Plasma Level of Circular RNA hsa_circ_0000190 Correlates with Tumor Progression and Poor Treatment Response in Advanced Lung Cancers
Abstract
:1. Introduction
2. Results
2.1. Identification of Potential Secretory circRNA Biomarker Candidates Using RNA-Seq
2.2. Validation of Expression of hsa_circ_0000190 and hsa_circ_0001649 in LC Cell Lines
2.3. RT-ddPCR Detection of hsa_circ_0000190 and hsa_circ_0001649 Secreted by LC Cell Lines and in Human Blood Plasma
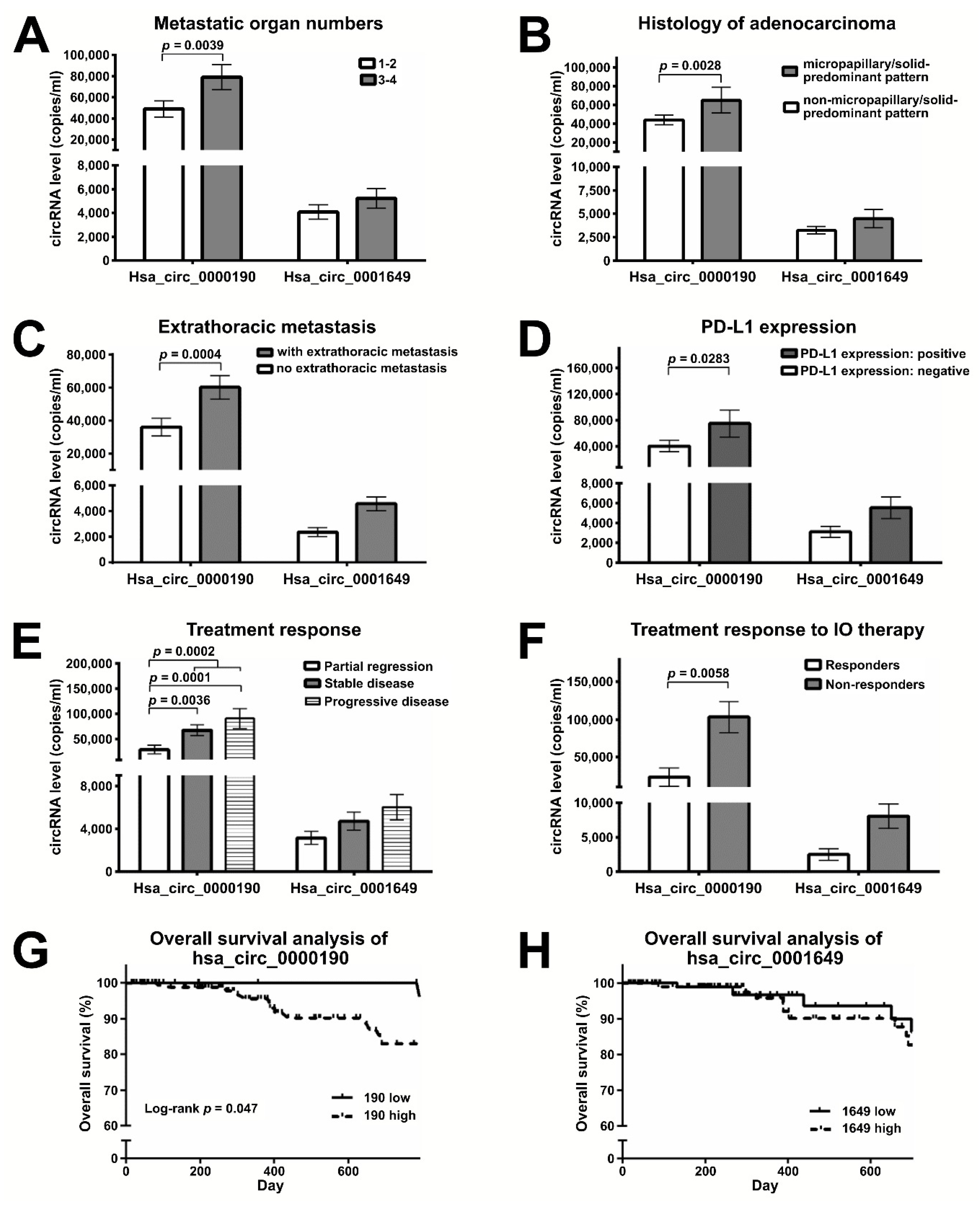
2.4. Expression of hsa_circ_0000190 and hsa_circ_0001649 in LC Patients with Different Stages and Tumor Sizes
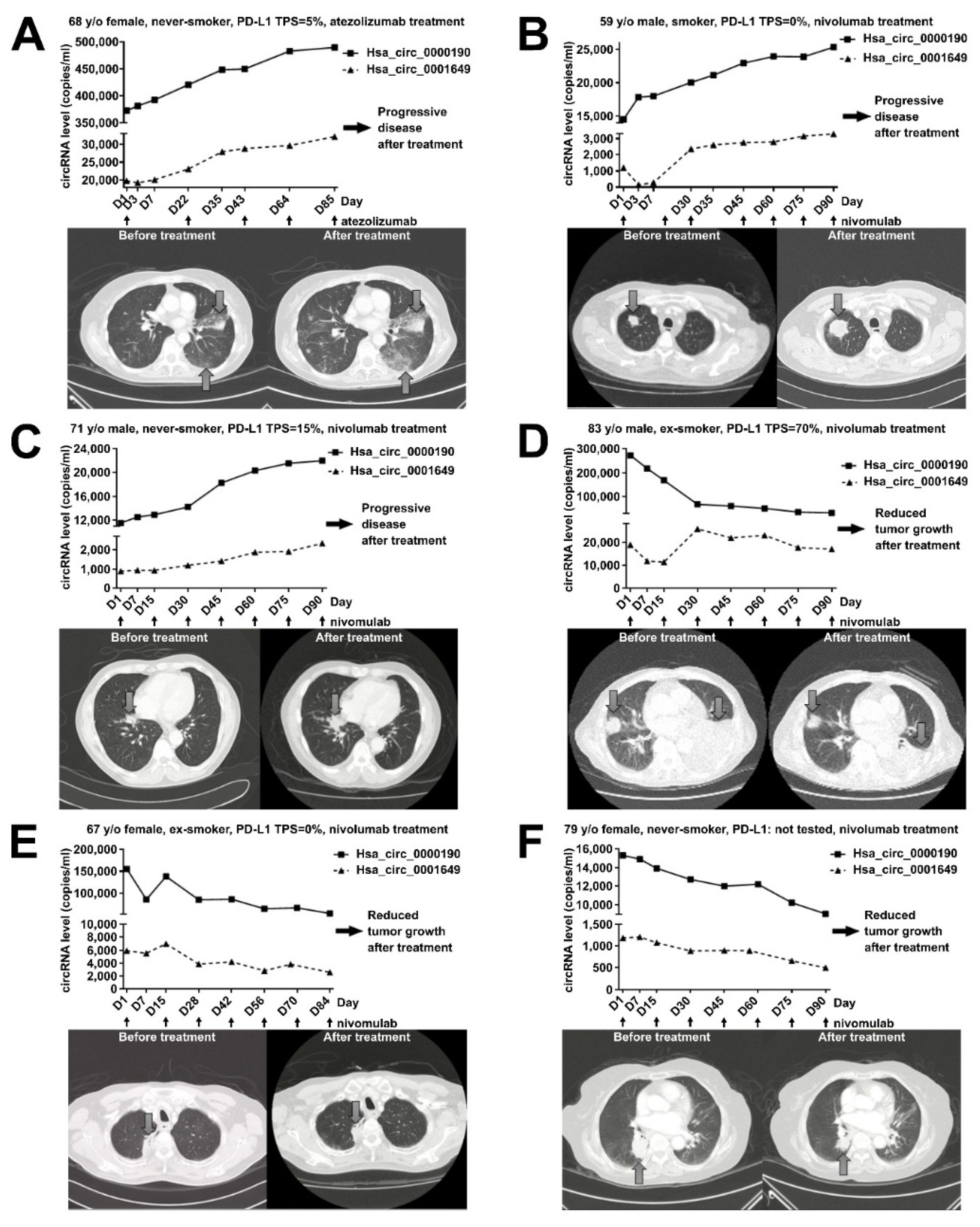
2.5. Plasma Levels of Hsa_circ_0000190 Negatively Correlate with the Response to Immunotherapy
2.6. Monitoring the Treatment Response by Plasma Levels of circRNAs in LC Patients Receiving Immunotherapy
2.7. Bioinformatics Analysis of Potential Downstream Network for Hsa_circ_0000190 and Hsa_circ_0001649 in LC
3. Discussion
4. Methods
4.1. Patient Population
4.2. Cell Culture
4.3. RNA Isolation and qRT-PCR
4.4. qRT-PCT and RT-ddPCR for Detecting Plasma circRNA
4.5. RNA-Seq
4.6. Evaluation of LC Treatment Efficacy
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ettinger, D.S.; Akerley, W.; Borghaei, H.; Chang, A.C.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Ganti, A.K.; Govindan, R.; et al. Non-small cell lung cancer. J. Natl. Compr. Canc. Netw. 2012, 10, 1236–1271. [Google Scholar] [CrossRef]
- Tan, C.-S.; Gilligan, D.; Pacey, S. Treatment approaches for egfr-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol. 2015, 16, e447–e459. [Google Scholar] [CrossRef]
- Hanna, N.; Johnson, D.; Temin, S.; Baker, S., Jr.; Brahmer, J.; Ellis, P.M.; Giaccone, G.; Hesketh, P.J.; Jaiyesimi, I.; Leighl, N.B.; et al. Systemic therapy for stage iv non-small-cell lung cancer: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 2017, 35, 3484–3515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M. Update of epidermal growth factor receptor-tyrosine kinase inhibitors in non-small-cell lung cancer. J. Chin. Med. Assoc. JCMA 2013, 76, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.M.; Whang-Peng, J.; Chen, C.M. First-line systemic therapy for metastatic non-small-cell lung cancer—A review. J. Exp. Clin. Med. 2011, 3, 116–120. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [Green Version]
- Ribas, A. Tumor immunotherapy directed at pd-1. N. Engl. J. Med. 2012, 366, 2517–2519. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.H.; Ho, H.L.; Tsai, C.M.; Shih, J.F.; Chiu, C.H.; Lai, S.L.; Lee, Y.C.; Perng, R.P.; Whang-Peng, J.; Chou, T.Y.; et al. The association between tumor epidermal growth factor receptor (egfr) mutation and multiple primary malignancies in patients with adenocarcinoma of the lungs. Am. J. Clin. Oncol. 2015, 38, 147–151. [Google Scholar] [CrossRef]
- Kramer, M.C.; Liang, D.; Tatomer, D.C.; Gold, B.; March, Z.M.; Cherry, S.; Wilusz, J.E. Combinatorial control of drosophila circular rna expression by intronic repeats, hnrnps, and sr proteins. Genes Dev. 2015. [Google Scholar] [CrossRef] [Green Version]
- Su, M.; Xiao, Y.; Ma, J.; Tang, Y.; Tian, B.; Zhang, Y.; Li, X.; Wu, Z.; Yang, D.; Zhou, Y.; et al. Circular rnas in cancer: Emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol. Cancer 2019, 18, 90. [Google Scholar] [CrossRef]
- Zong, L.; Sun, Q.; Zhang, H.; Chen, Z.; Deng, Y.; Li, D.; Zhang, L. Increased expression of circrna_102231 in lung cancer and its clinical significance. Biomed. Pharmacother. 2018, 102, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; He, D.; Peng, Z.; Peng, W.; Shi, W.; Wang, J.; Li, B.; Zhang, C.; Duan, C. Circular RNAs in cancer: An emerging key player. J. Hematol. Oncol. 2017, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Zhang, M.; Zheng, X.; Yi, P.; Lan, C.; Xu, M. The circular rna cirs-7 (cdr1as) acts as a risk factor of hepatic microvascular invasion in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 17–27. [Google Scholar] [CrossRef]
- Greene, J.; Baird, A.M.; Brady, L.; Lim, M.; Gray, S.G.; McDermott, R.; Finn, S.P. Circular RNAs: Biogenesis, function and role in human diseases. Front. Mol. Biosci. 2017, 4, 38. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal rnas with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Xu, T.; Wu, J.; Han, P.; Zhao, Z.; Song, X. Circular RNA expression profiles and features in human tissues: A study using RNA-seq data. BMC Genom. 2017, 18, 680. [Google Scholar] [CrossRef]
- Li, T.; Shao, Y.; Fu, L.; Xie, Y.; Zhu, L.; Sun, W.; Yu, R.; Xiao, B.; Guo, J. Plasma circular RNA profiling of patients with gastric cancer and their droplet digital rt-pcr detection. J. Mol. Med. 2018, 96, 85–96. [Google Scholar] [CrossRef]
- Zheng, M.; Hou, L.; Ma, Y.; Zhou, L.; Wang, F.; Cheng, B.; Wang, W.; Lu, B.; Liu, P.; Lu, W.; et al. Exosomal let-7d-3p and mir-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Mol. Cancer 2019, 18, 76. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Lee, J.H.; Diefenbach, R.J.; Kefford, R.F.; Rizos, H. Liquid biomarkers in melanoma: Detection and discovery. Mol. Cancer 2018, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.C.; Jiang, P.; Zheng, Y.W.; Liao, G.J.; Sun, H.; Wong, J.; Siu, S.S.; Chan, W.C.; Chan, S.L.; Chan, A.T.; et al. Cancer genome scanning in plasma: Detection of tumor-associated copy number aberrations, single-nucleotide variants, and tumoral heterogeneity by massively parallel sequencing. Clin. Chem. 2013, 59, 211–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hench, I.B.; Hench, J.; Tolnay, M. Liquid biopsy in clinical management of breast, lung, and colorectal cancer. Front. Med. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Asmitananda, T.; Gao, L.; Gai, D.; Song, Z.; Zhang, Y.; Ren, H.; Yang, T.; Chen, T.; Chen, M. Biomarkers in the lung cancer diagnosis: A clinical perspective. Neoplasma 2012, 59, 500–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.H.; Tseng, P.C.; Lee, Y.C.; Perng, R.P.; Whang-Peng, J.; Chen, Y.M. A prospective study of the use of circulating markers as predictors for epidermal growth factor receptor-tyrosine kinase inhibitor treatment in pulmonary adenocarcinoma. Cancer Biomark. Sect. A Dis. Markers 2016, 16, 19–29. [Google Scholar] [CrossRef]
- Yao, J.T.; Zhao, S.H.; Liu, Q.P.; Lv, M.Q.; Zhou, D.X.; Liao, Z.J.; Nan, K.J. Over-expression of circrna_100876 in non-small cell lung cancer and its prognostic value. Pathol. Res. Pract. 2017, 213, 453–456. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Wei, S.; Chen, Y.; Chen, Y.; Fan, X.; Han, S.; Wu, G. Hsa_circ_0013958: A circular RNA and potential novel biomarker for lung adenocarcinoma. FEBS J. 2017, 284, 2170–2182. [Google Scholar] [CrossRef]
- Olmedillas-Lopez, S.; Garcia-Arranz, M.; Garcia-Olmo, D. Current and emerging applications of droplet digital pcr in oncology. J. Mol. Diagn. 2017, 21, 493–510. [Google Scholar] [CrossRef]
- Zhang, X.L.; Xu, L.L.; Wang, F. Hsa_circ_0020397 regulates colorectal cancer cell viability, apoptosis and invasion by promoting the expression of the mir-138 targets tert and pd-l1. Cell Biol. Int. 2017, 41, 1056–1064. [Google Scholar] [CrossRef]
- Dragomir, M.; Calin, G.A. Circular RNAs in cancer—Lessons learned from micrornas. Front. Oncol. 2018, 8, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Liu, F.B.; Huang, M.; Xie, K.; Xie, Q.S.; Liu, C.H.; Shen, M.J.; Huang, Q. Circular RNA cirs-7 promotes the proliferation and metastasis of pancreatic cancer by regulating mir-7-mediated egfr/stat3 signaling pathway. Hepatobiliary Pancreat. Dis. Int. HBPD Int. 2019, 18, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, G.; Zhou, X.; Song, X.; Gao, H.; Ma, C.; Chang, H.; Li, H.; Liu, F.F.; Lu, J.; et al. Mir-1299 suppresses cell proliferation of hepatocellular carcinoma (hcc) by targeting cdk6. Biomed. Pharmacother. 2016, 83, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Meng, L.; Liu, S.; Ding, P.; Chang, S.; Ju, Y.; Liu, F.; Gu, L.; Lian, Y.; Geng, C. Circular RNA cirs-7 maintains metastatic phenotypes as a cerna of mir-1299 to target mmps. Mol. Cancer Res. 2018, 16, 1665–1675. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lu, Z.; Wang, N.; Zhang, M.; Zeng, X.; Zhao, W. MicroRNA-1299 is a negative regulator of stat3 in colon cancer. Oncol. Rep. 2017, 37, 3227–3234. [Google Scholar] [CrossRef] [Green Version]
- Silveyra, P.; DiAngelo, S.L.; Floros, J. An 11-nt sequence polymorphism at the 3’utr of human sftpa1 and sftpa2 gene variants differentially affect gene expression levels and miRNA regulation in cell culture. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L106–L119. [Google Scholar] [CrossRef] [Green Version]
- Boeri, M.; Verri, C.; Conte, D.; Roz, L.; Modena, P.; Facchinetti, F.; Calabro, E.; Croce, C.M.; Pastorino, U.; Sozzi, G. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 3713–3718. [Google Scholar] [CrossRef] [Green Version]
- Castro, D.; Moreira, M.; Gouveia, A.M.; Pozza, D.H.; De Mello, R.A. MicroRNAs in lung cancer. Oncotarget 2017, 8, 81679. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Guan, Z.; Cuk, K.; Brenner, H.; Zhang, Y. Circulating microRNA biomarkers for lung cancer detection in western populations. Cancer Med. 2018, 7, 4849–4862. [Google Scholar] [CrossRef]
- Tian, F.; Yu, C.T.; Ye, W.D.; Wang, Q. Cinnamaldehyde induces cell apoptosis mediated by a novel circular RNA hsa_circ_0043256 in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1260–1266. [Google Scholar] [CrossRef]
- Chen, L.; Nan, A.; Zhang, N.; Jia, Y.; Li, X.; Ling, Y.; Dai, J.; Zhang, S.; Yang, Q.; Yi, Y.; et al. Circular rna 100146 functions as an oncogene through direct binding to mir-361-3p and mir-615-5p in non-small cell lung cancer. Mol. Cancer 2019, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.H.; Luo, L.; Wampfler, J.A.; Wang, Y.; Liu, D.; Chen, Y.M.; Adjei, A.A.; Midthun, D.E.; Yang, P. 5-year overall survival in patients with lung cancer eligible or ineligible for screening according to us preventive services task force criteria: A prospective, observational cohort study. Lancet Oncol. 2019, 20, 1098–1108. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Yokose, T.; Nakayama, H. Prognostic contribution of non-predominant solid and micropapillary components in lung adenocarcinomas. J. Thorac. Dis. 2017, 9, 504–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.T.; Luo, Y.H.; Chien, C.S.; Wu, C.H.; Tseng, P.C.; Chiou, S.H.; Lee, Y.C.; Whang-Peng, J.; Chen, Y.M. Induced pluripotent stem cell-conditioned medium suppressed melanoma tumorigenicity through the enhancement of natural-killer cellular immunity. J. Immunother. 2016, 39, 153–159. [Google Scholar] [CrossRef]
- Chen, Y.M. Immune checkpoint inhibitors for nonsmall cell lung cancer treatment. J. Chin. Med. Assoc. JCMA 2017, 80, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Chiou, S.H.; Wang, M.L.; Chou, Y.T.; Chen, C.J.; Hong, C.F.; Hsieh, W.J.; Chang, H.T.; Chen, Y.S.; Lin, T.W.; Hsu, H.S.; et al. Coexpression of oct4 and nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010, 70, 10433–10444. [Google Scholar] [CrossRef] [Green Version]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (oak): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Chu, Y.W.; Yang, P.C.; Yang, S.C.; Shyu, Y.C.; Hendrix, M.J.; Wu, R.; Wu, C.W. Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am. J. Respir. Cell Mol. Biol. 1997, 17, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, T.; Zhao, Q.; Xiao, B.; Guo, J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin. Chim. Acta Int. J. Clin. Chem. 2017, 466, 167–171. [Google Scholar] [CrossRef]
- Qin, M.; Liu, G.; Huo, X.; Tao, X.; Sun, X.; Ge, Z.; Yang, J.; Fan, J.; Liu, L.; Qin, W. Hsa_circ_0001649: A circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer Biomark. Sect. A Dis. Markers 2016, 16, 161–169. [Google Scholar] [CrossRef] [Green Version]





| □ | Total No. | p-Value | |
|---|---|---|---|
| Hsa_circ_ 0000190 | Hsa_circ_ 0001649 | ||
| Patient no. | 231 | <0.0001 * | 0.938 |
| Healthy controls | 41 | ||
| Gender (%) | |||
| Male | 156 (67.5) | 0.213 | >0.999 |
| Female | 75 (32.5) | ||
| Mean age (range, year) | 63.8 (35–90) | 0.552 | >0.999 |
| With smoking history | 110 | 0.922 | 0.997 |
| Performance status (ECOG) (%) | |||
| 0 | 118 (51.1) | 0.489 | 0.335 |
| 1 | 95 (41.1) | ||
| 2 | 18 (7.8) | ||
| Lung cancer stage (%) | |||
| I | 62 (26.8) | I-II vs. III-IV <0.0001 * | I-II vs. III-IV 0.924 |
| II | 3 (1.3) | ||
| IIIa | 11 (4.8) | ||
| IIIb | 11 (4.8) | ||
| IV | 144 (62.3) | ||
| Histology (%) | |||
| Adenocarcinoma | 195 (84.4) | 0.386 | 0.915 |
| Squamous cell carcinoma | 19 (8.2) | ||
| Non-adeno/non-sqcc NSCLC | 15 (6.5) | ||
| SCLC | 2 (0.9) | ||
| Primary tumor size (range, cm) | 3.1 (0.6–9.5) | ||
| Metastatic organs, mean (range) | 1 (0–4) | <0.0001 * | 0.899 |
| Extrathoracic metastasis | 125 (44.7) | 0.0004 * | 0.93 |
| Positive PD-L1 expression (negative) | 33 (56) | 0.0283 * | 0.981 |
| Immunotherapy | 50 | Responder vs. non-responder | |
| Pembrolizumab | 14 | 0.0058 * | 0.972 |
| Nivolumab | 24 | ||
| Atezolizumab | 9 | ||
| Durvalumab | 3 | ||
| Systemic treatment response | |||
| Complete remission (CR) | 0 | CR/PR vs. SD/PD 0.0002 * > 0.999 | |
| Partial remission (PR) | 37 | ||
| Stable disease (SD) | 69 | ||
| Progressive disease (PD) | 27 | ||
| EGFR mutation | EGFR mutation vs. wild type | ||
| with any mutation | 73 | 0.243 | 0.987 |
| with exon 19 deletion | 27 | ||
| with L858R mutation | 38 | ||
| Wild type | 95 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.-H.; Yang, Y.-P.; Chien, C.-S.; Yarmishyn, A.A.; Ishola, A.A.; Chien, Y.; Chen, Y.-M.; Huang, T.-W.; Lee, K.-Y.; Huang, W.-C.; et al. Plasma Level of Circular RNA hsa_circ_0000190 Correlates with Tumor Progression and Poor Treatment Response in Advanced Lung Cancers. Cancers 2020, 12, 1740. https://doi.org/10.3390/cancers12071740
Luo Y-H, Yang Y-P, Chien C-S, Yarmishyn AA, Ishola AA, Chien Y, Chen Y-M, Huang T-W, Lee K-Y, Huang W-C, et al. Plasma Level of Circular RNA hsa_circ_0000190 Correlates with Tumor Progression and Poor Treatment Response in Advanced Lung Cancers. Cancers. 2020; 12(7):1740. https://doi.org/10.3390/cancers12071740
Chicago/Turabian StyleLuo, Yung-Hung, Yi-Ping Yang, Chian-Shiu Chien, Aliaksandr A. Yarmishyn, Afeez Adekunle Ishola, Yueh Chien, Yuh-Min Chen, Tsai-Wang Huang, Kang-Yun Lee, Wen-Chien Huang, and et al. 2020. "Plasma Level of Circular RNA hsa_circ_0000190 Correlates with Tumor Progression and Poor Treatment Response in Advanced Lung Cancers" Cancers 12, no. 7: 1740. https://doi.org/10.3390/cancers12071740







