Y Chromosome Loss Is a Frequent Event in Barrett’s Adenocarcinoma and Associated with Poor Outcome
Abstract
1. Introduction
2. Results
2.1. Patient Samples and Correlation with Clinical Data
2.2. Y Chromosome Status in Cell Lines
2.3. Y Chromosome Status in the Patients’ Cohort
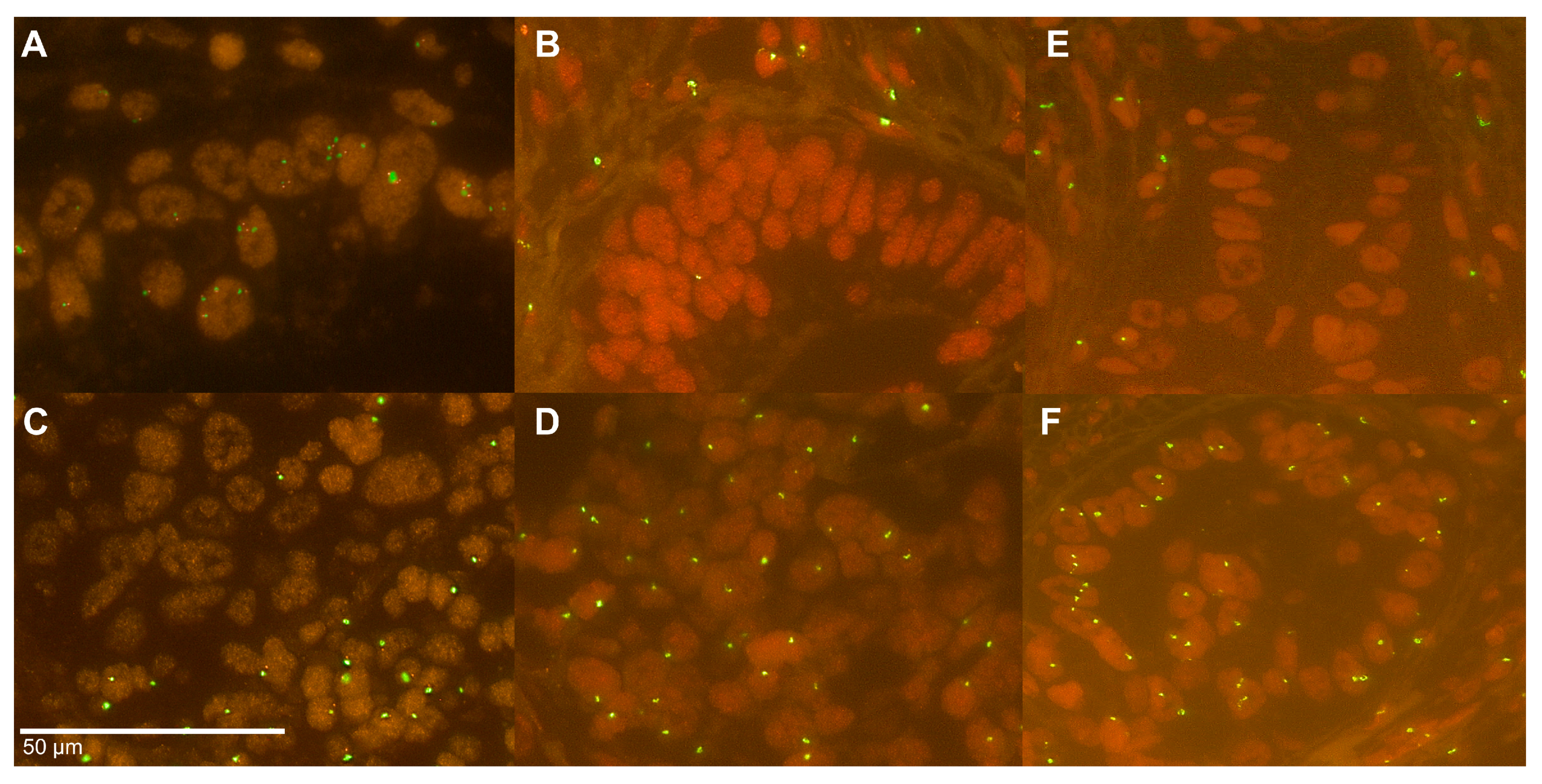
2.4. Heterogeneity of the Y Chromosome Status
2.5. Y Chromosome Status and Correlation with Immunohistochemical and Molecular Markers
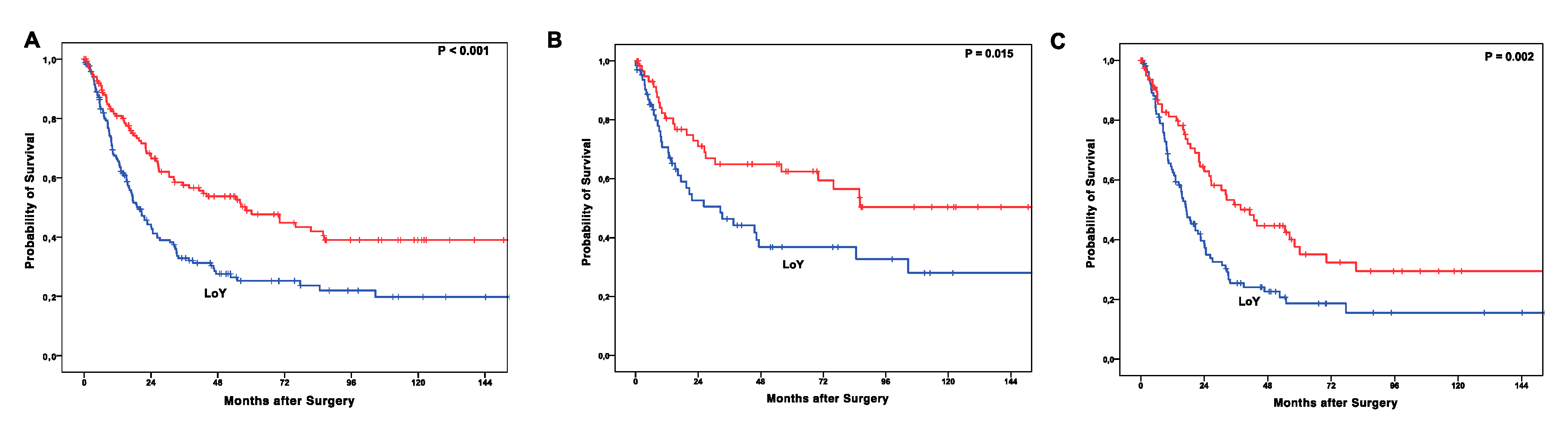
2.6. LoY Correlation to Patients’ Outcome
3. Discussion
4. Materials and Methods
4.1. Patients and Tumor Samples
4.2. Cell Culture
4.3. Formalin-Fixation and Paraffin-Embedding of Cell Line Pellets
4.4. TMA Construction
4.5. Fluorescence In Situ Hybridization
4.6. Analysis of Heterogeneity of Y Chromosome Status
4.7. Immunohistochemistry
4.8. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lepage, C.; Rachet, B.; Jooste, V.; Faivre, J.; Coleman, M.P. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am. J. Gastroenterol. 2008, 103, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Edgren, G.; Adami, H.O.; Weiderpass, E.; Nyren, O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut 2013, 62, 1406–1414. [Google Scholar] [CrossRef]
- Pohl, H.; Sirovich, B.; Welch, H.G. Esophageal adenocarcinoma incidence: Are we reaching the peak? Cancer Epidemiol. Biomarkers Prev. 2010, 19, 1468–1470. [Google Scholar] [CrossRef]
- Barrett, J.C. Mechanisms of multistep carcinogenesis and carcinogen risk assessment. Environ. Health Perspect 1993, 100, 9–20. [Google Scholar] [CrossRef]
- Koch-Institut, R. Krebs in Deutschland für 2013/2014. Available online: https://edoc.rki.de/handle/176904/3270 (accessed on 30 June 2020).
- Tustumi, F.; Kimura, C.M.; Takeda, F.R.; Uema, R.H.; Salum, R.A.; Ribeiro-Junior, U.; Cecconello, I. Prognostic Factors and Survival Analysis in Esophageal Carcinoma. Arq. Bras. Cir. Dig. 2016, 29, 138–141. [Google Scholar] [CrossRef]
- Sukocheva, O.A.; Li, B.; Due, S.L.; Hussey, D.J.; Watson, D.I. Androgens and esophageal cancer: What do we know? World J. Gastroenterol. 2015, 21, 6146–6156. [Google Scholar] [CrossRef]
- Palethorpe, H.M.; Drew, P.A.; Smith, E. Androgen Signaling in Esophageal Adenocarcinoma Cell Lines In Vitro. Dig. Dis. Sci. 2017, 62, 3402–3414. [Google Scholar] [CrossRef]
- Asanuma, K.; Iijima, K.; Shimosegawa, T. Gender difference in gastro-esophageal reflux diseases. World J. Gastroenterol. 2016, 22, 1800–1810. [Google Scholar] [CrossRef]
- Case, L.K.; Wall, E.H.; Dragon, J.A.; Saligrama, N.; Krementsov, D.N.; Moussawi, M.; Zachary, J.F.; Huber, S.A.; Blankenhorn, E.P.; Teuscher, C. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome. Res. 2013, 23, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Wesley, J.D.; Tessmer, M.S.; Paget, C.; Trottein, F.; Brossay, L. A Y chromosome-linked factor impairs NK T development. J. Immunol. 2007, 179, 3480–3487. [Google Scholar] [CrossRef]
- Charchar, F.J.; Bloomer, L.D.; Barnes, T.A.; Cowley, M.J.; Nelson, C.P.; Wang, Y.; Denniff, M.; Debiec, R.; Christofidou, P.; Nankervis, S.; et al. Inheritance of coronary artery disease in men: An analysis of the role of the Y chromosome. Lancet 2012, 379, 915–922. [Google Scholar] [CrossRef]
- Teuscher, C.; Noubade, R.; Spach, K.; McElvany, B.; Bunn, J.Y.; Fillmore, P.D.; Zachary, J.F.; Blankenhorn, E.P. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc. Natl. Acad. Sci. USA 2006, 103, 8024–8029. [Google Scholar] [CrossRef] [PubMed]
- Case, L.K.; Toussaint, L.; Moussawi, M.; Roberts, B.; Saligrama, N.; Brossay, L.; Huber, S.A.; Teuscher, C. Chromosome y regulates survival following murine coxsackievirus b3 infection. G3 (Bethesda) 2012, 2, 115–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sauter, G.; Moch, H.; Wagner, U.; Novotna, H.; Gasser, T.C.; Mattarelli, G.; Mihatsch, M.J.; Waldman, F.M. Y chromosome loss detected by FISH in bladder cancer. Cancer Genet. Cytogenet 1995, 82, 163–169. [Google Scholar] [CrossRef]
- Forsberg, L.A. Loss of chromosome Y (LOY) in blood cells is associated with increased risk for disease and mortality in aging men. Hum. Genet. 2017, 136, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Persons, D.L.; Croughan, W.S.; Borelli, K.A.; Cherian, R. Interphase cytogenetics of esophageal adenocarcinoma and precursor lesions. Cancer Genet. Cytogenet 1998, 106, 11–17. [Google Scholar] [CrossRef]
- Cestari, R.; Villanacci, V.; Rossi, E.; Della Casa, D.; Missale, G.; Conio, M.; Grigolato, P.; Bassotti, G. Fluorescence in situ hybridization to evaluate dysplasia in Barrett’s esophagus: A pilot study. Cancer Lett. 2007, 251, 278–287. [Google Scholar] [CrossRef]
- Doak, S.H.; Jenkins, G.J.; Parry, E.M.; D’Souza, F.R.; Griffiths, A.P.; Toffazal, N.; Shah, V.; Baxter, J.N.; Parry, J.M. Chromosome 4 hyperploidy represents an early genetic aberration in premalignant Barrett’s oesophagus. Gut 2003, 52, 623–628. [Google Scholar] [CrossRef]
- Beuzen, F.; Dubois, S.; Flejou, J.F. Chromosomal numerical aberrations are frequent in oesophageal and gastric adenocarcinomas: A study using in-situ hybridization. Histopathology 2000, 37, 241–249. [Google Scholar] [CrossRef]
- Altaf, K.; Xiong, J.J.; la Iglesia, D.; Hickey, L.; Kaul, A. Meta-analysis of biomarkers predicting risk of malignant progression in Barrett’s oesophagus. Br. J. Surg. 2017, 104, 493–502. [Google Scholar] [CrossRef]
- Nones, K.; Waddell, N.; Wayte, N.; Patch, A.M.; Bailey, P.; Newell, F.; Holmes, O.; Fink, J.L.; Quinn, M.C.J.; Tang, Y.H.; et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nat. Commun. 2014, 5, 5224. [Google Scholar] [CrossRef] [PubMed]
- Eischen, C.M. Genome Stability Requires p53. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Agahozo, M.C.; Timmermans, M.A.M.; Sleddens, H.F.B.M.; Foekens, R.; Trapman-Jansen, A.M.A.C.; Schröder, C.P.; van Leeuwen-Stok, E.; Martens, J.W.M.; Dinjens, W.N.M.; van Deurzen, C.H.M. Loss of Y-Chromosome during Male Breast Carcinogenesis. Cancers 2020, 12, 631. [Google Scholar] [CrossRef]
- Hollows, R.; Wei, W.; Cazier, J.B.; Mehanna, H.; Parry, G.; Halford, G.; Murray, P. Association between loss of Y chromosome and poor prognosis in male head and neck squamous cell carcinoma. Head Neck 2019, 41, 993–1006. [Google Scholar] [CrossRef]
- Arseneault, M.; Monlong, J.; Vasudev, N.S.; Laskar, R.S.; Safisamghabadi, M.; Harnden, P.; Egevad, L.; Nourbehesht, N.; Panichnantakul, P.; Holcatova, I.; et al. Loss of chromosome Y leads to down regulation of KDM5D and KDM6C epigenetic modifiers in clear cell renal cell carcinoma. Sci. Rep. 2017, 7, 44876. [Google Scholar] [CrossRef]
- Thompson, D.J.; Genovese, G.; Halvardson, J.; Ulirsch, J.C.; Wright, D.J.; Terao, C.; Davidsson, O.B.; Day, F.R.; Sulem, P.; Jiang, Y.; et al. Genetic predisposition to mosaic Y chromosome loss in blood. Nature 2019, 575, 652–657. [Google Scholar] [CrossRef]
- Persani, L.; Bonomi, M.; Lleo, A.; Pasini, S.; Civardi, F.; Bianchi, I.; Campi, I.; Finelli, P.; Miozzo, M.; Castronovo, C.; et al. Increased loss of the Y chromosome in peripheral blood cells in male patients with autoimmune thyroiditis. J. Autoimmun. 2012, 38, J193–J196. [Google Scholar] [CrossRef]
- Stone, J.F.; Sandberg, A.A. Sex chromosome aneuploidy and aging. Mutat. Res. 1995, 338, 107–113. [Google Scholar] [CrossRef]
- Dumanski, J.P.; Rasi, C.; Lonn, M.; Davies, H.; Ingelsson, M.; Giedraitis, V.; Lannfelt, L.; Magnusson, P.K.; Lindgren, C.M.; Morris, A.P.; et al. Mutagenesis. Smoking is associated with mosaic loss of chromosome Y. Science 2015, 347, 81–83. [Google Scholar] [CrossRef]
- Rozhok, A.I.; DeGregori, J. Toward an evolutionary model of cancer: Considering the mechanisms that govern the fate of somatic mutations. Proc. Nat. Acad. Sci. USA 2015, 112, 8914–8921. [Google Scholar] [CrossRef]
- Komura, K.; Jeong, S.H.; Hinohara, K.; Qu, F.; Wang, X.; Hiraki, M.; Azuma, H.; Lee, G.S.; Kantoff, P.W.; Sweeney, C.J. Resistance to docetaxel in prostate cancer is associated with androgen receptor activation and loss of KDM5D expression. Proc. Natl. Acad. Sci. USA 2016, 113, 6259–6264. [Google Scholar] [CrossRef]
- Dunford, A.; Weinstock, D.M.; Savova, V.; Schumacher, S.E.; Cleary, J.P.; Yoda, A.; Sullivan, T.J.; Hess, J.M.; Gimelbrant, A.A.; Beroukhim, R.; et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat. Genet. 2017, 49, 10–16. [Google Scholar] [CrossRef]
- Vaz, C.V.; Correia, S.; Cardoso, H.J.; Figueira, M.I.; Marques, R.; Maia, C.J.; Socorro, S. The Emerging Role of Regucalcin as a Tumor Suppressor: Facts and Views. Curr. Mol. Med. 2016, 16, 607–619. [Google Scholar] [CrossRef]
- Weng, S.; Stoner, S.A.; Zhang, D.E. Sex chromosome loss and the pseudoautosomal region genes in hematological malignancies. Oncotarget 2016, 7, 72356–72372. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, Y.M.; Zhao, J.J.; Chen, Y.B.; Jiang, S.S.; Yan, S.M.; Zhao, B.W.; Pan, K.; Wang, D.D.; Lv, L.; et al. Decreased expression of transcription elongation factor A-like 7 is associated with gastric adenocarcinoma prognosis. PLoS ONE 2013, 8, e54671. [Google Scholar] [CrossRef] [PubMed]
- Chien, J.; Narita, K.; Rattan, R.; Giri, S.; Shridhar, R.; Staub, J.; Beleford, D.; Lai, J.; Roberts, L.R.; Molina, J.; et al. A role for candidate tumor-suppressor gene TCEAL7 in the regulation of c-Myc activity, cyclin D1 levels and cellular transformation. Oncogene 2008, 27, 7223–7234. [Google Scholar] [CrossRef][Green Version]
- Wong, H.Y.; Wang, G.M.; Croessmann, S.; Zabransky, D.J.; Chu, D.; Garay, J.P.; Cidado, J.; Cochran, R.L.; Beaver, J.A.; Aggarwal, A.; et al. TMSB4Y is a candidate tumor suppressor on the Y chromosome and is deleted in male breast cancer. Oncotarget 2015, 6, 44927–44940. [Google Scholar] [CrossRef]
- Dumanski, J.P.; Lambert, J.C.; Rasi, C.; Giedraitis, V.; Davies, H.; Grenier-Boley, B.; Lindgren, C.M.; Campion, D.; Dufouil, C.; The European Alzheimer’s Disease Initiative Investigators; et al. Mosaic Loss of Chromosome Y in Blood Is Associated with Alzheimer Disease. Am. J. Hum. Genet. 2016, 98, 1208–1219. [Google Scholar] [CrossRef]
- Korkmaz, D.T.; Demirhan, O.; Abat, D.; Demirberk, B.; Tunc, E.; Kuleci, S. Microchimeric Cells, Sex Chromosome Aneuploidies and Cancer. Pathol. Oncol. Res. 2015, 21, 1157–1165. [Google Scholar] [CrossRef]
- Noveski, P.; Madjunkova, S.; Sukarova Stefanovska, E.; Matevska Geshkovska, N.; Kuzmanovska, M.; Dimovski, A.; Plaseska-Karanfilska, D. Loss of Y Chromosome in Peripheral Blood of Colorectal and Prostate Cancer Patients. PLoS ONE 2016, 11, e0146264. [Google Scholar] [CrossRef]
- Menke-Pluymers, M.B.; van Drunen, E.; Vissers, K.J.; Mulder, A.H.; Tilanus, H.W.; Hagemeijer, A. Cytogenetic analysis of Barrett’s mucosa and adenocarcinoma of the distal esophagus and cardia. Cancer Genet. Cytogenet. 1996, 90, 109–117. [Google Scholar] [CrossRef]
- Chaves, P.; Crespo, M.; Ribeiro, C.; Laranjeira, C.; Pereira, A.D.; Suspiro, A.; Cardoso, P.; Leitao, C.N.; Soares, J. Chromosomal analysis of Barrett’s cells: Demonstration of instability and detection of the metaplastic lineage involved. Mod. Pathol. 2007, 20, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Holscher, A.H.; Schneider, P.M.; Gutschow, C.; Schroder, W. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann. Surg. 2007, 245, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Schallenberg, S.; Bork, J.; Essakly, A.; Alakus, H.; Buettner, R.; Hillmer, A.M.; Bruns, C.; Schroeder, W.; Zander, T.; Loeser, H.; et al. Loss of the SWI/SNF-ATPase subunit members SMARCF1 (ARID1A), SMARCA2 (BRM), SMARCA4 (BRG1) and SMARCB1 (INI1) in oesophageal adenocarcinoma. BMC Cancer 2020, 20, 12. [Google Scholar] [CrossRef]
- Schneider, P.M.; Metzger, R.; Schaefer, H.; Baumgarten, F.; Vallbohmer, D.; Brabender, J.; Wolfgarten, E.; Bollschweiler, E.; Baldus, S.E.; Dienes, H.P.; et al. Response evaluation by endoscopy, rebiopsy, and endoscopic ultrasound does not accurately predict histopathologic regression after neoadjuvant chemoradiation for esophageal cancer. Ann. Surg. 2008, 248, 902–908. [Google Scholar] [CrossRef]
- Helbig, D.; Ihle, M.A.; Putz, K.; Tantcheva-Poor, I.; Mauch, C.; Buttner, R.; Quaas, A. Oncogene and therapeutic target analyses in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget 2016, 7, 21763–21774. [Google Scholar] [CrossRef]
- Simon, R.; Mirlacher, M.; Sauter, G. Tissue microarrays. Methods Mol. Med. 2005, 114, 257–268. [Google Scholar] [CrossRef]
- Loeser, H.; Waldschmidt, D.; Kuetting, F.; Heydt, C.; Zander, T.; Plum, P.; Alakus, H.; Buettner, R.; Quaas, A. Copy-number variation and protein expression of DOT1L in pancreatic adenocarcinoma as a potential drug target. Mol. Clin. Oncol. 2017, 6, 639–642. [Google Scholar] [CrossRef][Green Version]
- Essakly, A.; Loeser, H.; Kraemer, M.; Alakus, H.; Chon, S.H.; Zander, T.; Buettner, R.; Hillmer, A.M.; Bruns, C.J.; Schroeder, W.; et al. PIK3CA and KRAS Amplification in Esophageal Adenocarcinoma and their Impact on the Inflammatory Tumor Microenvironment and Prognosis. Transl. Oncol. 2019, 13, 157–164. [Google Scholar] [CrossRef]
- Plum, P.S.; Gebauer, F.; Kramer, M.; Alakus, H.; Berlth, F.; Chon, S.H.; Schiffmann, L.; Zander, T.; Buttner, R.; Holscher, A.H.; et al. HER2/neu (ERBB2) expression and gene amplification correlates with better survival in esophageal adenocarcinoma. BMC Cancer 2019, 19, 38. [Google Scholar] [CrossRef]
- Schiffmann, L.M.; Loeser, H.; Jacob, A.S.; Maus, M.; Fuchs, H.; Zhao, Y.; Tharun, L.; Essakly, A.; Iannos Damanakis, A.; Zander, T.; et al. Dickkopf-2 (DKK2) as Context Dependent Factor in Patients with Esophageal Adenocarcinoma. Cancers 2020, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Quaas, A.; Heydt, C.; Gebauer, F.; Alakus, H.; Loeser, H.; Buettner, R.; Hillmer, A.; Bruns, C.; Merkelbach-Bruse, S.; Zander, T.; et al. Genomic Characterization of TP53-Wild-Type Esophageal Carcinoma. Transl. Oncol. 2019, 12, 154–161. [Google Scholar] [CrossRef]

| Cliniopathological Parameter | Y Chromosome | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Loss | Presence | p Value | |||||
| age group | <65 yrs | 210 | 52.8% | 117 | 56.0% | 93 | 44.0% | |
| >65 yrs | 190 | 47.2% | 91 | 47.9% | 99 | 52.1% | 0.124 | |
| tumor stage | pT1/2 | 109 | 27.4% | 52 | 48.6% | 56 | 51.4% | |
| pT3/4 | 289 | 72.6% | 157 | 54.3% | 132 | 45.7% | 0.314 | |
| lymph node metastasis | pN0 | 159 | 39.9% | 68 | 42.8% | 91 | 57.2% | |
| pN+ | 239 | 60.1% | 142 | 59.4% | 97 | 40.6% | 0.001 | |
| grading | G1 | 4 | 1.4% | 0 | 0.0% | 4 | 100.0% | |
| G2 | 164 | 56.0% | 70 | 48.5% | 85 | 51.5% | ||
| G3 | 123 | 42.3% | 73 | 59.3% | 50 | 40.7% | 0.031 | |
| neoadjuvant treatment | no | 177 | 44.3% | 87 | 49.2% | 90 | 50.8% | |
| yes | 223 | 55.7% | 123 | 55.2% | 100 | 44.8% | 0.268 | |
| TP53 | wildtype | 122 | 41.5% | 53 | 43.4% | 69 | 56.6% | |
| mutation | 172 | 58.5% | 106 | 61.6% | 66 | 38.4% | 0.003 | |
| KRAS | negative | 318 | 81.3% | 159 | 50.0% | 159 | 50.0% | |
| positive | 73 | 18.7% | 50 | 68.5% | 23 | 31.5% | 0.004 | |
| HER2/neu | negative | 247 | 87.0% | 130 | 52.6% | 117 | 47.4% | |
| positive | 37 | 13.0% | 22 | 59.5% | 15 | 40.5% | 0.483 | |
| ARID1a | loss | 40 | 10.4% | 15 | 37.5% | 25 | 62.5% | |
| presence | 343 | 89.6% | 188 | 54.8% | 155 | 45.2% | 0.045 | |
| BRG1 | loss | 16 | 4.1% | 5 | 31.3% | 11 | 68.8% | |
| presence | 371 | 95.9% | 202 | 54.4% | 169 | 45.6% | 0.078 | |
| GATA6 | negative | 340 | 89.5% | 175 | 51.5% | 165 | 48.5% | |
| positive | 40 | 10.5% | 23 | 57.5% | 17 | 42.5% | 0.507 | |
| PIK3CA | negative | 335 | 94.1% | 181 | 54.0% | 154 | 46.0% | |
| positive | 21 | 5.9% | 8 | 38.1% | 13 | 61.9% | 0.180 | |
| CD3 infiltrating cells | low | 203 | 70.2% | 112 | 55.2% | 91 | 44.8% | 0.699 |
| high | 86 | 29.8% | 45 | 52.3% | 41 | 47.7% | ||
| LAG3 | negative | 290 | 93.9% | 159 | 54.8% | 131 | 45.2% | |
| positive | 19 | 6.1% | 5 | 26.3% | 14 | 73.7% | 0.018 | |
| Clinicopathological Parameter | Hazard Ratio | 95% Confidence Interval | p Value | ||
|---|---|---|---|---|---|
| lower | upper | ||||
| age group | <65 yrs. vs. >65 yrs | 1.435 | 0.978 | 2.105 | 0.065 |
| tumor stage | pT1/2 vs. pT3/4 | 1.195 | 0.704 | 2.027 | 0.51 |
| lymph node metastasis | pN0 vs. pN+ | 2.877 | 1.839 | 4.502 | <0.001 |
| grading | G1 vs. G2/3 | 1.572 | 1.082 | 2.284 | 0018 |
| TP53 | wildtype vs. mutation | 1.120 | 0.756 | 1.658 | 0.573 |
| LoY | presence vs. loss | 1.835 | 2.725 | 1.233 | 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loeser, H.; Wölwer, C.B.; Alakus, H.; Chon, S.-H.; Zander, T.; Buettner, R.; Hillmer, A.M.; Bruns, C.J.; Schroeder, W.; Gebauer, F.; et al. Y Chromosome Loss Is a Frequent Event in Barrett’s Adenocarcinoma and Associated with Poor Outcome. Cancers 2020, 12, 1743. https://doi.org/10.3390/cancers12071743
Loeser H, Wölwer CB, Alakus H, Chon S-H, Zander T, Buettner R, Hillmer AM, Bruns CJ, Schroeder W, Gebauer F, et al. Y Chromosome Loss Is a Frequent Event in Barrett’s Adenocarcinoma and Associated with Poor Outcome. Cancers. 2020; 12(7):1743. https://doi.org/10.3390/cancers12071743
Chicago/Turabian StyleLoeser, Heike, Christina B. Wölwer, Hakan Alakus, Seung-Hun Chon, Thomas Zander, Reinhard Buettner, Axel M. Hillmer, Christiane J. Bruns, Wolfgang Schroeder, Florian Gebauer, and et al. 2020. "Y Chromosome Loss Is a Frequent Event in Barrett’s Adenocarcinoma and Associated with Poor Outcome" Cancers 12, no. 7: 1743. https://doi.org/10.3390/cancers12071743
APA StyleLoeser, H., Wölwer, C. B., Alakus, H., Chon, S.-H., Zander, T., Buettner, R., Hillmer, A. M., Bruns, C. J., Schroeder, W., Gebauer, F., & Quaas, A. (2020). Y Chromosome Loss Is a Frequent Event in Barrett’s Adenocarcinoma and Associated with Poor Outcome. Cancers, 12(7), 1743. https://doi.org/10.3390/cancers12071743







