC cells and important cytokines releasers [160,161]. |
References
- Global Observatory of Cancer. Available online: http://gco.iarc.fr (accessed on 3 April 2020).
- American Society of Cancer. Available online: https://cancerstatisticscenter.cancer.org/ (accessed on 14 April 2020).
- Pauk, N.; Kubík, A.; Zatloukal, P.; Křepela, E. Lung Cancer in Women. Lung Cancer 2005, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.J.; Henley, S.J.; Calle, E.E. Tobacco Use and Cancer: An Epidemiologic Perspective for Geneticists. Oncogene 2002, 21, 7307–7325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.-Y. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Jin, R.; Zhao, J.; Li, W.; Shen, H. Risk of COVID-19 for Cancer Patients. Lancet. Oncol. 2020, 21, e180. [Google Scholar] [CrossRef]
- Carr, L.L.; Jacobson, S.; Lynch, D.A.; Foreman, M.G.; Flenaugh, E.L.; Hersh, C.P.; Sciurba, F.C.; Wilson, D.O.; Sieren, J.C.; Mulhall, P.; et al. Features of COPD as Predictors of Lung Cancer. Chest 2018, 153, 1326–1335. [Google Scholar] [CrossRef]
- Griffin, J.P.; Tolley, E.A.; Zaman, M.K.; Niell, H.B.; Cole, H.F.J.; Weiman, D.S. Chronic Obstructive Pulmonary Disease with Lung Cancer: Prevalence, Severity, and Common Pathogenesis. J. Cancer Res. 2016, 4, 1–6. [Google Scholar]
- Marchesani, W. Über Den Primären Bronchialkrebs. Frankf. Z. Path. 1924, 30, 158–190. [Google Scholar]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [Green Version]
- Basumallik, N.; Agarwal, M. Cancer, Lung Small Cell (Oat Cell). StatPearls 2020. [Google Scholar]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-Small-Cell Lung Cancer. Nat. Rev. Dis. Prim. 2015, 1, 1–16. [Google Scholar] [CrossRef]
- Sutherland, K.D.; Berns, A. Cell of Origin of Lung Cancer. Mol. Oncol. 2010, 4, 397–403. [Google Scholar] [CrossRef]
- Beadsmoore, C.J.; Screaton, N.J. Classification, Staging and Prognosis of Lung Cancer. Eur. J. Radiol. 2003, 45, 8–17. [Google Scholar] [CrossRef]
- Tsim, S.; O’Dowd, C.A.; Milroy, R.; Davidson, S. Staging of Non-Small Cell Lung Cancer (NSCLC): A Review. Respir. Med. 2010, 104, 1767–1774. [Google Scholar] [CrossRef] [Green Version]
- Tockman, M.S. Survival and Mortality from Lung Cancer in a Screened Population: The Johns Hopkins Study. Chest 1986, 89, 324–325. [Google Scholar] [CrossRef]
- Tamura, T.; Kurishima, K.; Nakazawa, K.; Kagohashi, K.; Ishikawa, H.; Satoh, H. Specific Organ Metastases and Survival in Metastatic Non-Small-Cell Lung Cancer. Mol. Clin. Oncol. 2015, 3, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Rami-Porta, R.; Asamura, H.; Travis, W.; Rusch, V. Lung. In Cancer Staging Manual, 8th ed.; Edge, S.B., Greene, F.L., Schilsky, R.L., Gaspar, L.E., Washington, M.K., Sullivan, D.C., Brookland, R.K., Brierley, J.D., Balch, C.M., Compton, C.C., et al., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 431–457. [Google Scholar]
- Farver, C.F.; Zander, D.S. Molecular Basis of Pulmonary Disease. In Molecular Pathology: The Molecular Basis of Human Disease; Coleman, W.B., Tsongalis, G.J., Eds.; Elsevier Inc.: San Diego, CA, USA; Burlington, VT, USA; London, UK, 2009; pp. 305–364. [Google Scholar]
- Li, C.; Lu, H. Adenosquamous Carcinoma of the Lung. Onco. Targets. 2018, 11, 4829–4835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Association for the Study of Lung Cancer. The TNM Classification for Lung Cancer, 8th ed.; International Association for the Study of Lung Cancer: Denver, CO, USA, 2018. [Google Scholar]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for Staging Non-Small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed; American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143, e211S–e250S. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.; Adjei, A.A.; Gridelli, C.; Reck, M.; Kerr, K.; Felip, E. Metastatic Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2012, 23, vii56–vii64. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.J.; Howington, J.; Feigenberg, S.; Movsas, B.; Pisters, K. Treatment of Non-Small Cell Lung Cancer Stage I and Stage II: ACCP Evidence-Based Clinical Practice Guidelines, 2nd Edition. Chest 2007, 132, e234S–e242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, M.S. Improving Cancer Immunotherapy through Nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef]
- US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT02379845?term (accessed on 29 April 2020).
- US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT02213744?term (accessed on 29 April 2020).
- US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT00377936?term (accessed on 29 April 2020).
- US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT01644890?term (accessed on 29 April 2020).
- Smith, A.D. Big Moment for Nanotech: Oncology Therapeutics Poised for a Leap. Available online: https://www.onclive.com/publications/oncology-live/2013/june-2013/big-moment-for-nanotech-oncology-therapeutics-poised-for-a-leap (accessed on 15 April 2020).
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Son, S.; Park, K.S.; Zou, W.; Shea, L.D.; Moon, J.J. Cancer Nanomedicine for Combination Cancer Immunotherapy. Nat. Rev. Mater. 2019, 4, 398–414. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The Entry of Nanoparticles into Solid Tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.N.; Li, X.D.; Chang, J. Multimodality Imaging in Nanomedicine and Nanotheranostics. Cancer Biol. Med. 2016, 13, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Zappa, C.; Mousa, S.A. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Melosky, B. Editorial: Update on the Treatment of Metastatic Non-Small Cell Lung Cancer (NSCLC) in New Era of Personalised Medicine. Front. Oncol. 2017, 7, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Fornaguera, C.; Guerra-rebollo, M.; Lázaro, M.Á.; Castells-sala, C.; Meca-cortés, O.; Ramos-pérez, V.; Cascante, A.; Rubio, N.; Blanco, J.; Borrós, S. MRNA Delivery System for Targeting Antigen-Presenting Cells In Vivo. Adv. Healthc Mater. 2018, 1800335, 1–11. [Google Scholar] [CrossRef]
- Sharma, S.V.; Bell, D.W.; Settleman, J.; Haber, D.A. Epidermal Growth Factor Receptor Mutations in Lung Cancer. Nat. Rev. Cancer 2007, 7, 169–181. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Godeshala, S.; Miryala, B. Bioreducible Poly (Amino Ethers) Based MTOR SiRNA Delivery for Lung Cancer. Pharm. Res. 2018, 35, 1–20. [Google Scholar] [CrossRef]
- Tan, A.C. Targeting the PI3K/Akt/MTOR Pathway in Non-Small Cell Lung Cancer (NSCLC). Thorac. Cancer 2020, 11, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Bhavana, V.; Janardhana, S.; Sudharshan, S. Natural Anticancer Compounds and Their Derivatives in Clinical Trials; Springer: Singapore, 2017. [Google Scholar]
- Boulikas, T.; Vougiouka, M. Recent Clinical Trials Using Cisplatin, Carboplatin and Their Combination Chemotherapy Drugs (Review). Oncol. Rep. 2004, 11, 559–595. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ju, D.; Chang, C.; Reddy, P.M.; Velmurugan, B.K. A Review on the Effects of Current Chemotherapy Drugs and Natural Agents in Treating Non–Small Cell Lung Cancer. Biomed. 2017, 7, 12–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsudomi, T.; Suda, K.; Yatabe, Y. Surgery for NSCLC in the Era of Personalized. Nat. Rev. Clin. Oncol. 2013, 10, 235–244. [Google Scholar] [CrossRef]
- Saadeddin, A. Radiotherapy for NSCLC: Review of Conventional and New Treatment Techniques. J. Infect. Public Health 2012, 5, S45–S49. [Google Scholar] [CrossRef] [Green Version]
- Burdett, S.; Rydzewska, L.; Tierney, J.; Fisher, D.; Mkb, P.; Arriagada, R.; Pignon, J.P.; Le Pechoux, C. Postoperative Radiotherapy for Non-Small Cell Lung Cancer (Review). Cochrane Database Syst. Rev. 2016, 11, 1–45. [Google Scholar]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K. Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc. Natl. Acad. Sci. 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [Green Version]
- Fumarola, C.; Bonelli, M.A.; Petronini, P.G.; Alfieri, R.R. Targeting PI3K/AKT/MTOR Pathway in Non Small Cell Lung Cancer. Biochem. Pharm. 2014, 90, 197–207. [Google Scholar] [CrossRef]
- Park, H.J.; Park, H.S.; Cha, Y.J.; Lee, S.; Jeung, H.C.; Cho, J.Y.; Kim, H.J.; Byun, M.K. Efficacy of Adjuvant Chemotherapy for Completely Resected Stage IB Non-Small Cell Lung Cancer: A Retrospective Study. J. Thorac. Dis. 2018, 10, 2279–2287. [Google Scholar] [CrossRef] [Green Version]
- McDonald, F.; De Waele, M.; Hendriks, L.E.L.; Faivre-Finn, C.; Dingemans, A.M.C.; Van Schil, P.E. Management of Stage I and II Nonsmall Cell Lung Cancer. Eur. Respir. J. 2017, 49, 889–902. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.G.; Shin, J.H.; Shim, H.S.; Lee, C.Y.; Kim, D.J.; Kim, Y.S.; Chung, K.Y. Autophagy Contributes to the Chemo-Resistance of Non-Small Cell Lung Cancer in Hypoxic Conditions. Respir. Res. 2015, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Pei, F.; Yang, F.; Li, L.; Amin, A.D.; Liu, S.; Ross Buchan, J.; Cho, W.C. Role of Autophagy and Apoptosis in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2017, 18, 367. [Google Scholar] [CrossRef] [PubMed]
- Cordani, M.; Somoza, Á. Targeting Autophagy Using Metallic Nanoparticles: A Promising Strategy for Cancer Treatment. Cell. Mol. Life Sci. 2019, 76, 1215–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, S.; Zhou, T.; Yang, P.; Wang, Y.; Zhang, P.; Chen, K.; Ren, L.; Ye, S. Gold Nanoparticles Enhance TRAIL Sensitivity through Drp1-Mediated Apoptotic and Autophagic Mitochondrial Fission in NSCLC Cells. Int. J. Nanomed. 2017, 12, 2531–2551. [Google Scholar] [CrossRef] [Green Version]
- FDA. FDA-approved Drugs Database. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 29 April 2020).
- National Cancer Institute FDA-approved Drugs for, N.S.C.L.C. 17 May. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/everolimus (accessed on 17 May 2020).
- Sacco, P.C.; Gridelli, C. An Update on the Developing Mitotic Inhibitors for the Treatment of Non-Small Cell Carcinoma. Expert Opin. Emerg. Drugs 2017, 22, 213–222. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, X.; Yang, Y.; Dai, W. Advances in Mitotic Inhibitors for Cancer Treatment. Mini Rev. Med. Chem. 2006, 6, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Manikhas, G.M.; Orlov, S.; Afanasyev, B.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane®, a Novel Cremophor®-Free, Albumin-Bound Particle Form of Paclitaxel for the Treatment of Advanced Non-Small-Cell Lung Cancer. Ann. Oncol. 2006, 17, 1263–1268. [Google Scholar] [CrossRef]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Dostdar, S.A.; Sokolov, A.V.; Brzecka, A.; Sukocheva, O.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; et al. Extracellular Vesicles in Cancer Nanomedicine. Semin. Cancer Biol. 2019, 0–1. [Google Scholar] [CrossRef]
- Srivastava, A.; Amreddy, N.; Babu, A.; Panneerselvam, J.; Mehta, M.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Razaq, M.; Riedinger, N.; et al. Nanosomes Carrying Doxorubicin Exhibit Potent Anticancer Activity against Human Lung Cancer Cells. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Coccè, V.; Franzè, S.; Brini, A.T.; Giannì, A.B.; Pascucci, L.; Ciusani, E.; Alessandri, G.; Farronato, G.; Cavicchini, L.; Sordi, V.; et al. In Vitro Anticancer Activity of Extracellular Vesicles (Evs) Secreted by Gingival Mesenchymal Stromal Cells Primed with Paclitaxel. Pharmaceutics 2019, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Petrella, F.; Coccè, V.; Masia, C.; Milani, M.; Salè, E.O.; Alessandri, G.; Parati, E.; Sisto, F.; Pentimalli, F.; Brini, A.T.; et al. Paclitaxel-Releasing Mesenchymal Stromal Cells Inhibit in Vitro Proliferation of Human Mesothelioma Cells. Biomed. Pharm. 2017, 87, 755–758. [Google Scholar] [CrossRef]
- Saari, H.; Lázaro-Ibáñez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle and Exosome-Mediated Drug Delivery Enhances the Cytotoxicity of Paclitaxel in Autologous Prostate Cancer Cells. J. Control. Release 2015, 220, 727–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmidis, P. Chemotherapy in NSCLC: Historical Review. Lung Cancer 2002, 38, S19–S22. [Google Scholar] [CrossRef]
- Chang, A. Lung Cancer Chemotherapy, Chemoresistance and the Changing Treatment Landscape for NSCLC. Lung Cancer 2011, 71, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L.; Zunino, F. Overview of Tumor Cell Chemoresistance Mechanisms. Methods Mol. Med. 2005, 111, 127–148. [Google Scholar]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
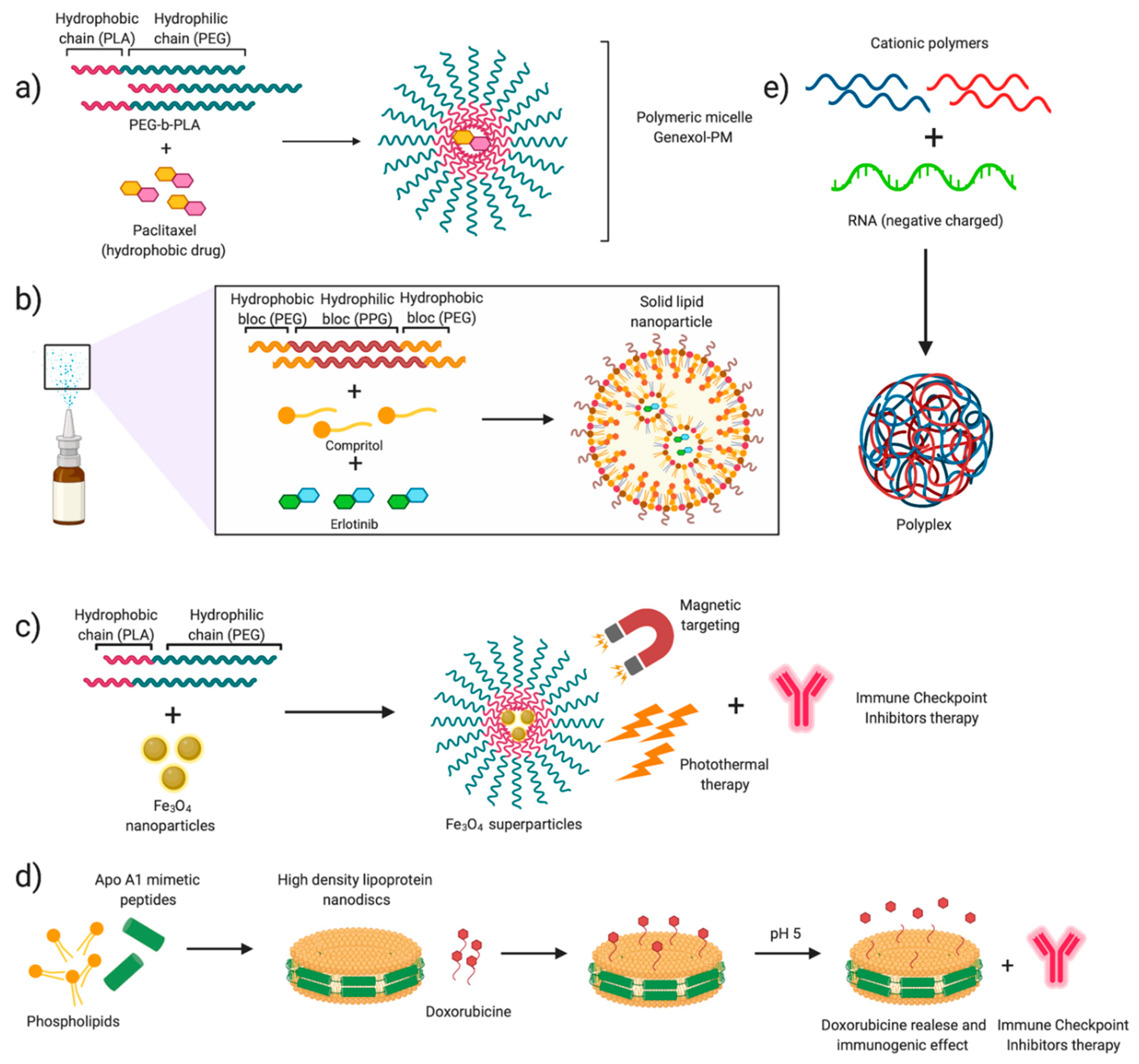
- Kim, D.W.; Kim, S.Y.; Kim, H.K.; Kim, S.W.; Shin, S.W.; Kim, J.S.; Park, K.; Lee, M.Y.; Heo, D.S. Multicenter Phase II Trial of Genexol-PM, a Novel Cremophor-Free, Polymeric Micelle Formulation of Paclitaxel, with Cisplatin in Patients with Advanced Non-Small-Cell Lung Cancer. Ann. Oncol. 2007, 18, 2009–2014. [Google Scholar] [CrossRef]
- Ahn, H.K.; Jung, M.; Sym, S.J.; Shin, D.B.; Kang, S.M.; Kyung, S.Y.; Park, J.W.; Jeong, S.H.; Cho, E.K. A Phase II Trial of Cremorphor EL-Free Paclitaxel (Genexol-PM) and Gemcitabine in Patients with Advanced Non-Small Cell Lung Cancer. Cancer Chemother. Pharm. 2014, 74, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Mukai, H.; Kogawa, T.; Matsubara, N.; Naito, Y.; Sasaki, M.; Hosono, A. A First-in-Human Phase 1 Study of Epirubicin-Conjugated Polymer Micelles (K-912/NC-6300) in Patients with Advanced or Recurrent Solid Tumors. Invest. New Drugs 2017, 35, 307–314. [Google Scholar] [CrossRef]
- Subbiah, V.; Combest, A.; Grilley-Olson, J.; Sharma, N.; Andrews, E.; Bobe, I.; Balkissoon, J.; Camp, A.; Masada, A.; Reitsma, D.; et al. Phase Ib/II Trial of NC-6004 (Nanoparticle Cisplatin) plus Gemcitabine (G) in Patients (Pts) with Advanced Solid Tumors. Eur. J. Cancer 2016, 69, S118–S119. [Google Scholar] [CrossRef]
- Ueno, T.; Endo, K.; Hori, K.; Ozaki, N.; Tsuji, A.; Kondo, S.; Wakisaka, N.; Murono, S.; Kataoka, K.; Kato, Y.; et al. Assessment of Antitumor Activity and Acute Peripheral Neuropathy of 1,2-Diaminocyclohexane Platinum (II)-Incorporating Micelles (NC-4016). Int. J. Nanomed. 2014, 9, 3005–3012. [Google Scholar] [CrossRef] [Green Version]
- Budde, L.S.; Hanna, N.H. Antimetabolites in the Management of Non-Small Cell Lung Cancer. Curr. Treat. Options Oncol. 2005, 6, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Pao, W.; Girard, N. New Driver Mutations in Non-Small-Cell Lung Cancer. Lancet Oncol. 2011, 12, 175–180. [Google Scholar] [CrossRef]
- Thirumaran, R.; Prendergast, G.C.; Gilman, P.B. Cytotoxic Chemotherapy in Clinical Treatment of Cancer. In Cancer Immunotherapy: Immune Suppression and Tumor Growth; Prendergast, G.C., Jaffee, E.M., Eds.; Elsevier Inc.: Burlington, VT, USA; San Diego, CA USA; London UK, 2007; pp. 101–116. [Google Scholar]
- Dubey, S.; Schiller, J.H. Three Emerging New Drugs for NSCLC: Pemetrexed, Bortezomib, and Cetuximab. Oncologist 2005, 10, 282–291. [Google Scholar] [CrossRef]
- Hanna, N.; Shepherd, F.A.; Fossella, F.V.; Pereira, J.R.; Demarinis, F.; Von Pawel, J.; Gatzemeier, U.; Tsao, T.C.Y.; Pless, M.; Muller, T.; et al. Randomized Phase III Trial of Pemetrexed versus Docetaxel in Patients with Non-Small-Cell Lung Cancer Previously Treated with Chemotherapy. J. Clin. Oncol. 2004, 22, 1589–1597. [Google Scholar] [CrossRef]
- Curtin, N.J.; Hughes, A.N. Pemetrexed Disodium, a Novel Antifolate with Multiple Targets. Lancet Oncol. 2001, 2, 298–306. [Google Scholar] [CrossRef]
- Okamoto, I.; Fukuoka, M. S-1: A New Oral Fluoropyrimidine in the Treatment of Patients with Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2009, 10, 290–294. [Google Scholar] [CrossRef]
- Yumine, K.; Kawahara, M. Phase II Study of S-1, a Novel Oral Fluorouracil, in Advanced Non-Small-Cell Lung Cancer. Br. J. Cancer 2001, 85, 939–943. [Google Scholar]
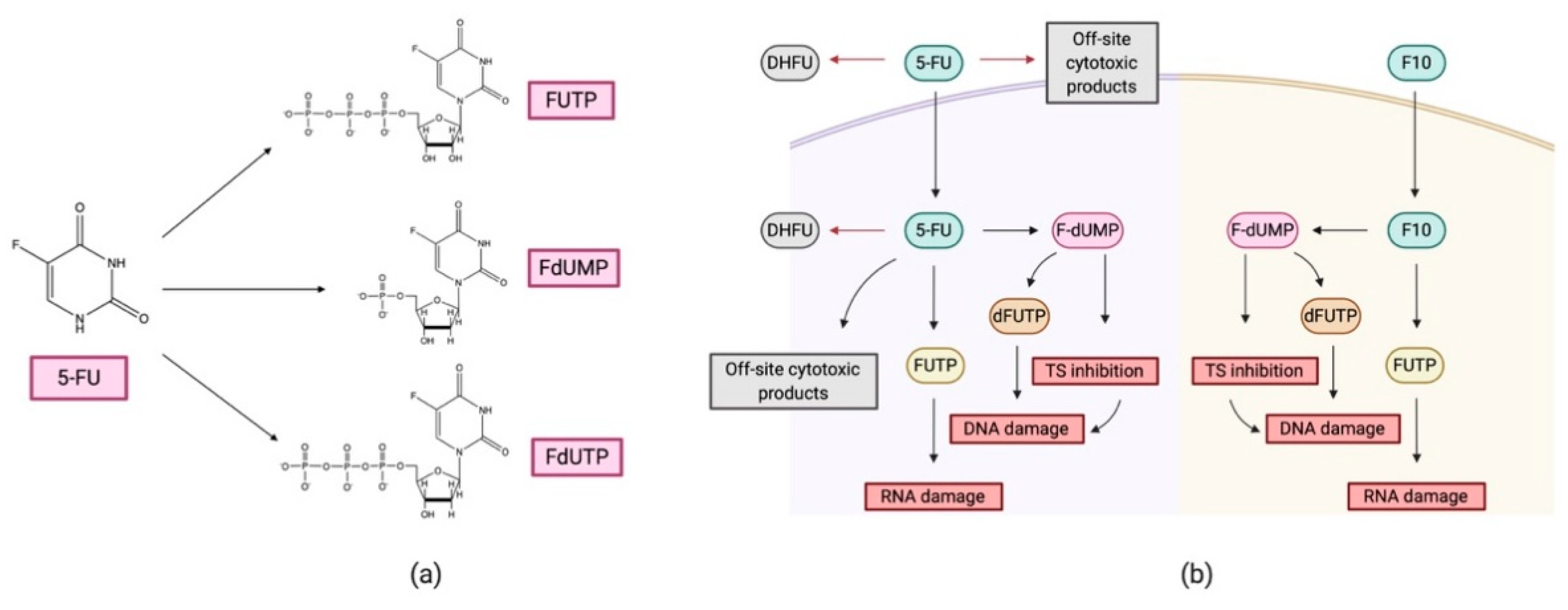
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Miller, L.D.; Chou, J.W.; Dominijanni, A.; Mutkus, L.; Marini, F.; Ruiz, J.; Dotson, T.; Thomas, K.W.; Parks, G.; et al. Dysregulated Pyrimidine Biosynthesis Contributes to 5-FU Resistance in SCLC Patient-Derived Organoids but Response to a Novel Polymeric Fluoropyrimidine, CF10. Cancers 2020, 12, 788–803. [Google Scholar] [CrossRef] [Green Version]
- Jorge, A.F.; Aviñó, A.; Pais, A.A.C.C.; Eritja, R.; Fàbrega, C. DNA-Based Nanoscaffolds as Vehicles for 5-Fluoro-2′-Deoxyuridine Oligomers in Colorectal Cancer Therapy. Nanoscale 2018, 10, 7238–7249. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, K.; Hu, C.; Gao, M.; Zhang, J.; Wang, Y.; Chen, Y.; Song, Z.; Wang, Z. Evaluation of 5-Fluorouracil-Treated Lung Cancer Cells by Atomic Force Microscopy. Anal. Methods 2019, 11, 4977–4982. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Debinski, W.; Milligan, C.; Caudell, D.; Pardee, T.S. The Applications of the Novel Polymeric Fluoropyrimidine F10 in Cancer Treatment: Current Evidence. Futur. Oncol. 2016, 12, 2009–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, F.R.; Suda, K.; Wiens, J.; Bunn, P.A. New and Emerging Targeted Treatments in Advanced Non-Small-Cell Lung Cancer. Lancet 2016, 388, 1012–1024. [Google Scholar] [CrossRef]
- Weinstein, I.B.; Joe, A. Oncogene Addiction. Cancer Res. 2008, 68, 3077–3080. [Google Scholar] [CrossRef] [Green Version]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or Chemotherapy for Non-Small-Cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha Santos, G.; Shepherd, F.A.; Tsao, M.S. EGFR Mutations and Lung Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 49–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riely, G.J.; Marks, J.; Pao, W. KRAS Mutations in Non–Small Cell Lung Cancer. Proc. Am. Thorac Soc. 2009, 41, 711–716. [Google Scholar] [CrossRef]
- Román, M.; Baraibar, I.; López, I.; Nadal, E.; Rolfo, C.; Vicent, S.; Gil-Bazo, I. KRAS Oncogene in Non-Small Cell Lung Cancer: Clinical Perspectives on the Treatment of an Old Target. Mol. Cancer 2018, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, C.J.; Stock, G.; Tay, R.; Dawod, M.; Gomes, F.; Califano, R. Targeted Therapy for RET-Rearranged Non-Small Cell Lung Cancer: Clinical Development and Future Directions. Onco. Targets. 2019, 12, 7857–7864. [Google Scholar] [CrossRef] [Green Version]
- Bronte, G.; Ulivi, P.; Verlicchi, A.; Cravero, P.; Delmonte, A.; Crinò, L. Targeting RET-Rearranged Non-Small-Cell Lung Cancer: Future Prospects. Lung Cancer Targets. 2019, 10, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Harashima, H. Integration of Nano Drug-Delivery System with Cancer Immunotherapy. Ther. Deliv. 2017, 8, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shi, K.; Hao, Y.; Yang, C.; Zha, R.; Yi, C.; Qian, Z. Advances in Nanotechnology-Based Delivery Systems for EGFR Tyrosine Kinases Inhibitors in Cancer Therapy. Asian J. Pharm. Sci. 2020, 15, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Tomasini, P.; Khobta, N.; Greillier, L.; Barlesi, F. Ipilimumab: Its Potential in Non-Small Cell Lung Cancer. Adv. Med. Oncol. 2012, 4, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellmann, M.D.; Paz Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; De La Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiary, Z.; Barar, J.; Aghanejad, A.; Saei, A.A.; Nemati, E.; Ezzati Nazhad Dolatabadi, J.; Omidi, Y. Microparticles Containing Erlotinib-Loaded Solid Lipid Nanoparticles for Treatment of Non-Small Cell Lung Cancer. Drug Dev. Ind. Pharm. 2017, 43, 1244–1253. [Google Scholar] [CrossRef]
- Han, W.; Shi, L.; Ren, L.; Zhou, L.; Li, T.; Qiao, Y.; Wang, H. A Nanomedicine Approach Enables Co-Delivery of Cyclosporin A and Gefitinib to Potentiate the Therapeutic Efficacy in Drug-Resistant Lung Cancer. Signal Transduct. Target. 2018, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ricciuti, B.; Lamberti, G.; Andrini, E.; Genova, C.; De Giglio, A.; Bianconi, V.; Sahebkar, A.; Chiari, R.; Pirro, M. Antibody–Drug Conjugates for Lung Cancer in the Era of Personalized Oncology; Elsevier Ltd.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Langer, C.J. Emerging Immunotherapies in the Treatment of Non-Small Cell Lung Cancer (NSCLC): The Role of Immune Checkpoint Inhibitors. Am. J. Clin. Oncol. Cancer Clin. Trials 2015, 38, 422–430. [Google Scholar] [CrossRef]
- Davies, M. New Modalities of Cancer Treatment for NSCLC: Focus on Immunotherapy. Cancer Manag. Res. 2014, 6, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Gkolfinopoulos, S.; Mountzios, G. Recent Clinical Trials of Immunotherapy in Non-Small-Cell Lung Cancer. Immunotherapy 2019, 11, 461–466. [Google Scholar] [CrossRef]
- Malhotra, J.; Jabbour, S.K.; Aisner, J. Current State of Immunotherapy for Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2017, 6, 196–211. [Google Scholar] [CrossRef] [Green Version]
- Teng, M.W.L.; Kershaw, M.H.; Smyth, M.J. Cancer Immunoediting: From Surveillance to Escape. In Cancer Immunotherapy: Immune Suppression and Tumor Growth, 2nd ed.; Prendergast, G.C., Jaffee, E.M., Eds.; Elsevier Inc.: Burlington, VT, USA; San Diego, CA, USA; London UK, 2013; pp. 85–99. [Google Scholar]
- Kim, R.; Emi, M.; Tanabe, K. Cancer Immunoediting from Immune Surveillance to Immune Escape. Immunology 2007, 121, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.B.; Smyth, M.J. Review Series Immune Surveillance of Tumors. J. Clin. Invest. 2007, 117, 1137–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzberg, B.; Campo, M.J.; Gainor, J.F. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Oncologist 2017, 22, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. Cancer Clin. Trials 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazandjian, D.; Suzman, D.L.; Blumenthal, G.; Mushti, S.; He, K.; Libeg, M.; Keegan, P.; Pazdur, R. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer With Progression On or After Platinum-Based Chemotherapy. Oncologist 2016, 21, 634–642. [Google Scholar] [CrossRef] [Green Version]
- Sul, J.; Blumenthal, G.M.; Jiang, X.; He, K.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Patients With Metastatic Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. Oncologist 2016, 21, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Gravara, L.D.; Battiloro, C.; Cantile, R.; Letizia, A.; Vitiello, F.; Montesarchio, V.; Rocco, D. Chemotherapy and/or Immune Checkpoint Inhibitors in NSCLC First-Line Setting: What Is the Best Approach? Lung Cancer Manag. 2020, 9, LMT22. [Google Scholar] [CrossRef] [Green Version]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Kuai, R.; Yuan, W.; Son, S.; Nam, J.; Xu, Y.; Fan, Y.; Schwendeman, A.; Moon, J.J. Elimination of Established Tumors with Nanodisc-Based Combination Chemoimmunotherapy. Sci. Adv. 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer Vaccine Nanodiscs for Personalized Cancer Immunotherapy. Nat. Mater. 2017, 16, 489–498. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, Z. The Application of Nanotechnology in Immune Checkpoint Blockade for Cancer Treatment. J. Control. Release 2018, 290, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Zhou, F.; Doughty, A.; Hoover, A.R.; Nordquist, R.E.; Chen, W.R. Nanotechnology-Based Photoimmunological Therapies for Cancer. Cancer Lett. 2019, 442, 429–438. [Google Scholar] [CrossRef]
- Ge, R.; Liu, C.; Zhang, X.; Wang, W.; Li, B.; Liu, J.; Liu, Y.; Sun, H.; Zhang, D.; Hou, Y.; et al. Photothermal-Activatable Fe3O4 Superparticle Nanodrug Carriers with PD-L1 Immune Checkpoint Blockade for Anti-Metastatic Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2018, 10, 20342–20355. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Palucka, K. Cancer Vaccines on the Move. Nat. Rev. Clin. Oncol. 2018, 15, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Cuppens, K.; Vansteenkiste, J. Vaccination Therapy for Non-Small-Cell Lung Cancer. Curr. Opin. Oncol. 2014, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Jofré, M.; Uranga, R.; Torres Pombert, A.; Arango Prado, M.d.C.; Caballero Aguirrechu, I.; Pacheco, C.; Ortiz Reyes, R.M.; Chuecas, F.; Mas Bermejo, P.I. Therapeutic Vaccines for Advanced Non-Small Cell Lung Cancer. Cochrane Database Syst. Rev. 2019, 1–13. [Google Scholar]
- Xia, L.; Schrump, D.S.; Gildersleeve, J.C. Whole-Cell Cancer Vaccines Induce Large Antibody Responses to Carbohydrates and Glycoproteins. Cell Chem. Biol. 2016, 23, 1515–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, S.; Casey, D.; Labarthe, M.C.; Whelan, M.; Dalgleish, A.; Pandha, H.; Todryk, S. Immunotherapeutic Potential of Whole Tumour Cells. Cancer Immunol. Immunother. 2002, 51, 351–357. [Google Scholar] [CrossRef]
- Hirschowitz, E.A.; Mullins, A.; Prajapati, D.; Baeker, T.; Kloecker, G.; Foody, T.; Damron, K.; Love, C.; Yannelli, J.R. Pilot Study of 1650-G: A Simplified Cellular Vaccine for Lung Cancer. J. Thorac. Oncol. 2011, 6, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Peled, N.; Oton, A.B.; Hirsch, F.R.; Bunn, P. MAGE A3 Antigen-Specific Cancer Immunotherapeutic. Immunotherapy 2009, 1, 19–25. [Google Scholar] [CrossRef]
- Wada, S.; Yada, E.; Ohtake, J.; Sasada, T. Personalized Peptide Vaccines for Cancer Therapy: Current Progress and State of the Art. Expert Rev. Precis. Med. Drug Dev. 2017, 2, 371–381. [Google Scholar] [CrossRef]
- Sebastian, M.; von Boehmer, L.; Zippelius, A.; Mayer, F.; Reck, M.; Atanackovic, D.; Thomas, M.; Schneller, F.; Stoehlmacher, J.; Goekkurt, E.; et al. Messenger RNA Vaccination in NSCLC: Findings from a Phase I/IIa Clinical Trial. J. Clin. Oncol. 2011, 29, 2584. [Google Scholar] [CrossRef]
- Sebastian, M.; Papachristofilou, A.; Weiss, C.; Früh, M.; Cathomas, R.; Hilbe, W.; Wehler, T.; Rippin, G.; Koch, S.D.; Scheel, B.; et al. Phase Ib Study Evaluating a Self-Adjuvanted MRNA Cancer Vaccine (RNActive®®) Combined with Local Radiation as Consolidation and Maintenance Treatment for Patients with Stage IV Non-Small Cell Lung Cancer. BMC Cancer 2014, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vansteenkiste, J.; Zielinski, M.; Linder, A.; Dahabreh, J.; Gonzalez, E.E.; Malinowski, W.; Lopez-Brea, M.; Vanakesa, T.; Jassem, J.; Kalofonos, H.; et al. Adjuvant MAGE-A3 Immunotherapy in Resected Non–Small-Cell Lung Cancer: Phase II Randomized Study Results. J. Clin. Oncol. 2013, 31, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Quoix, E.; Ramlau, R.; Westeel, V.; Papai, Z.; Madroszyk, A.; Riviere, A.; Koralewski, P.; Breton, J.L.; Stoelben, E.; Braun, D.; et al. Therapeutic Vaccination with TG4010 and First-Line Chemotherapy in Advanced Non-Small-Cell Lung Cancer: A Controlled Phase 2B Trial. Lancet Oncol. 2011, 12, 1125–1133. [Google Scholar] [CrossRef]
- Butts, C.; Murray, N.; Maksymiuk, A.; Goss, G.; Marshall, E.; Soulières, D.; Cormier, Y.; Ellis, P.; Price, A.; Sawhney, R.; et al. Randomized Phase IIB Trial of BLP25 Liposome Vaccine in Stage IIIB and IV Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2005, 23, 6674–6681. [Google Scholar] [CrossRef] [Green Version]
- Sangha, R.; Butts, C. L-BLP25: A Peptide Vaccine Strategy in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2007, 13, 4652–4655. [Google Scholar] [CrossRef] [Green Version]
- Midoux, P.; Pichon, C. Lipid-Based MRNA Vaccine Delivery Systems. Expert Rev. Vaccines 2014, 14, 221–234. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.-G.; Lévy, J.-P.; Meulien, P. Induction of Virus-Specific Cytotoxic T Lymphocytes in Vivo by Liposome-Entrapped MRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef]
- Fiedler, K.; Lazzaro, S.; Lutz, J.; Rauch, S.; Heidenreich, R. MRNA Cancer Vaccines. Recent Results Cancer Res 2016, 209, 61–84. [Google Scholar]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines-a New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauffman, K.J.; Webber, M.J.; Anderson, D.G. Materials for Non-Viral Intracellular Delivery of Messenger RNA Therapeutics. J. Control. Release 2016, 240, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Reesor, E.K.G.; Xu, Y.; Zope, H.R.; Zetter, B.R.; Shi, J. Biomaterials for MRNA Delivery. Biomater. Sci. 2015, 3, 1519–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotow, J.; Bivona, T.G. Understanding and Targeting Resistance Mechanisms in NSCLC. Nat. Rev. Cancer 2017, 17, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Li, W.; Liu, X.H.; Sun, M.; Zhang, M.L.; Wu, W.Q.; Xie, W.P.; Hou, Y.Y. Long Non-Coding RNA MEG3 Inhibits NSCLC Cells Proliferation and Induces Apoptosis by Affecting P53 Expression. BMC Cancer 2013, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, S.; Iyer, A.K.; Weiler, J.; Morrissey, D.V.; Amiji, M.M. Combination of SiRNA-Directed Gene Silencing with Cisplatin Reverses Drug Resistance in Human Non-Small Cell Lung Cancer. Mol. Nucleic Acids 2013, 2, e110–e121. [Google Scholar] [CrossRef]
- Zhang, Y.; Schwerbrock, N.M.J.; Rogers, A.B.; Kim, W.Y.; Huang, L. Codelivery of VEGF SiRNA and Gemcitabine Monophosphate in a Single Nanoparticle Formulation for Effective Treatment of NSCLC. Mol. Ther. 2013, 21, 1559–1569. [Google Scholar] [CrossRef] [Green Version]
- Dosta, P.; Ramos, V.; Borrós, S. Stable and Efficient Generation of Poly (β-Amino Ester) s for RNAi Delivery. Mol. Syst. Des. Eng. 2018, 3, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Dosta, P.; Segovia, N.; Cascante, A.; Ramos, V.; Borrós, S. Surface Charge Tunability as a Powerful Strategy to Control Electrostatic Interaction for High Efficiency Silencing, Using Tailored Oligopeptide-Modified Poly(Beta-Amino Ester)s (PBAEs). Acta Biomater. 2015, 20, 82–93. [Google Scholar] [CrossRef]
- Segovia, N.; Dosta, P.; Cascante, A.; Ramos, V.; Borrós, S. Oligopeptide-Terminated Poly (β-Amino Ester)s for Highly Efficient Gene Delivery and Intracellular Localization. Acta Biomater. 2014, 10, 2147–2158. [Google Scholar] [CrossRef]
- Xiao, D.; He, J. Epithelial Mesenchymal Transition and Lung Cancer. J. Thorac. Dis. 2010, 2, 154–159. [Google Scholar] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The MiR-200 Family and MiR-205 Regulate Epithelial to Mesenchymal Transition by Targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Zambre, A.; Mukherjee, S.; Ghoshdastidar, S.; Jiang, Y.; Joshi, T.; Upendran, A.; Kannan, R. Silencing AXL by Covalent SiRNA-Gelatin-Antibody Nanoconjugate Inactivates MTOR/EMT Pathway and Stimulates P53 for TKI Sensitization in NSCLC. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102007. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.; Nair, A.; Veerappan, S.; Sen, D. Translatable Gene Therapy for Lung Cancer Using Crispr CAS9—an Exploratory Review. Cancer Gene. 2020, 27, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Lin, X.; Zhao, Z. Applications of CRISPR/Cas9 Technology in the Treatment of Lung Cancer. Trends Mol. Med. 2019, 25, 1039–1049. [Google Scholar] [CrossRef]
- Greco, C.; Rosenzweig, K.; Cascini, G.L.; Tamburrini, O. Current Status of PET/CT for Tumour Volume Definition in Radiotherapy Treatment Planning for Non-Small Cell Lung Cancer (NSCLC). Lung Cancer 2007, 57, 125–134. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; DeCamp, M.M.; Kohman, L.J.; Silvestri, G.A. Lung Cancer. Invasive Staging: The Guidelines. Chest 2003, 123, 167S–175S. [Google Scholar] [CrossRef]
- Gabrilovich, D. Mechanisms and Functional Significance of Tumour-Induced Dendritic-Cell Defects. Nat. Rev. Immunol. 2004, 4, 941–952. [Google Scholar] [CrossRef]
- Macri, C.; Dumont, C.; Johnston, A.P.R.; Mintern, J.D. Targeting Dendritic Cells: A Promising Strategy to Improve Vaccine Effectiveness. Clin. Transl. Immunol. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Waldhauer, I.; Steinle, A. NK Cells and Cancer Immunosurveillance. Oncogene 2008, 27, 5932–5943. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, T.; Kimoto, Y. NK Cells and Cancer. Jpn. J. Cancer Chemother. 1986, 13, 3327–3333. [Google Scholar]
- Trojan, A.; Urosevic, M.; Dummer, R.; Giger, R.; Weder, W.; Stahel, R.A. Immune Activation Status of CD8+ T Cells Infiltrating Non-Small Cell Lung Cancer. Lung Cancer 2004, 44, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Atanackovic, D.; Altorki, N.K.; Stockert, E.; Williamson, B.; Jungbluth, A.A.; Ritter, E.; Santiago, D.; Ferrara, C.A.; Matsuo, M.; Selvakumar, A.; et al. Vaccine-Induced CD4+T Cell Responses to MAGE-3 Protein in Lung Cancer Patients. J. Immunol. 2004, 172, 3289–3296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arenberg, D.A.; Keane, M.P.; DiGiovine, B.; Kunkel, S.L.; Strom, S.R.B.; Burdick, M.D.; Iannettoni, M.D.; Strieter, R.M. Macrophage Infiltration in Human Non-Small-Cell Lung Cancer: The Role of CC Chemokines. Cancer Immunol. Immunother. 2000, 49, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| NSCLC Subtype | Percentage (% Over NSCLC Cases) | Characteristics | Ref. |
|---|---|---|---|
| Adenocarcinoma | 40 | Presents frequent histologic heterogeneity. Mainly affects the outer edges of the lung. | [10,19] |
| Squamous cell carcinoma | 30 | Centrally located in the larger bronchi of the lung. The incidence is linked with smoking more than for other NSCLC cancers. | [10,19] |
| Large cell carcinoma | 10 | Non-differentiated type of lung cancer that lacks the architecture of squamous or glandular differentiation. It usually affects the peripherical part of the lung. | [10,19] |
| Adenosquamous carcinoma | <5 | It is a subtype presenting components of both adenocarcinoma and squamous cell carcinoma. | [10,19,20] |
| Sarcomatoid carcinoma | <1 | Centrally located in the larger bronchi of the lung and the peripherical part of the lung. Hard to diagnose due to its unclear characteristics which are common to other cancer subtypes. | [10,19] |
| Stage | Tumor | Lymph Node | Metastasis |
|---|---|---|---|
| Occult carcinoma | TX | N0 | M0 |
| Stage 0 | Tis | N0 | M0 |
| Stage IA | T1mi-c | N0 | M0 |
| Stage IB | T2a | N0 | M0 |
| Stage IIA | T2b | N0–N1 | M0 |
| Stage IIB | T1, T2, T3 | N0–N1 | M0 |
| Stage IIIA | T1–T4 | N0–N2 | M0 |
| Stage IIIB | T1a–T4 | N2–N3 | M0 |
| Stage IVA | Any T | Any N | M1a–b |
| Stage IVB | Any T | Any N | M1c |
| Generic Name (Brand Name) | Mechanism of Action |
|---|---|
| Carboplatin (Paraplatin®) | Alkylating agent |
| Docetaxel (Taxotere®) | Mitotic inhibitor |
| Doxorubicin Hydrochloride (Adryamycin®, Rubex®) | Topoisomerase inhibitor |
| Gemcitabine Hydrochloride (Gemzar®) | Antimetabolite |
| Lurtotecan (OSI-211) | Topoisomerase inhibitor |
| Mechlorethamine Hydrochloride (Mustargen®) | Alkylating agent |
| Methotrexate (TrexallTM, Rheumatrex®) | Antimetabolite |
| Paclitaxel (Taxol®) | Mitotic inhibitor |
| Paclitaxel–Albumin-stabilized Nanoparticle Formulation (Abraxane®) | Mitotic inhibitor |
| Pemetrexed Disodium (Alimta®) | Antimetabolite |
| Vinorelbine Tartrate (Navelbine®) | Tubuline-binding agent |
| Drug | Nano Delivery System | NSCLC Stage | Phase | Clinical Trial |
|---|---|---|---|---|
| Doxorubicin Hydrochloride (Adryamycin®, Rubex ®) | Pegylated Liposome | IIIB–IV | II | NCT01051362 |
| Aerosolized Liposome | IIIB | I | NCT00020124 | |
| Paclitaxel | Liposome | IIIB–IV | IV | NCT02996214 |
| Polymeric micelle (Genexol-PM®) | IV | II | NCT01023347 | |
| NCT01770795 | ||||
| Camptothecin | Aerosolized Liposome | IIIB–IV | Pre-clinical | NCT00277082 |
| Lurtotecan | Liposome | IIIB | I | NCT00006036 |
| Generic Name (Brand Name) | Mechanism | Ref. |
|---|---|---|
| Atezolizumab (Tecentriq®) * | PD-L1 | [55,56] |
| Durvalumab (Imfinzi®) * | PD-L1 | [55,56] |
| Nivolumab (Opdivo®) * | PD1 | [55,56] |
| Pembrolizumab (Keytruda®) * | PD1 | [55,56] |
| Ipilimumab (Yervoy®) | CTLA-4 | [97,98] |
| Vaccine | Components (Brand/Clinical Trial Name) | NSCLC Stage | Clinical Study Phase | Clinical Trial |
|---|---|---|---|---|
| Cellular vaccine | Allogenic tumoral cells (1650-G) | I–II | II | NCT00654030, NCT00601796 |
| Autologous engineered dendritic cells (MIDRIX4-LUNG) | III | I | NCT04082182 | |
| Autologous mRNA/DNA transfected dendritic cells (MIDRIXNEO-LUNG) | III–IV | I | NCT04078269 | |
| Allogenic mRNA-transfected dendritic cells (AST-VAC2) | III–IV | I | NCT03371485 | |
| Allogenic engineered dendritic cells irradiated with seven active agents (NY-ESO-1, MAGE C1, 4MAGE C2, TPGB, Survivn, MUC1, Melan-A antigen (PDC*lung01) | N.S. | I–II | NCT03970746 | |
| Autologous dendritic cells pulsed with allogenic tumor cells | III | II | NCT00103116 | |
| Allogenic whole tumor cells (Lucanix ®) | III–IV | III | NCT00676507, NCT01058785 | |
| Autologous dendritic cells pulsed with allogenic tumor cells (MelCancerVac®) | III–IV | II | NCT00442754 | |
| Autologous dendritic cells pulsed with p53 peptide | III | II | NCT00019929 | |
| Engineered autologous killed tumor cells | IV | I–II | NCT01159288, NCT02439450 | |
| Allogeneic CD4+ memory Th1-like T-cells (Allostim®) | II–IV | I–II | NCT01065441 | |
| Autologous dendritic cells pulsed with allogenic tumor cells (DVAC/LuCa) | IV | I–II | NCT02470468 | |
| Allogenic lymphocytes | I–IV | I | NCT00161187 | |
| Protein vaccine | MUC1 | III | I–II | NCT01720836, NCT03353675, NCT00415818 NCT03623750 |
| Heat shock protein (gp96-Ig) | III–IV | I | NCT00503568 | |
| Tumor antigen-loaded dendritic cell-derived exosomes | III–IV | II | NCT01159288 | |
| Anti-idiotype vaccine | IIA–III | II | NCT00006470 | |
| Recombinant PRAME protein | I–IIIA | II | NCT01853878 | |
| Peptide vaccine | IDO peptide | III–IV | I | NCT01219348 |
| HLA-A*0201 restricted 9-mer epitopes (Vx001) | IV | II | NCT01935154 | |
| Short lived proteins (SLiPs) and defective ribosomal products (DRiPs) | III–IV | I | NCT00850785, NCT01909752 | |
| Synthetic peptides encoding hTERT (UV1) | III | I–II | NCT01789099 | |
| MUC1 peptide (Tecemotide/L-BLP25/Stimuvax®) | III | III | NCT00409188, NCT00960115, NCT00157196, NCT00828009, NCT00157209 | |
| UCP2 and UCP4 (telomerase derived peptides) | III | I–II | NCT02818426 | |
| Epitope Peptide Restricted to HLA-A*02 | III–IV | I | NCT01069640, NCT01069575 | |
| GV1001 (Synthetic peptides encoding hTERT) | III | N.E. (already approved in Korea for pancreatic cancer) | NCT00509457 | |
| (MAGE3 epitope) (Astuprotimut-R (GSK-249553)) | IB–II | II | NCT00290355 | |
| Wilms tumor 1 (WT1) analog peptide (DSP-7888) | III–IV | I | NCT03715985 | |
| Peptides derived from a patient’s tumor individual neo-antigens (NeoPepVac, GRT-C901 and GRT-R902, GEN-009, NEO-PV-01) | III–IV | I | NCT03715985, NCT03639714, NCT03794128, NCT03953235, NCT03633110, NCT02897765, NCT03380871 | |
| Tedopi® (OSE2101) | III–IV | III | NCT02654587 | |
| RAS peptide | II–IV | I–II | NCT00019006, NCT00019331, NCT00003125 | |
| Arginase-1 peptide | Generic | I | NCT03689192 | |
| YE-NEO-001 Neoepitope yeast vaccine (YE-NEO-001) | Generic | I | NCT03552718 | |
| MAGE-12 peptide | IV | I | NCT00020267 | |
| Patient specific neoepitopes | ||||
| mRNA vaccine | NY-ESO-1, MAGE C1, 4MAGE C2, TPGB, Survivn, MUC1 (RNActive®) | III–IV | I–II | NCT03164772, NCT00923312 |
| KRAS gene vaccine V941 (mRNA-5671) | III–IV | I | NCT03948763 | |
| Personalized vaccine against patient’s mutations (RO7198457) | III–IV | I | NCT03289962 | |
| DNA vaccine | NY-ESO-1 plasmid DNA (pPJV7611) to increase immunogenicity of tumor cells | III–IV | I–II | NCT00199849 |
| Plasmid encoding neoepitopes (VB10.NEO) | III–IV | I–II | NCT03548467 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Fernández, C.; Fornaguera, C.; Borrós, S. Nanomedicine in Non-Small Cell Lung Cancer: From Conventional Treatments to Immunotherapy. Cancers 2020, 12, 1609. https://doi.org/10.3390/cancers12061609
García-Fernández C, Fornaguera C, Borrós S. Nanomedicine in Non-Small Cell Lung Cancer: From Conventional Treatments to Immunotherapy. Cancers. 2020; 12(6):1609. https://doi.org/10.3390/cancers12061609
Chicago/Turabian StyleGarcía-Fernández, Coral, Cristina Fornaguera, and Salvador Borrós. 2020. "Nanomedicine in Non-Small Cell Lung Cancer: From Conventional Treatments to Immunotherapy" Cancers 12, no. 6: 1609. https://doi.org/10.3390/cancers12061609
APA StyleGarcía-Fernández, C., Fornaguera, C., & Borrós, S. (2020). Nanomedicine in Non-Small Cell Lung Cancer: From Conventional Treatments to Immunotherapy. Cancers, 12(6), 1609. https://doi.org/10.3390/cancers12061609