Fluoropyrimidine Modulation of the Anti-Tumor Immune Response―Prospects for Improved Colorectal Cancer Treatment
Abstract
1. Introduction
2. Results
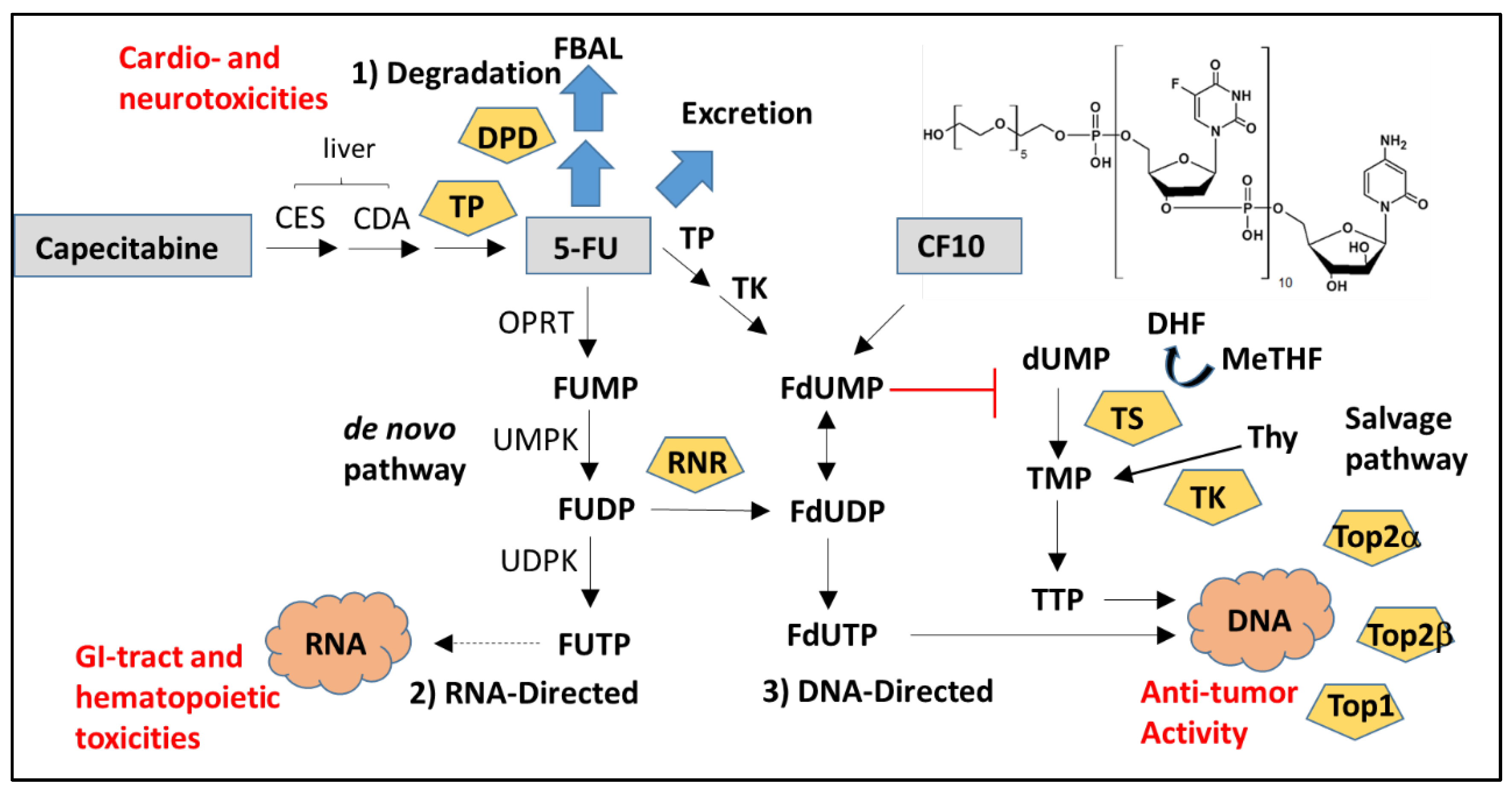
2.1. Cytotoxic Mechanism of 5-FU
2.1.1. DNA-Directed Effects of 5-FU
2.1.2. RNA-Directed Effects of 5-FU
2.1.3. Effects of 5-FU Degradation Metabolites
3. 5-FU Modulates the Anti-Tumor Immune Response
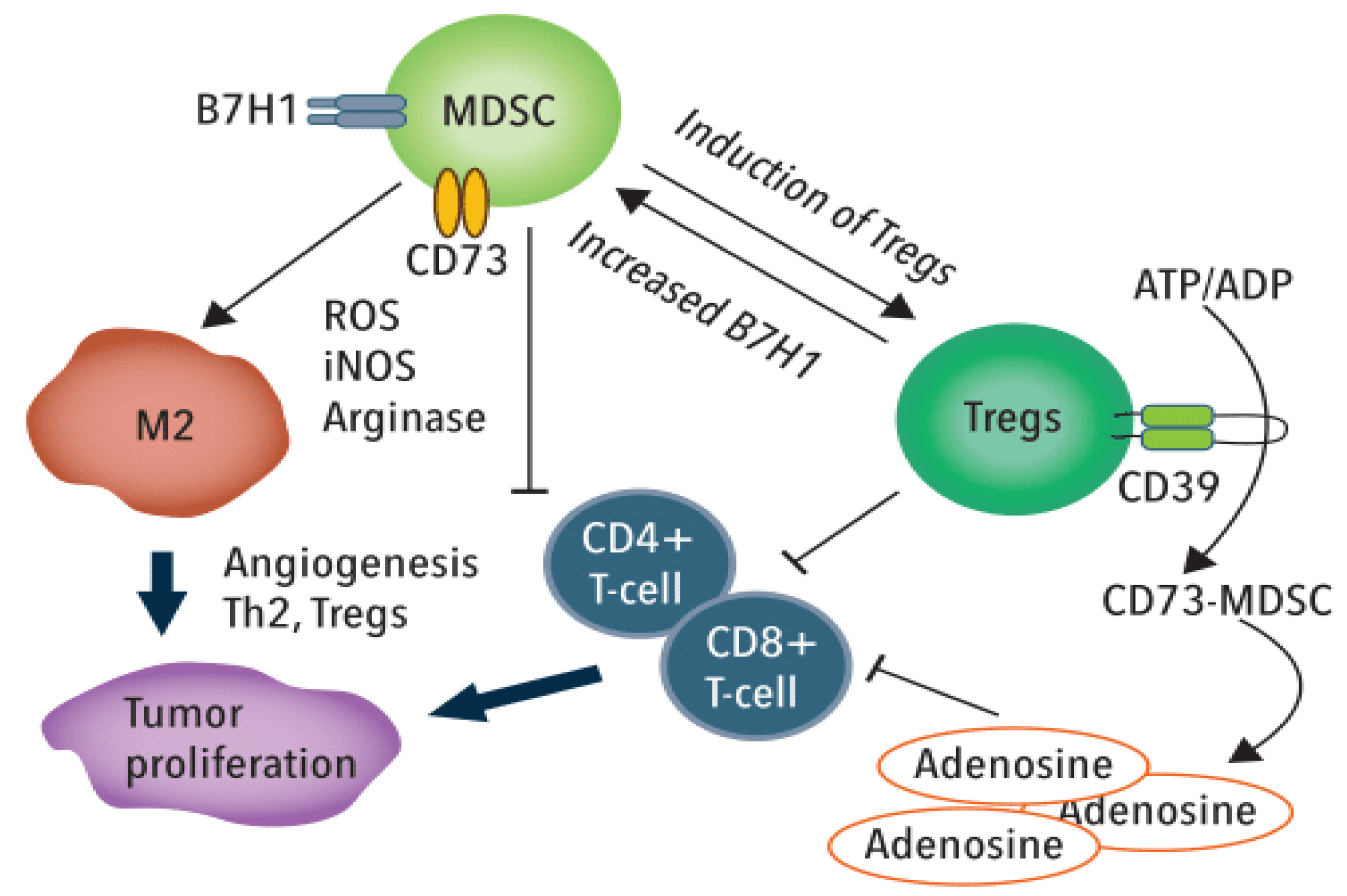
3.1. 5-FU Effects to MDSCs and TRegs
3.2. 5-FU Stimulation of Immunogenic Cell Death
3.3. 5-FU Effects on Immune Cells Are Dynamic
4. Modulation of 5-FU-Induced Anti-Tumor Immunity
4.1. Direct and Indirect Modulation of 5-FU by IFNs
4.2. Combining 5-FU-Based Chemotherapy with Immune Checkpoint Blockade
5. Novel Chemical Approaches to Modulating 5-FU’s Anti-Tumor Response
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Heidelberger, C.; Chaudhuri, N.K.; Danneberg, P.; Mooren, D.; Griesbach, L.; Duschinsky, R.; Schnitzer, R.J.; Pleven, E.; Scheiner, J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Punt, C.J.; Koopman, M.; Vermeulen, L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.M.; Danenberg, P.V.; Johnston, P.G.; Lenz, H.J.; Ladner, R.D. Standing the test of time: Targeting thymidylate biosynthesis in cancer therapy. Nat. Rev. Clin. Oncol. 2014, 11, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Grem, J.L. Mechanisms of Action and Modulation of Fluorouracil. Semin. Radiat. Oncol. 1997, 7, 249–259. [Google Scholar] [CrossRef]
- Skillings, J.R.; Levine, M.; Rayner, H.L.; Eisenhauer, E.; Erlichman, C.; Germond, C.; Kerr, I.; Lofters, W.; Maroun, J.; Yoshida, S. Levamisole and 5-fluorouracil therapy for resected colon cancer: A new indication. CMAJ 1991, 144, 297–301. [Google Scholar]
- Renoux, G. The general immunopharmacology of levamisole. Drugs 1980, 20, 89–99. [Google Scholar] [CrossRef]
- AbdAlla, E.E.; Blair, G.E.; Jones, R.A.; Sue-Ling, H.M.; Johnston, D. Mechanism of synergy of levamisole and fluorouracil: Induction of human leukocyte antigen class I in a colorectal cancer cell line. J. Natl Cancer Inst. 1995, 87, 489–496. [Google Scholar] [CrossRef]
- Wadler, S.; Schwartz, E.L. Antineoplastic activity of the combination of interferon and cytotoxic agents against experimental and human malignancies: A review. Cancer Res. 1990, 50, 3473–3486. [Google Scholar]
- Thirion, P.; Piedbois, P.; Buyse, M.; O’Dwyer, P.J.; Cunningham, D.; Man, A.; Greco, F.A.; Colucci, G.; Kohne, C.H.; Di Constanzo, F.; et al. Alpha-interferon does not increase the efficacy of 5-fluorouracil in advanced colorectal cancer. Br. J. Cancer 2001, 84, 611–620. [Google Scholar] [CrossRef][Green Version]
- Rustum, Y.M. Clinical implications of 5-FU modulation. Oncology (Williston Park) 1999, 13, 22–25. [Google Scholar]
- Gmeiner, W.H. Novel chemical strategies for thymidylate synthase inhibition. Curr. Med. Chem. 2005, 12, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Hanawalt, P.C. A balanced perspective on unbalanced growth and thymineless death. Front. Microbiol. 2015, 6, 504. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.F.; Zheng, Y.F.; Ming, P.P.; Deng, X.X.; Ge, W.; Wu, Y.G. Immune checkpoint inhibitors in non-small-cell lung cancer: Current status and future directions. Brief. Funct Genom. 2019, 18, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Queirolo, P.; Boutros, A.; Tanda, E.; Spagnolo, F.; Quaglino, P. Immune-checkpoint inhibitors for the treatment of metastatic melanoma: A model of cancer immunotherapy. Semin. Cancer Biol. 2019, 59, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, G.; Guan, H.; Yu, Y.; Lu, J.; Yu, J. Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 87. [Google Scholar] [CrossRef]
- Hermel, D.J.; Sigal, D. The Emerging Role of Checkpoint Inhibition in Microsatellite Stable Colorectal Cancer. J. Pers. Med. 2019, 9, 5. [Google Scholar] [CrossRef]
- Carrato, A. Adjuvant treatment of colorectal cancer. Gastrointest. Cancer Res. 2008, 2 (Suppl. S4), S42–S46. [Google Scholar]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Lawrence, T.S.; Davis, M.A.; Maybaum, J. Dependence of 5-fluorouracil-mediated radiosensitization on DNA-directed effects. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 519–523. [Google Scholar] [CrossRef]
- Gmeiner, W.H. Entrapment of DNA topoisomerase-DNA complexes by nucleotide/nucleoside analogs. Cancer Drug Resist. 2019, 2, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Mojardin, L.; Botet, J.; Quintales, L.; Moreno, S.; Salas, M. New insights into the RNA-based mechanism of action of the anticancer drug 5’-fluorouracil in eukaryotic cells. PLoS ONE 2013, 8, e78172. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.M.; Watson, A.J.; Potten, C.S.; Jackman, A.L.; Hickman, J.A. Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil: Evidence for the involvement of RNA perturbation. Proc. Natl. Acad. Sci. USA 1997, 94, 1795–1799. [Google Scholar] [CrossRef]
- Sun, X.X.; Dai, M.S.; Lu, H. 5-fluorouracil activation of p53 involves an MDM2-ribosomal protein interaction. J. Biol. Chem. 2007, 282, 8052–8059. [Google Scholar] [CrossRef] [PubMed]
- Van Groeningen, C.J.; Peters, G.J.; Leyva, A.; Laurensse, E.; Pinedo, H.M. Reversal of 5-fluorouracil-induced myelosuppression by prolonged administration of high-dose uridine. J. Natl. Cancer Inst. 1989, 81, 157–162. [Google Scholar] [CrossRef]
- Soares, P.M.; Mota, J.M.; Souza, E.P.; Justino, P.F.; Franco, A.X.; Cunha, F.Q.; Ribeiro, R.A.; Souza, M.H. Inflammatory intestinal damage induced by 5-fluorouracil requires IL-4. Cytokine 2013, 61, 46–49. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Debinski, W.; Milligan, C.; Caudell, D.; Pardee, T.S. The applications of the novel polymeric fluoropyrimidine F10 in cancer treatment: Current evidence. Future Oncol. 2016, 12, 2009–2020. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Miller, L.D.; Chou, J.W.; Dominijanni, A.; Mutkus, L.; Marini, F.; Ruiz, J.; Dotson, T.; Thomas, K.W.; Parks, G.; et al. Dysregulated Pyrimidine Biosynthesis Contributes to 5-FU Resistance in SCLC Patient-Derived Organoids but Response to a Novel Polymeric Fluoropyrimidine, CF10. Cancers 2020, 12, 788. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Bastos, D.A.; Ribeiro, S.C.; de Freitas, D.; Hoff, P.M. Combination therapy in high-risk stage II or stage III colon cancer: Current practice and future prospects. Ther. Adv. Med. Oncol. 2010, 2, 261–272. [Google Scholar] [CrossRef]
- Wolmark, N.; Fisher, B.; Rockette, H.; Redmond, C.; Wickerham, D.L.; Fisher, E.R.; Jones, J.; Glass, A.; Lerner, H.; Lawrence, W.; et al. Postoperative adjuvant chemotherapy or BCG for colon cancer: Results from NSABP protocol C-01. J. Natl. Cancer Inst. 1988, 80, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Shikina, A.; Shinto, E.; Hashiguchi, Y.; Ueno, H.; Naito, Y.; Okamoto, K.; Kubo, T.; Fukazawa, S.; Yamamoto, J.; Hase, K. Differential clinical benefits of 5-fluorouracil-based adjuvant chemotherapy for patients with stage III colorectal cancer according to CD133 expression status. Jpn. J. Clin. Oncol. 2014, 44, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.J.; Lin, J.K.; Chen, W.S.; Jiang, J.K.; Teng, H.W.; Yen, C.C.; Lin, T.C.; Yang, S.H. Adjuvant FOLFOX treatment for stage III colon cancer: How many cycles are enough? Springerplus 2016, 5, 1318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohelnikova-Duchonova, B.; Melichar, B.; Soucek, P. FOLFOX/FOLFIRI pharmacogenetics: The call for a personalized approach in colorectal cancer therapy. World J. Gastroenterol. 2014, 20, 10316–10330. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef]
- Fang, H.; Ang, B.; Xu, X.; Huang, X.; Wu, Y.; Sun, Y.; Wang, W.; Li, N.; Cao, X.; Wan, T. TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell. Mol. Immunol. 2014, 11, 150–159. [Google Scholar] [CrossRef]
- Vincent, J.; Mignot, G.; Chalmin, F.; Ladoire, S.; Bruchard, M.; Chevriaux, A.; Martin, F.; Apetoh, L.; Rebe, C.; Ghiringhelli, F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010, 70, 3052–3061. [Google Scholar] [CrossRef]
- Vincent, J.; Mignot, G.; Chalmin, F.; Chang, C.T.; Ho, T.Y.; Lin, H.; Liang, J.A.; Huang, H.C.; Li, C.C.; Lo, H.Y.; et al. 5-Fluorouracil induced intestinal mucositis via nuclear factor-kappaB activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS ONE 2012, 7, e31808. [Google Scholar] [CrossRef]
- Li, X.; Slayton, W.B. Molecular mechanisms of platelet and stem cell rebound after 5-fluorouracil treatment. Exp. Hematol. 2013, 41, 635–645 e63. [Google Scholar] [CrossRef]
- Garg, M.B.; Lincz, L.F.; Adler, K.; Scorgie, F.E.; Ackland, S.P.; Sakoff, J.A. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: A multivariate analysis. Br. J. Cancer 2012, 107, 1525–1533. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kawasaki, T. Active transport of 5-fluorouracil and its energy coupling in Ehrlich ascites tumor cells. J. Biochem. 1981, 90, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Weckbecker, G. Biochemical pharmacology and analysis of fluoropyrimidines alone and in combination with modulators. Pharm. Ther. 1991, 50, 367–424. [Google Scholar] [CrossRef]
- Saif, M.W.; Syrigos, K.; Mehra, R.; Mattison, L.K.; Diasio, R.B. Dihydropyrimidine Dehydrogenase Deficiency (DPD) in Gi Malignancies: Experience of 4-Years. Pak. J. Med. Sci. 2007, 23, 832–839. [Google Scholar]
- Lee, A.M.; Shi, Q.; Pavey, E.; Alberts, S.R.; Sargent, D.J.; Sinicrope, F.A.; Berenberg, J.L.; Goldberg, R.M.; Diasio, R.B. DPYD variants as predictors of 5-fluorouracil toxicity in adjuvant colon cancer treatment (NCCTG N0147). J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- El-Rayes, B. Managing Severe Toxicities Associated with 5-FU and Capecitabine: An Oncologists’s Perspective. Medscape Educ. Oncol. 2017. Available online: https://www.medscape.org/viewarticle/872909_sidebar2 (accessed on 15 May 2020).
- Lee, J.J.; Beumer, J.H.; Chu, E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother. Pharm. 2016, 78, 447–464. [Google Scholar] [CrossRef]
- Noordhuis, P.; Holwerda, U.; Van der Wilt, C.L.; Van Groeningen, C.J.; Smid, K.; Meijer, S.; Pinedo, H.M.; Peters, G.J. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 2004, 15, 1025–1032. [Google Scholar] [CrossRef]
- Saif, M.W.; Diasio, R.B. Benefit of uridine triacetate (Vistogard) in rescuing severe 5-fluorouracil toxicity in patients with dihydropyrimidine dehydrogenase (DPYD) deficiency. Cancer Chemother. Pharm. 2016, 78, 151–156. [Google Scholar] [CrossRef]
- Houghton, J.A.; Tillman, D.M.; Harwood, F.G. Ratio of 2’-deoxyadenosine-5’-triphosphate/thymidine-5’-triphosphate influences the commitment of human colon carcinoma cells to thymineless death. Clin. Cancer Res. 1995, 1, 723–730. [Google Scholar]
- Mani, C.; Pai, S.; Papke, C.M.; Palle, K.; Gmeiner, W.H. Thymineless Death by the Fluoropyrimidine Polymer F10 Involves Replication Fork Collapse and Is Enhanced by Chk1 Inhibition. Neoplasia 2018, 20, 1236–1245. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Sordet, O.; Zhang, H.L.; Kohlhagen, G.; Antony, S.; Gmeiner, W.H.; Pommier, Y. A novel polypyrimidine antitumor agent FdUMP[10] induces thymineless death with topoisomerase I-DNA complexes. Cancer Res. 2005, 65, 4844–4851. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, W.H.; Reinhold, W.C.; Pommier, Y. Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP[10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Mol. Cancer Ther. 2010, 9, 3105–3114. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.A.; Harwood, F.G.; Tillman, D.M. Thymineless death in colon carcinoma cells is mediated via fas signaling. Proc. Natl. Acad. Sci. USA 1997, 94, 8144–8149. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.A.; Harwood, F.G.; Gibson, A.A.; Tillman, D.M. The fas signaling pathway is functional in colon carcinoma cells and induces apoptosis. Clin. Cancer Res. 1997, 3, 2205–2209. [Google Scholar]
- Gmeiner, W.H.; Jennings-Gee, J.; Stuart, C.H.; Pardee, T.S. Thymineless death in F10-treated AML cells occurs via lipid raft depletion and Fas/FasL co-localization in the plasma membrane with activation of the extrinsic apoptotic pathway. Leuk. Res. 2015, 39, 229–235. [Google Scholar] [CrossRef][Green Version]
- Hassin, D.; Garber, O.G.; Meiraz, A.; Schiffenbauer, Y.S.; Berke, G. Cytotoxic T lymphocyte perforin and Fas ligand working in concert even when Fas ligand lytic action is still not detectable. Immunology 2011, 133, 190–196. [Google Scholar] [CrossRef]
- Sahasrabudhe, P.V.; Pon, R.T.; Gmeiner, W.H. Solution structures of 5-fluorouracil-substituted DNA and RNA decamer duplexes. Biochemistry 1996, 35, 13597–13608. [Google Scholar] [CrossRef]
- Sahasrabudhe, P.V.; Sun, J.; Gmeiner, W.H. The 5′ stem-loop from human U4 snRNA adopts a novel conformation stabilized by G-C and G-U base pairs. J. Biomol. Struct. Dyn. 1997, 14, 567–577. [Google Scholar] [CrossRef]
- Sahasrabudhe, P.V.; Gmeiner, W.H. Solution structures of 5-fluorouracil-substituted RNA duplexes containing G-U wobble base pairs. Biochemistry 1997, 36, 5981–5991. [Google Scholar] [CrossRef]
- Sahasrabudhe, P.V.; Pon, R.T.; Gmeiner, W.H. Effects of site-specific substitution of 5-fluorouridine on the stabilities of duplex DNA and RNA. Nucleic. Acids Res. 1995, 23, 3916–3921. [Google Scholar] [CrossRef]
- Warny, M.; Helby, J.; Nordestgaard, B.G.; Birgens, H.; Bojesen, S.E. Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLoS Med. 2018, 15, e1002685. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Baccanari, D.P.; Rustum, Y.M.; Davis, S.T.; Tansik, R.L.; Porter, D.J.; Spector, T. Alpha-fluoro-beta-alanine: Effects on the antitumor activity and toxicity of 5-fluorouracil. Biochem. Pharm. 2000, 59, 953–960. [Google Scholar] [CrossRef]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef]
- Guo, X.D.; Harold, N.; Saif, M.W.; Schuler, B.; Szabo, E.; Hamilton, J.M.; Monahan, B.P.; Quinn, M.G.; Cliatt, J.; Nguyen, D.; et al. Pharmacokinetic and pharmacodynamic effects of oral eniluracil, fluorouracil and leucovorin given on a weekly schedule. Cancer Chemother. Pharm. 2003, 52, 79–85. [Google Scholar] [CrossRef]
- Yamashita, K.; Yada, H.; Ariyoshi, T. Neurotoxic effects of alpha-fluoro-beta-alanine (FBAL) and fluoroacetic acid (FA) on dogs. J. Toxicol. Sci 2004, 29, 155–166. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Funakoshi, T.; Horimatsu, T.; Miyamoto, S.; Matsubara, T.; Yanagita, M.; Nakagawa, S.; Yonezawa, A.; Matsubara, K.; Muto, M. Accumulation of alpha-fluoro-beta-alanine and fluoro mono acetate in a patient with 5-fluorouracil-associated hyperammonemia. Cancer Chemother. Pharm. 2017, 79, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, M.; Kishton, R.J.; Restifo, N.P. Metabolic reprograming of anti-tumor immunity. Curr. Opin. Immunol. 2017, 46, 14–22. [Google Scholar] [CrossRef]
- Bracci, L.; Schiavoni, G.; Sistigu, A.; Belardelli, F. Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014, 21, 15–25. [Google Scholar] [CrossRef]
- Proietti, E.; Moschella, F.; Capone, I.; Belardelli, F. Exploitation of the propulsive force of chemotherapy for improving the response to cancer immunotherapy. Mol. Oncol. 2012, 6, 1–14. [Google Scholar] [CrossRef]
- Ito, S.E.; Shirota, H.; Kasahara, Y.; Saijo, K.; Ishioka, C. IL-4 blockade alters the tumor microenvironment and augments the response to cancer immunotherapy in a mouse model. Cancer Immunol. Immunother. 2017, 66, 1485–1496. [Google Scholar] [CrossRef]
- Li, H.L.; Lu, L.; Wang, X.S.; Qin, L.Y.; Wang, P.; Qiu, S.P.; Wu, H.; Huang, F.; Zhang, B.B.; Shi, H.L.; et al. Alteration of Gut Microbiota and Inflammatory Cytokine/Chemokine Profiles in 5-Fluorouracil Induced Intestinal Mucositis. Front. Cell. Infect. Microbiol. 2017, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, D.; Larmonier, N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. 2014, 74, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Till, B.; Gao, Q. Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology 2017, 6, e1331807. [Google Scholar] [CrossRef] [PubMed]
- Corthay, A. How do regulatory T cells work? Scand. J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef]
- Ohta, A.; Sitkovsky, M. Extracellular adenosine-mediated modulation of regulatory T cells. Front. Immunol. 2014, 5, 304. [Google Scholar] [CrossRef]
- Vigano, S.; Alatzoglou, D.; Irving, M.; Menetrier-Caux, C.; Caux, C.; Romero, P.; Coukos, G. Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front. Immunol 2019, 10, 925. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Zhang, X.; Kelaria, S.; Kerstetter, J.; Wang, J. The functional and prognostic implications of regulatory T cells in colorectal carcinoma. J. Gastrointest. Oncol. 2015, 6, 307–313. [Google Scholar] [CrossRef]
- Walker, L.S. Treg and CTLA-4: Two intertwining pathways to immune tolerance. J. Autoimmun. 2013, 45, 49–57. [Google Scholar] [CrossRef]
- OuYang, L.Y.; Wu, X.J.; Ye, S.B.; Zhang, R.X.; Li, Z.L.; Liao, W.; Pan, Z.Z.; Zheng, L.M.; Zhang, X.S.; Wang, Z.; et al. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J. Transl. Med. 2015, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Cai, T.T.; Wu, X.J.; Liu, Y.N.; He, J.; Zhang, X.S.; Ma, G.; Li, J. Tumour YAP1 and PTEN expression correlates with tumour-associated myeloid suppressor cell expansion and reduced survival in colorectal cancer. Immunology 2018, 155, 263–272. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, M.; Yu, T.; Liu, X.; Zhang, P. Intratumoral regulatory T cells are associated with suppression of colorectal carcinoma metastasis after resection through overcoming IL-17 producing T cells. Cell. Immunol. 2014, 287, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Xu, Y.; Ying, M.; Li, Q.; Huang, L.; Li, D.; Cai, S.; Li, B. FOXP3+ Tregs: Heterogeneous phenotypes and conflicting impacts on survival outcomes in patients with colorectal cancer. Immunol. Res. 2015, 61, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Le, H.K.; Graham, L.; Cha, E.; Morales, J.K.; Manjili, M.H.; Bear, H.D. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int. Immunopharmacol. 2009, 9, 900–909. [Google Scholar] [CrossRef]
- Orecchioni, S.; Talarico, G.; Labanca, V.; Calleri, A.; Mancuso, P.; Bertolini, F. Vinorelbine, cyclophosphamide and 5-FU effects on the circulating and intratumoural landscape of immune cells improve anti-PD-L1 efficacy in preclinical models of breast cancer and lymphoma. Br. J. Cancer 2018, 118, 1329–1336. [Google Scholar] [CrossRef]
- Limagne, E.; Euvrard, R.; Thibaudin, M.; Rebe, C.; Derangere, V.; Chevriaux, A.; Boidot, R.; Vegran, F.; Bonnefoy, N.; Vincent, J.; et al. Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer Res. 2016, 76, 5241–5252. [Google Scholar] [CrossRef] [PubMed]
- Kanterman, J.; Sade-Feldman, M.; Biton, M.; Ish-Shalom, E.; Lasry, A.; Goldshtein, A.; Hubert, A.; Baniyash, M. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res. 2014, 74, 6022–6035. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Formica, V.; Cereda, V.; Jochems, C.; Richards, J.; Grenga, I.; Orlandi, A.; Ferroni, P.; Guadagni, F.; Schlom, J. The association of clinical outcome and peripheral T-cell subsets in metastatic colorectal cancer patients receiving first-line FOLFIRI plus bevacizumab therapy. Oncoimmunology 2016, 5, e1188243. [Google Scholar] [CrossRef]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef]
- Pages, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Krijgsman, D.; de Vries, N.L.; Skovbo, A.; Andersen, M.N.; Swets, M.; Bastiaannet, E.; Vahrmeijer, A.L.; van de Velde, C.J.H.; Heemskerk, M.H.M.; Hokland, M.; et al. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: The peripheral blood immune cell profile. Cancer Immunol. Immunother. 2019, 68, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Rocca, Y.S.; Roberti, M.P.; Arriaga, J.M.; Amat, M.; Bruno, L.; Pampena, M.B.; Huertas, E.; Loria, F.S.; Pairola, A.; Bianchini, M.; et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun. 2013, 19, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.P.; Xie, M.Z.; Li, K.Z.; Li, J.L.; Cai, Z.M.; Hu, B.L. Prognostic value of peripheral blood natural killer cells in colorectal cancer. BMC Gastroenterol. 2020, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, C.; Claus, M.; Wildemann, B.; Wingert, S.; Korporal, M.; Romisch, J.; Meuer, S.; Watzl, C.; Giese, T. Exposure of NK cells to intravenous immunoglobulin induces IFN gamma release and degranulation but inhibits their cytotoxic activity. Clin. Immunol. 2009, 133, 393–401. [Google Scholar] [CrossRef]
- Cui, F.; Qu, D.; Sun, R.; Nan, K. Circulating CD16+CD56+ nature killer cells indicate the prognosis of colorectal cancer after initial chemotherapy. Med. Oncol. 2019, 36, 84. [Google Scholar] [CrossRef]
- Shinko, D.; McGuire, H.M.; Diakos, C.I.; Pavlakis, N.; Clarke, S.J.; Byrne, S.N.; Charles, K.A. Mass Cytometry Reveals a Sustained Reduction in CD16+ Natural Killer Cells Following Chemotherapy in Colorectal Cancer Patients. Front. Immunol. 2019, 10, 2584. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Panaretakis, T.; Joza, N.; Modjtahedi, N.; Tesniere, A.; Vitale, I.; Durchschlag, M.; Fimia, G.M.; Kepp, O.; Piacentini, M.; Froehlich, K.U.; et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008, 15, 1499–1509. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Binder, R.J. The heat shock protein-CD91 pathway mediates tumor immunosurveillance. Oncoimmunology 2014, 3, e28222. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Martins, I.; Ma, Y.; Kepp, O.; Galluzzi, L.; Kroemer, G. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy 2013, 9, 1624–1625. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Criollo, A.; Ortiz, C.; Lidereau, R.; Mariette, C.; Chaput, N.; Mira, J.P.; Delaloge, S.; et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol. Rev. 2007, 220, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.; Lei, J.; Sun, Y.; Yan, F.; Chen, B.; Zhang, L.; Lu, Z.; Cao, R.; Lin, Y.Y.; Wang, C.C.; et al. Induction of enhanced immunogenic cell death through ultrasound-controlled release of doxorubicin by liposome-microbubble complexes. Oncoimmunology 2018, 7, e1446720. [Google Scholar] [CrossRef] [PubMed]
- Castle, J.C.; Loewer, M.; Boegel, S.; de Graaf, J.; Bender, C.; Tadmor, A.D.; Boisguerin, V.; Bukur, T.; Sorn, P.; Paret, C.; et al. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genom. 2014, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, B. TP53 mutation in colorectal cancer. Hum. Mutat. 2003, 21, 271–276. [Google Scholar] [CrossRef]
- Bunz, F.; Hwang, P.M.; Torrance, C.; Waldman, T.; Zhang, Y.; Dillehay, L.; Williams, J.; Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Investig. 1999, 104, 263–269. [Google Scholar] [CrossRef]
- Kandioler, D.; Mittlbock, M.; Kappel, S.; Puhalla, H.; Herbst, F.; Langner, C.; Wolf, B.; Tschmelitsch, J.; Schippinger, W.; Steger, G.; et al. TP53 Mutational Status and Prediction of Benefit from Adjuvant 5-Fluorouracil in Stage III Colon Cancer Patients. EBioMedicine 2015, 2, 825–830. [Google Scholar] [CrossRef]
- Pardee, T.S.; Gomes, E.; Jennings-Gee, J.; Caudell, D.; Gmeiner, W.H. Unique dual targeting of thymidylate synthase and topoisomerase1 by FdUMP[10] results in high efficacy against AML and low toxicity. Blood 2012, 119, 3561–3570. [Google Scholar] [CrossRef] [PubMed]
- Arshad, U.; Ploylearmsaeng, S.A.; Karlsson, M.O.; Doroshyenko, O.; Langer, D.; Schomig, E.; Kunze, S.; Guner, S.A.; Skripnichenko, R.; Ullah, S.; et al. Prediction of exposure-driven myelotoxicity of continuous infusion 5-fluorouracil by a semi-physiological pharmacokinetic-pharmacodynamic model in gastrointestinal cancer patients. Cancer Chemother. Pharm. 2020, 85, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Delea, T.E.; Vera-Llonch, M.; Edelsberg, J.S.; McGarry, L.; Anton, S.; Ulcickas-Yood, M.; Oster, G. The incidence and cost of hospitalization for 5-FU toxicity among Medicare beneficiaries with metastatic colorectal cancer. Value Health 2002, 5, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Randall, T.D.; Weissman, I.L. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood 1997, 89, 3596–3606. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, Z.; Wang, H.; Ma, W.; Zhou, C.; Zhang, S. Repeated cycles of 5-fluorouracil chemotherapy impaired anti-tumor functions of cytotoxic T cells in a CT26 tumor-bearing mouse model. BMC Immunol. 2016, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D. In vitro data supporting interferon plus cytotoxic agent combinations. Semin. Oncol. 1991, 18, 58–61. [Google Scholar] [PubMed]
- Schwartz, E.L.; Hoffman, M.; O’Connor, C.J.; Wadler, S. Stimulation of 5-fluorouracil metabolic activation by interferon-alpha in human colon carcinoma cells. Biochem. Biophys. Res. Commun. 1992, 182, 1232–1239. [Google Scholar] [CrossRef]
- Van der Wilt, C.L.; Smid, K.; Aherne, G.W.; Noordhuis, P.; Peters, G.J. Biochemical mechanisms of interferon modulation of 5-fluorouracil activity in colon cancer cells. Eur. J. Cancer 1997, 33, 471–478. [Google Scholar] [CrossRef]
- Grem, J.L.; McAtee, N.; Murphy, R.F.; Balis, F.M.; Steinberg, S.M.; Hamilton, J.M.; Sorensen, J.M.; Sartor, O.; Kramer, B.S.; Goldstein, L.J.; et al. A pilot study of interferon alfa-2a in combination with fluorouracil plus high-dose leucovorin in metastatic gastrointestinal carcinoma. J. Clin. Oncol. 1991, 9, 1811–1820. [Google Scholar] [CrossRef]
- Yee, L.K.; Allegra, C.J.; Steinberg, S.M.; Grem, J.L. Decreased catabolism of fluorouracil in peripheral blood mononuclear cells during combination therapy with fluorouracil, leucovorin, and interferon alpha-2a. J. Natl. Cancer Inst. 1992, 84, 1820–1825. [Google Scholar] [CrossRef]
- Raderer, M.; Scheithauer, W. Treatment of advanced colorectal cancer with 5-fluorouracil and interferon-alpha: An overview of clinical trials. Eur. J. Cancer 1995, 31A, 1002–1008. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Goncalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Aquino, A.; Prete, S.P.; Greiner, J.W.; Giuliani, A.; Graziani, G.; Turriziani, M.; De Filippi, R.; Masci, G.; Bonmassar, E.; De Vecchis, L. Effect of the combined treatment with 5-fluorouracil, gamma-interferon or folinic acid on carcinoembryonic antigen expression in colon cancer cells. Clin. Cancer Res. 1998, 4, 2473–2481. [Google Scholar]
- Wiesenfeld, M.; O’Connell, M.J.; Wieand, H.S.; Gonchoroff, N.J.; Donohue, J.H.; Fitzgibbons, R.J., Jr.; Krook, J.E.; Mailliard, J.A.; Gerstner, J.B.; Pazdur, R. Controlled clinical trial of interferon-gamma as postoperative surgical adjuvant therapy for colon cancer. J. Clin. Oncol 1995, 13, 2324–2329. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Park, Y.M.; Kim, N.Y.; Yun, H.K.; Lee, K.J.; Kim, B.H.; Park, S.J.; Yeon, J.W.; Jung, G. Combination treatment with intrahepatic arterial infusion and intratumoral injection chemotherapy in patients with far-advanced hepatocellular carcinoma and arterioportal or arteriovenous shunts: Preliminary results. Korean J. Hepatol. 2011, 17, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.X.; Auh, S.L. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011, 71, 2488–2496. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Zhang, W.Y.; Tan, N.Y.; Khatoo, M.; Suter, M.A.; Tripathi, S.; Cheung, F.S.; Lim, W.K.; Tan, P.H.; Ngeow, J.; et al. The DNA Structure-Specific Endonuclease MUS81 Mediates DNA Sensor STING-Dependent Host Rejection of Prostate Cancer Cells. Immunity 2016, 44, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Regairaz, M.; Zhang, Y.W.; Fu, H.; Agama, K.K.; Tata, N.; Agrawal, S.; Aladjem, M.I.; Pommier, Y. Mus81-mediated DNA cleavage resolves replication forks stalled by topoisomerase I-DNA complexes. J. Cell Biol. 2011, 195, 739–749. [Google Scholar] [CrossRef]
- Li, T.; Cheng, H.; Yuan, H.; Xu, Q.; Shu, C.; Zhang, Y.; Xu, P.; Tan, J.; Rui, Y.; Li, P.; et al. Antitumor Activity of cGAMP via Stimulation of cGAS-cGAMP-STING-IRF3 Mediated Innate Immune Response. Sci. Rep. 2016, 6, 19049. [Google Scholar] [CrossRef]
- Benmerzoug, S.; Rose, S.; Bounab, B.; Gosset, D.; Duneau, L.; Chenuet, P.; Mollet, L.; Le Bert, M.; Lambers, C.; Geleff, S.; et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat. Commun. 2018, 9, 5226. [Google Scholar] [CrossRef]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef]
- Klarquist, J.; Hennies, C.M.; Lehn, M.A.; Reboulet, R.A.; Feau, S.; Janssen, E.M. STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J. Immunol. 2014, 193, 6124–6134. [Google Scholar] [CrossRef]
- Lemos, H.; Huang, L.; McGaha, T.L.; Mellor, A.L. Cytosolic DNA sensing via the stimulator of interferon genes adaptor: Yin and Yang of immune responses to DNA. Eur. J. Immunol. 2014, 44, 2847–2853. [Google Scholar] [CrossRef] [PubMed]
- Lemos, H.; Mohamed, E.; Huang, L.; Ou, R.; Pacholczyk, G.; Arbab, A.S.; Munn, D.; Mellor, A.L. STING Promotes the Growth of Tumors Characterized by Low Antigenicity via IDO Activation. Cancer Res. 2016, 76, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Jain, C.; Velcheti, V. Role of immune-checkpoint inhibitors in lung cancer. Ther. Adv. Respir. Dis. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Zaanan, A.; Sinicrope, F.A. Microsatellite instability testing and its role in the management of colorectal cancer. Curr. Treat. Options Oncol. 2015, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Klempner, S.J.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Kang, W.K.; Kim, K.M.; Lee, J. Correlating programmed death ligand 1 (PD-L1) expression, mismatch repair deficiency, and outcomes across tumor types: Implications for immunotherapy. Oncotarget 2017, 8, 77415–77423. [Google Scholar] [CrossRef] [PubMed]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef]
- Fabrizio, D.A.; George, T.J., Jr.; Dunne, R.F.; Frampton, G.; Sun, J.; Gowen, K.; Kennedy, M.; Greenbowe, J.; Schrock, A.B.; Hezel, A.F.; et al. Beyond microsatellite testing: Assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J. Gastrointest. Oncol. 2018, 9, 610–617. [Google Scholar] [CrossRef]
- Van Der Kraak, L.; Goel, G.; Ramanan, K.; Kaltenmeier, C.; Zhang, L.; Normolle, D.P.; Freeman, G.J.; Tang, D.; Nason, K.S.; Davison, J.M.; et al. 5-Fluorouracil upregulates cell surface B7-H1 (PD-L1) expression in gastrointestinal cancers. J. Immunother. Cancer 2016, 4, 65. [Google Scholar] [CrossRef]
- Bedi, D.; Henderson, H.J.; Manne, U.; Samuel, T. Camptothecin Induces PD-L1 and Immunomodulatory Cytokines in Colon Cancer Cells. Medicines 2019, 6, 51. [Google Scholar] [CrossRef]
- Grasselly, C.; Denis, M.; Bourguignon, A.; Talhi, N.; Mathe, D.; Tourette, A.; Serre, L.; Jordheim, L.P.; Matera, E.L.; Dumontet, C. The Antitumor Activity of Combinations of Cytotoxic Chemotherapy and Immune Checkpoint Inhibitors Is Model-Dependent. Front. Immunol. 2018, 9, 2100. [Google Scholar] [CrossRef]
- Dosset, M.; Vargas, T.R.; Lagrange, A.; Boidot, R.; Vegran, F.; Roussey, A.; Chalmin, F.; Dondaine, L.; Paul, C.; Lauret Marie-Joseph, E.; et al. PD-1/PD-L1 pathway: An adaptive immune resistance mechanism to immunogenic chemotherapy in colorectal cancer. Oncoimmunology 2018, 7, e1433981. [Google Scholar] [CrossRef] [PubMed]
- Tintelnot, J.; Stein, A. Immunotherapy in colorectal cancer: Available clinical evidence, challenges and novel approaches. World J. Gastroenterol. 2019, 25, 3920–3928. [Google Scholar] [CrossRef] [PubMed]
- Shahda, S.; Noonan, A.M.; Bekaii-Saab, T.S.; O’Neil, B.H.; Sehdev, A.; Shaib, W.L.; Helft, P.R.; Loehrer, P.J.; Tong, Y.; Liu, Z.; et al. A phase II study of pembrolizumab in combination with mFOLFOX6 for patients with advanced colorectal cancer. J. Clin. Oncol. 2017, 35 (Suppl. S15), 3541. [Google Scholar] [CrossRef]
- Kim, R.; Chaves, J.; Kavan, P.; Fakih, M.; Kortmansky, J.; Spencer, K.; Wong, L.; Tehfe, M.; Li, J.J.; Lee, M.; et al. Pembrolizumab (pembro) plus mFOLFOX or FOLFIRI in patients with metastatic colorectal cancer (mCRC): KEYNOTE-651 cohorts B and D. Ann. Oncol. 2019, 30 (Suppl. S5), v229–v230. [Google Scholar] [CrossRef]
- Ensminger, W.D.; Rosowsky, A.; Raso, V.; Levin, D.C.; Glode, M.; Come, S.; Steele, G.; Frei, E., III. A clinical-pharmacological evaluation of hepatic arterial infusions of 5-fluoro-2’-deoxyuridine and 5-fluorouracil. Cancer Res. 1978; 38, 3784–3792. [Google Scholar]
- Ensminger, W.D. Intrahepatic arterial infusion of chemotherapy: Pharmacologic principles. Semin. Oncol. 2002, 29, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, W.H.; Willingham, M.C.; Bourland, J.D.; Hatcher, H.C.; Smith, T.L.; D’Agostino, R.B., Jr.; Blackstock, W. F10 Inhibits Growth of PC3 Xenografts and Enhances the Effects of Radiation Therapy. J. Clin. Oncol. Res. 2014, 2, 1028. [Google Scholar]
- Gmeiner, W.H.; Lema-Tome, C.; Gibo, D.; Jennings-Gee, J.; Milligan, C.; Debinski, W. Selective anti-tumor activity of the novel fluoropyrimidine polymer F10 towards G48a orthotopic GBM tumors. J. Neurooncol. 2014, 116, 447–454. [Google Scholar] [CrossRef][Green Version]
- Pardee, T.S.; Stadelman, K.; Jennings-Gee, J.; Caudell, D.L.; Gmeiner, W.H. The poison oligonucleotide F10 is highly effective against acute lymphoblastic leukemia while sparing normal hematopoietic cells. Oncotarget 2014, 5, 4170–4179. [Google Scholar] [CrossRef]
- Dominijanni, A.; Gmeiner, W.H. Improved potency of F10 relative to 5-fluorouracil in colorectal cancer cells with p53 mutations. Cancer Drug Resist. 2018, 1, 48–58. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gmeiner, W.H. Fluoropyrimidine Modulation of the Anti-Tumor Immune Response―Prospects for Improved Colorectal Cancer Treatment. Cancers 2020, 12, 1641. https://doi.org/10.3390/cancers12061641
Gmeiner WH. Fluoropyrimidine Modulation of the Anti-Tumor Immune Response―Prospects for Improved Colorectal Cancer Treatment. Cancers. 2020; 12(6):1641. https://doi.org/10.3390/cancers12061641
Chicago/Turabian StyleGmeiner, William H. 2020. "Fluoropyrimidine Modulation of the Anti-Tumor Immune Response―Prospects for Improved Colorectal Cancer Treatment" Cancers 12, no. 6: 1641. https://doi.org/10.3390/cancers12061641
APA StyleGmeiner, W. H. (2020). Fluoropyrimidine Modulation of the Anti-Tumor Immune Response―Prospects for Improved Colorectal Cancer Treatment. Cancers, 12(6), 1641. https://doi.org/10.3390/cancers12061641





