PTEN Protein Loss and Loss-of-Function Mutations in Gastric Cancers: The Relationship with Microsatellite Instability, EBV, HER2, and PD-L1 Expression
Abstract
:1. Introduction
2. Results
2.1. Clinicopathological Characteristics of Patients with PTEN-Mutated GC
2.2. Clinicopathologic Characteristics of PTEN Wild-Type GC
2.3. PTEN Mutations in Relationship with PTEN Protein Loss, MSI, EBV, and PD-L1 Expression
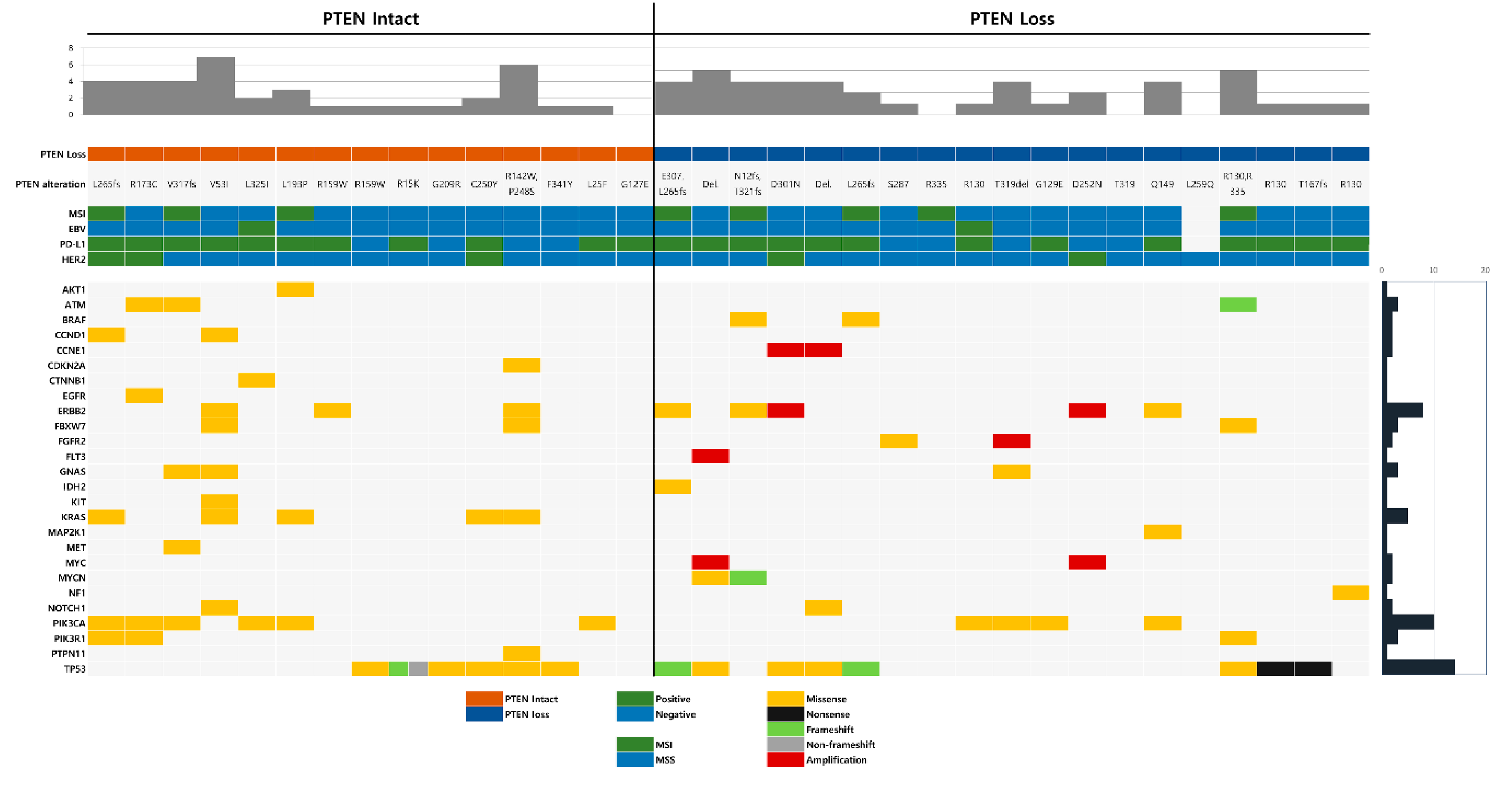
2.4. Co-Occurring Genetic Alterations Associated with PTEN Mutation
3. Discussion
4. Materials and Methods
4.1. Patients and Data Collection
4.2. Cancer Panel Test with NGS
4.3. Immunohistochemistry
4.4. MSI Test
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pellegrino, B.; Mateo, J.; Serra, V.; Balmana, J. Controversies in oncology: Are genomic tests quantifying homologous recombination repair deficiency (HRD) useful for treatment decision making? ESMO Open 2019, 4, e000480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, M.; He, Y.Y. PTEN in DNA damage repair. Cancer Lett. 2012, 319, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.T.; Yang, Z.; Lu, N.H. Roles of PTEN (Phosphatase and Tensin Homolog) in gastric cancer development and progression. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Bilbao, C.; Rodriguez, G.; Ramirez, R.; Falcon, O.; Leon, L.; Chirino, R.; Rivero, J.F.; Falcon, O., Jr.; Diaz-Chico, B.N.; Diaz-Chico, J.C.; et al. The relationship between microsatellite instability and PTEN gene mutations in endometrial cancer. Int. J. Cancer 2006, 119, 563–570. [Google Scholar] [CrossRef]
- Kanamori, Y.; Kigawa, J.; Itamochi, H.; Shimada, M.; Takahashi, M.; Kamazawa, S.; Sato, S.; Akeshima, R.; Terakawa, N. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin. Cancer Res. 2001, 7, 892–895. [Google Scholar] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Genome Atlas Research, N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kuang, L.G.; Zheng, H.C.; Li, J.Y.; Wu, D.Y.; Zhang, S.M.; Xin, Y. PTEN encoding product: A marker for tumorigenesis and progression of gastric carcinoma. World J. Gastroenterol. 2003, 9, 35–39. [Google Scholar] [CrossRef]
- Zheng, H.C.; Chen, Y.; Kuang, L.G.; Yang, L.; Li, J.Y.; Wu, D.Y.; Zhang, S.M.; Xin, Y. Expression of PTEN-encoding product in different stages of carcinogenesis and progression of gastric carcinoma. Zhonghua Zhong Liu Za Zhi 2003, 25, 13–16. [Google Scholar]
- Bai, Z.G.; Ye, Y.J.; Shen, D.H.; Lu, Y.Y.; Zhang, Z.T.; Wang, S. PTEN expression and suppression of proliferation are associated with Cdx2 overexpression in gastric cancer cells. Int. J. Oncol. 2013, 42, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, H.; Song, L.; Gao, X.; Chang, W.; Qin, X. Immunohistochemical expression of mTOR negatively correlates with PTEN expression in gastric carcinoma. Oncol. Lett. 2012, 4, 1213–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Cui, J.; Zhang, C.H.; Yang, D.J.; Chen, J.H.; Zan, W.H.; Li, B.; Li, Z.; He, Y.L. High-expression of DJ-1 and loss of PTEN associated with tumor metastasis and correlated with poor prognosis of gastric carcinoma. Int. J. Med Sci. 2013, 10, 1689–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Tamura, G.; Tsuchiya, T.; Endoh, Y.; Sakata, K.; Motoyama, T.; Usuba, O.; Kimura, W.; Terashima, M.; Nishizuka, S.; et al. Analysis of genetic and epigenetic alterations of the PTEN gene in gastric cancer. Virchows Arch. 2002, 440, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Huang, T.J.; Chen, F.M.; Hsieh, M.C.; Lin, S.R.; Hou, M.F.; Hsieh, J.S. Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in advanced gastric carcinomas. Virchows Arch. 2003, 442, 437–443. [Google Scholar] [CrossRef]
- Wen, Y.G.; Wang, Q.; Zhou, C.Z.; Qiu, G.Q.; Peng, Z.H.; Tang, H.M. Mutation analysis of tumor suppressor gene PTEN in patients with gastric carcinomas and its impact on PI3K/AKT pathway. Oncol. Rep. 2010, 24, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.M.; Araujo, J.J.; Harada, M.L.; Assumpcao, P.P.; Burbano, R.R.; Casartelli, C. Molecular study of the tumour suppressor gene PTEN in gastric adenocarcinoma in Brazil. Clin. Exp. Med. 2005, 5, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.G.; Chen, Y.J.; Perng, L.I.; Wang, N.M.; Kao, M.C.; Yang, T.Y.; Chang, C.P.; Tsai, C.H. Mutation analysis of the PTEN/MMAC1 gene in cancers of the digestive tract. Eur. J. Cancer 1999, 35, 647–651. [Google Scholar] [CrossRef]
- Forbes, S.A.; Beare, D.; Boutselakis, H.; Bamford, S.; Bindal, N.; Tate, J.; Cole, C.G.; Ward, S.; Dawson, E.; Ponting, L.; et al. COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017, 45, D777–D783. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.P.; Loukola, A.; Salovaara, R.; Nystrom-Lahti, M.; Peltomaki, P.; de la Chapelle, A.; Aaltonen, L.A.; Eng, C. PTEN mutational spectra, expression levels, and subcellular localization in microsatellite stable and unstable colorectal cancers. Am. J. Pathol. 2002, 161, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Guanti, G.; Resta, N.; Simone, C.; Cariola, F.; Demma, I.; Fiorente, P.; Gentile, M. Involvement of PTEN mutations in the genetic pathways of colorectal cancerogenesis. Hum. Mol. Genet. 2000, 9, 283–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Ye, Y.; Jiang, Q.; Chen, Y.; Lyu, X.; Li, J.; Wang, S.; Liu, T.; Cai, H.; Yao, K.; et al. Epstein-Barr virus-encoded microRNA BART1 induces tumour metastasis by regulating PTEN-dependent pathways in nasopharyngeal carcinoma. Nat. Commun. 2015, 6, 7353. [Google Scholar] [CrossRef] [Green Version]
- Hino, R.; Uozaki, H.; Murakami, N.; Ushiku, T.; Shinozaki, A.; Ishikawa, S.; Morikawa, T.; Nakaya, T.; Sakatani, T.; Takada, K.; et al. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res. 2009, 69, 2766–2774. [Google Scholar] [CrossRef] [Green Version]
- Dillon, L.M.; Miller, T.W. Therapeutic targeting of cancers with loss of PTEN function. Curr. Drug Targets 2014, 15, 65–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Cerniglia, M.; Xiu, J.; Grothey, A.; Pishvaian, M.J.; Hwang, J.J.; Marshall, J.; Vanderwalde, A.M.; Shields, A.F.; Lenz, H.-J.; Salem, M.E.; et al. Association of DNA damage response and repair genes (DDR) mutations and microsatellite instability (MSI), PD-L1 expression, tumor mutational burden (TMB) in gastroesophageal cancers. J. Clin. Oncol. 2019, 37, 60. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Jing, C.; Chang, X.; Ding, D.; Han, T.; Yang, J.; Lu, Z.; Hu, X.; Liu, Z.; Wang, J.; et al. Mutational landscape of gastric cancer and clinical application of genomic profiling based on target next-generation sequencing. J. Transl. Med. 2019, 17, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouw, K.W.; Goldberg, M.S.; Konstantinopoulos, P.A.; D’Andrea, A.D. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov. 2017, 7, 675–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, M.Y.; Seier, K.; Ostrovnaya, I.; Regazzi, A.M.; Kania, B.E.; Moran, M.M.; Cipolla, C.K.; Bluth, M.J.; Chaim, J.; Al-Ahmadie, H.; et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. J. Clin. Oncol. 2018, 36, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qin, X.; Fei, M.; Hou, W.; Greshock, J.; Bachman, K.E.; Kang, J.; Qin, C.Y. Loss and reduced expression of PTEN correlate with advanced-stage gastric carcinoma. Exp. Ther. Med. 2013, 5, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.H.; Lee, H.S.; Kim, W.H. Promoter methylation and silencing of PTEN in gastric carcinoma. Lab. Investig. 2002, 82, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Oda, K.; Stokoe, D.; Taketani, Y.; McCormick, F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005, 65, 10669–10673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Hong, M.; Kim, S.T.; Park, S.H.; Kang, W.K.; Kim, K.M.; Lee, J. The impact of concomitant genomic alterations on treatment outcome for trastuzumab therapy in HER2-positive gastric cancer. Sci. Rep. 2015, 5, 9289. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Lee, C.K.; Chon, H.J.; Kim, J.H.; Park, H.S.; Heo, S.J.; Kim, H.J.; Kim, T.S.; Kwon, W.S.; Chung, H.C.; et al. PTEN loss and level of HER2 amplification is associated with trastuzumab resistance and prognosis in HER2-positive gastric cancer. Oncotarget 2017, 8, 113494–113501. [Google Scholar] [CrossRef] [Green Version]
- Deguchi, Y.; Okabe, H.; Oshima, N.; Hisamori, S.; Minamiguchi, S.; Muto, M.; Sakai, Y. PTEN loss is associated with a poor response to trastuzumab in HER2-overexpressing gastroesophageal adenocarcinoma. Gastric Cancer 2017, 20, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linos, K.; Sheehan, C.E.; Ross, J.S. Correlation of HER2 and PTEN status with clinical outcome in esophageal (E), gastric (G), and gastroesophageal junction (GEJ) adenocarcinomas (ACs). J. Clin. Oncol. 2011, 29, 4066. [Google Scholar] [CrossRef]
- Lin, Y.K.; Fang, Z.; Jiang, T.Y.; Wan, Z.H.; Pan, Y.F.; Ma, Y.H.; Shi, Y.Y.; Tan, Y.X.; Dong, L.W.; Zhang, Y.J.; et al. Combination of Kras activation and PTEN deletion contributes to murine hepatopancreatic ductal malignancy. Cancer Lett. 2018, 421, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.J.; Marsh Durban, V.; Meniel, V.; Williams, G.T.; Clarke, A.R. PTEN loss and KRAS activation leads to the formation of serrated adenomas and metastatic carcinoma in the mouse intestine. J. Pathol. 2014, 233, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, T.; Brozick, J.; Babalola, K.; Budiu, R.; Tseng, G.; Vlad, A.M. Effects of Kras activation and Pten deletion alone or in combination on MUC1 biology and epithelial-to-mesenchymal transition in ovarian cancer. Oncogene 2016, 35, 5010–5020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piro, G.; Carbone, C.; Carbognin, L.; Pilotto, S.; Ciccarese, C.; Iacovelli, R.; Milella, M.; Bria, E.; Tortora, G. Revising PTEN in the Era of Immunotherapy: New Perspectives for an Old Story. Cancers 2019, 11, 1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruschoff, J.; Hanna, W.; Bilous, M.; Hofmann, M.; Osamura, R.Y.; Penault-Llorca, F.; van de Vijver, M.; Viale, G. HER2 testing in gastric cancer: A practical approach. Mod. Pathol. 2012, 25, 637–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulangara, K.; Hanks, D.A.; Waldroup, S.; Peltz, L.; Shah, S.; Roach, C.; Juco, J.W.; Emancipator, K.; Stanforth, D. Development of the combined positive score (CPS) for the evaluation of PD-L1 in solid tumors with the immunohistochemistry assay PD-L1 IHC 22C3 pharmDx. J. Clin. Oncol. 2017, 35, e14589. [Google Scholar] [CrossRef]

| Case | Age | Sex | Specimen | Histology | HER2 | EBV | MSI | PD-L1 CPS (≥1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 70 | M | Stomach, biopsy | TADC, WD | - | - | MSS | - |
| 2 | 58 | M | Stomach, biopsy | TADC, MD | - | - | MSI | - |
| 3 | 47 | F | Stomach, biopsy | TADC, PD | - | - | MSS | + |
| 4 | 65 | M | Stomach, resection | TADC, PD | - | + | MSS | + |
| 5 | 50 | F | Stomach, resection | TADC, MD | - | - | MSS | - |
| 6 | 53 | F | Stomach, biopsy | TADC, PD | - | - | MSS | + |
| 7 | 69 | M | Stomach, biopsy | TADC, MD | - | - | MSS | - |
| 8 | 61 | M | Stomach, resection | TADC, PD | - | - | MSS | - |
| 9 | 85 | M | Stomach, biopsy | TADC, PD | - | - | MSS | + |
| 10 | 69 | M | Stomach, resection | TADC, MD | + | - | MSS | + |
| 11 | 66 | F | Stomach, resection | TADC, MD | + | - | MSS | - |
| 12 | 41 | M | Stomach, resection | TADC, PD | - | - | MSS | - |
| 13 | 64 | F | Stomach, resection | TADC, PD | - | - | MSS | - |
| 14 | 64 | M | Stomach, biopsy | TADC, PD | - | - | MSS | + |
| 15 | 47 | M | Stomach, biopsy | TADC, PD | - | NA | NA | NA |
| 16 | 51 | M | Stomach, biopsy | TADC, PD | - | - | MSS | - |
| 17 | 56 | F | Stomach, biopsy | TADC, PD | - | - | MSS | + |
| 18 | 79 | F | Stomach, resection | TADC, MD | - | - | MSI | + |
| 19 | 77 | M | Stomach, biopsy | TADC, PD | - | - | MSS | + |
| 20 | 59 | M | Stomach, biopsy | TADC, MD | - | - | MSS | + |
| 21 | 66 | F | Stomach, biopsy | TADC, PD | - | - | MSS | + |
| 22 | 40 | F | Stomach, biopsy | SRC | - | - | MSS | + |
| 23 | 61 | F | Stomach, resection | PADC, MD | + | - | MSI | + |
| 24 | 78 | M | Stomach, resection | TADC, PD | - | - | MSI | + |
| 25 | 53 | M | Stomach, resection | TADC, MD | + | - | MSS | + |
| 26 | 76 | F | Stomach, resection | TADC, MD | - | - | MSI | + |
| 27 | 54 | M | Stomach, resection | PADC, MD | - | - | MSS | + |
| 28 | 79 | M | Stomach, resection | TADC, MD | - | - | MSI | + |
| 29 | 70 | M | Stomach, resection | TADC, MD | - | - | MSS | + |
| 30 | 60 | M | Stomach, resection | TADC, PD | - | + | MSS | + |
| 31 | 56 | F | Stomach, resection | TADC, MD | - | - | MSI | + |
| 32 | 76 | M | Stomach, resection | TADC, MD | + | - | MSS | + |
| 33 | 74 | M | Stomach, resection | TADC, MD | - | - | MSS | + |
| 34 | 76 | M | Stomach, resection | TADC, MD | - | - | MSI | + |
| PTEN-Mutated (n = 34) | PTEN WT (n = 288) | p-Value | |
|---|---|---|---|
| Sex | 0.9805 | ||
| Male | 22 (64.7%) | 181 (62.8%) | |
| Female | 12 (35.3%) | 107 (37.2%) | |
| Age (median, range) | 64 (40–85) | 61 (29–85) | 0.175 |
| PTEN IHC | 5.232 × 10−10 | ||
| Intact | 15 (44.1%) | 253 (87.8%) | |
| loss | 19 (55.9%) | 35 (12.2%) | |
| MSI status | 3.936 × 10−8 | ||
| MSI | 8 (23.5%) | 9 (3.1%) | |
| MSS | 25 (73.5%) | 279 (96.9%) | |
| NA | 1 (3.0%) | 0 (0%) | |
| EBV | 0.0071 | ||
| positive | 2 (5.9%) | 7 (2.4%) | |
| negative | 31 (91.2%) | 281 (97.6%) | |
| NA | 1 (2.9%) | 0 (0%) | |
| HER2 | 0.7945 | ||
| positive | 5 (14.7%) | 39 (13.5%) | |
| negative | 29 (85.3%) | 249 (86.5%) | |
| PD-L1 CPS | 0.111 | ||
| ≥1 | 24 (70.6%) | 182 (63.2%) | |
| 0 | 9 (26.5%) | 105 (36.5%) | |
| NA | 1 (2.9%) | 1 (0.3%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Kang, S.Y.; Kim, D.; Heo, Y.J.; Kim, K.-M. PTEN Protein Loss and Loss-of-Function Mutations in Gastric Cancers: The Relationship with Microsatellite Instability, EBV, HER2, and PD-L1 Expression. Cancers 2020, 12, 1724. https://doi.org/10.3390/cancers12071724
Kim B, Kang SY, Kim D, Heo YJ, Kim K-M. PTEN Protein Loss and Loss-of-Function Mutations in Gastric Cancers: The Relationship with Microsatellite Instability, EBV, HER2, and PD-L1 Expression. Cancers. 2020; 12(7):1724. https://doi.org/10.3390/cancers12071724
Chicago/Turabian StyleKim, Binnari, So Young Kang, Deokgeun Kim, You Jeong Heo, and Kyoung-Mee Kim. 2020. "PTEN Protein Loss and Loss-of-Function Mutations in Gastric Cancers: The Relationship with Microsatellite Instability, EBV, HER2, and PD-L1 Expression" Cancers 12, no. 7: 1724. https://doi.org/10.3390/cancers12071724
APA StyleKim, B., Kang, S. Y., Kim, D., Heo, Y. J., & Kim, K.-M. (2020). PTEN Protein Loss and Loss-of-Function Mutations in Gastric Cancers: The Relationship with Microsatellite Instability, EBV, HER2, and PD-L1 Expression. Cancers, 12(7), 1724. https://doi.org/10.3390/cancers12071724





