IGF-1R/mTOR Targeted Therapy for Ewing Sarcoma: A Meta-Analysis of Five IGF-1R-Related Trials Matched to Proteomic and Radiologic Predictive Biomarkers
Abstract
:1. Introduction
2. Results
2.1. Joint Suppression of IGF-1R/mTOR is Superior to Single-Agent IGF-1R mAbs
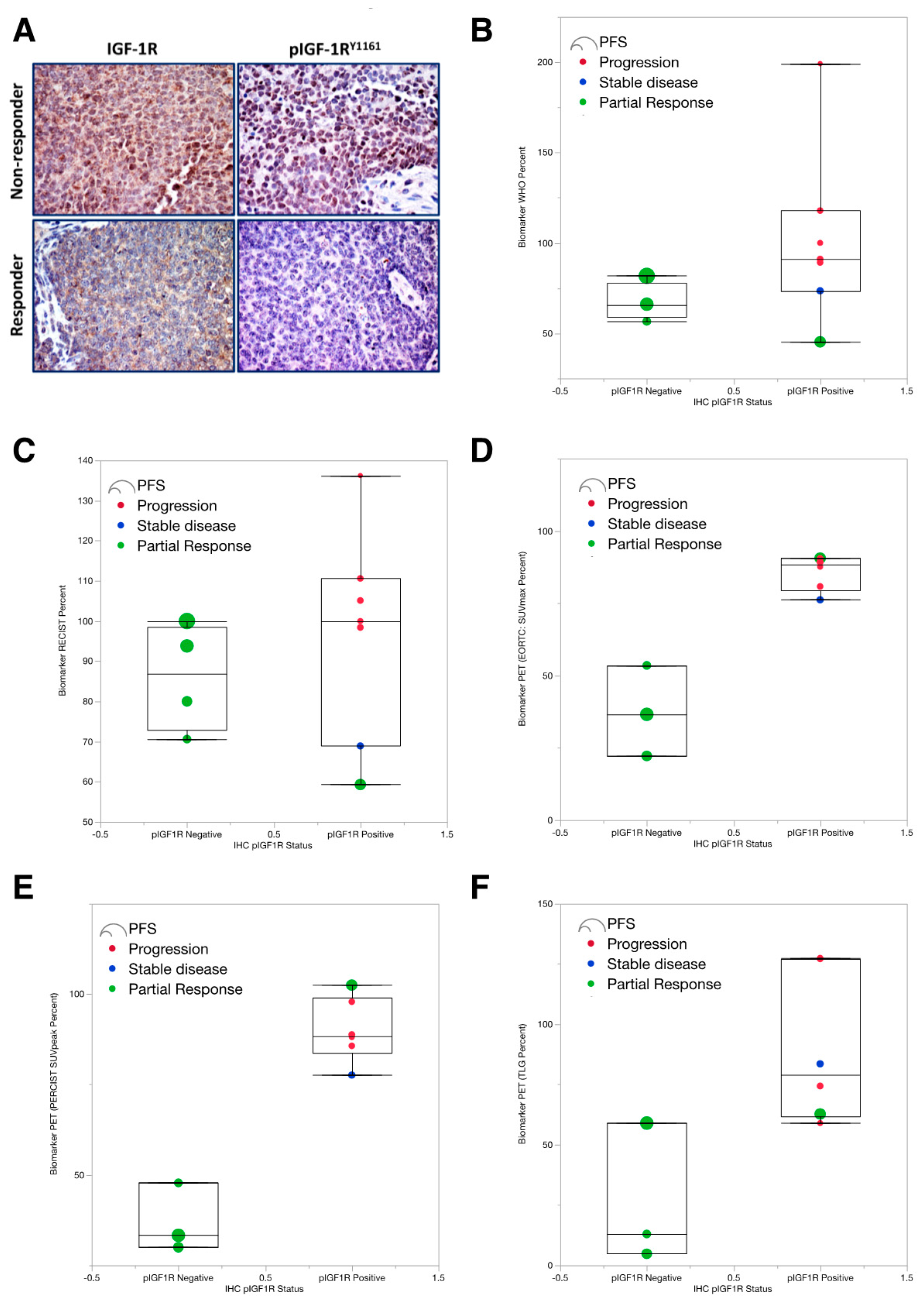
2.2. Early PET/CT Imaging Predicts Clinical Response at 6 Weeks and Progression-Free Survival (PFS)
2.3. Low pIGF-1R Predicts 6-Weeks Response to IGF-1R Targeted Abs
3. Discussion
4. Materials and Methods
4.1. Human Subjects
4.2. Statistical Analyses
4.3. CT & PET/CT Measurement and Interpretation
4.4. Immunohistochemical Staining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ES | Ewing sarcoma |
| IGF-1R | insulin-like growth factor receptor 1 |
| mTOR | mammalian target of rapamycin |
References
- Zucman, J.; Delattre, O.; Desmaze, C.; Plougastel, B.; Joubert, I.; Melot, T.; Peter, M.; De Jong, P.; Rouleau, G.; Aurias, A.; et al. Cloning and characterization of the Ewing’s sarcoma and peripheral neuroepithelioma t(11;22) translocation breakpoints. Genes Chromosom. Cancer 1992, 5, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Tomazou, E.M.; Sheffield, N.C.; Schmidl, C.; Schuster, M.; Schönegger, A.; Datlinger, P.; Kubicek, S.; Bock, C.; Kovar, H. Epigenome mapping reveals distinct modes of gene regulation and widespread enhancer reprogramming by the oncogenic fusion protein EWS-FLI1. Cell Rep. 2015, 10, 1082–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore IV, J.B.; Loeb, D.M.; Hong, K.U.; Sorensen, P.H.; Triche, T.J.; Lee, D.W.; Barbato, M.I.; Arceci, R.J. Epigenetic reprogramming and re-differentiation of a Ewing sarcoma cell line. Front. Cell Dev. Biol. 2015, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Riggi, N.; Knoechel, B.; Gillespie, S.M.; Rheinbay, E.; Boulay, G.; Suvà, M.L.; Rossetti, N.E.; Boonseng, W.E.; Oksuz, O.; Cook, E.B.; et al. EWS-FLI1 utilizes divergent chromatin remodeling mechanisms to directly activate or repress enhancer elements in Ewing sarcoma. Cancer Cell 2014, 26, 668–681. [Google Scholar] [CrossRef] [Green Version]
- Kolb, E.A.; Gorlick, R. Development of IGF-IR Inhibitors in Pediatric Sarcomas. Curr. Oncol. Rep. 2009, 11, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Sarantopoulos, J.; Patnaik, A.; Papadoupoulos, K.; Lin, C.-C.; Murphy, J.R.; Roth, B.; McCaffery, I.; Gorski, K.S.; Kaiser, B.; et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J. Clin. Oncol. 2009, 27, 5800–5807. [Google Scholar] [CrossRef]
- Prieur, A.; Tirode, F.; Cohen, P.; Delattre, O. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol. Cell Biol. 2004, 24, 7275–7283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo-Lozano, S.; Gokhale, P.C.; Soldatenkov, V.A.; Dritschilo, A.; Tirado, O.M.; Notario, V. Combined transcriptional and translational targeting of EWS/FLI-1 in Ewing’s sarcoma. Clin. Cancer Res. 2006, 12, 6781–6790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurzrock, R.; Patnaik, A.; Aisner, J.; Warren, T.; Leong, S.; Benjamin, R.; Eckhardt, S.G.; Eid, J.E.; Greig, G.; Habben, K.; et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin. Cancer Res. 2010, 16, 2458–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotlandi, K.; Benini, S.; Sarti, M.; Serra, M.; Lollini, P.-L.; Maurici, D.; Picci, P.; Manara, M.C.; Baldini, N. Insulin-like growth factor I receptor-mediated circuit in Ewing’s sarcoma/peripheral neuroectodermal tumor: A possible therapeutic target. Cancer Res. 1996, 56, 4570–4574. [Google Scholar]
- Fleuren, E.D.; Versleijen-Jonkers, Y.M.; Boerman, O.C.; Van der Graaf, W.T. Targeting receptor tyrosine kinases in osteosarcoma and Ewing sarcoma: Current hurdles and future perspectives. Biochim. Biophys. Acta. Rev. Cancer 2014, 1845, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Hess, K.R.; Khawaja, M.R.; Wagner, M.J.; Tang, C.; Naing, A.; Fu, S.; Janku, F.; Piha-Paul, S.; Tsimberidou, A.M.; et al. Evaluation of Novel Targeted Therapies in Aggressive Biology Sarcoma Patients after progression from US FDA approved Therapies. Sci. Rep. 2016, 6, 35448. [Google Scholar] [CrossRef] [Green Version]
- Livingston, J.A.; Hess, K.R.; Naing, A.; Hong, D.S.; Patel, S.; Benjamin, R.S.; Ludwig, J.A.; Conley, A.; Herzog, C.E.; Anderson, P.; et al. Validation of prognostic scoring and assessment of clinical benefit for patients with bone sarcomas enrolled in phase I clinical trials. Oncotarget 2016, 7, 64421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garofalo, C.; Manara, M.C.; Nicoletti, G.; Marino, M.T.; Lollini, P.-L.; Astolfi, A.; Pandini, G.; López-Guerrero, J.A.; Scharfer, K.-L.; Belfiore, A.; et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing’s sarcoma is dependent on insulin receptor signaling. Oncogene 2011, 30, 2730–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltran, P.J.; Chung, Y.A.; Moody, G.; Mitchell, P.; Cajulis, E.; Vonderfecht, S.; Kendall, R.; Radinsky, R.; Calzone, F.J. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing’s and osteogenic sarcoma models. J. Pharmacol. Exp. Ther. 2011, 337, 644–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamhamedi-Cherradi, S.E.; Menegaz, B.A.; Ramamoorthy, V.; Vishwamitra, D.; Wang, Y.; Maywald, R.L.; Buford, A.S.; Fokt, I.; Skora, S.; Wang, J.; et al. IGF-1R and mTOR Blockade: Novel Resistance Mechanisms and Synergistic Drug Combinations for Ewing Sarcoma. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Potratz, J.C.; Saunders, D.N.; Wai, D.H.; Ng, T.L.; McKinney, S.E.; Carboni, J.M.; Gottardis, M.M.; Triche, T.J.; Jürgens, H.; Pollak, M.N.; et al. Synthetic lethality screens reveal RPS6 and MST1R as modifiers of insulin-like growth factor-1 receptor inhibitor activity in childhood sarcomas. Cancer Res. 2010, 70, 8770–8781. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Harkavy, B.; Shen, N.; Helman, L.J. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 2007, 26, 1932–1940. [Google Scholar] [CrossRef] [Green Version]
- Hartley, D.; Cooper, G.M. Role of mTOR in the degradation of IRS-1: Regulation of PP2A activity. J. Cell. Biochem. 2002, 85, 304–314. [Google Scholar] [CrossRef]
- Haruta, T.; Uno, T.; Kawahara, J.; Takano, A.; Egawa, K.; Sharma, P.M.; Olefsky, J.M.; Kobayashi, M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol. Endocrinol. 2000, 14, 783–794. [Google Scholar] [CrossRef]
- Yoneyama, Y.; Inamitsu, T.; Chida, K.; Iemura, S.-I.; Natsume, T.; Maeda, T.; Hakuno, F.; Takahashi, S.-I. Serine Phosphorylation by mTORC1 Promotes IRS-1 Degradation through SCFbeta-TRCP E3 Ubiquitin Ligase. iScience 2018, 5, 1–18. [Google Scholar] [CrossRef]
- Mateo-Lozano, S.; Tirado, O.M.; Notario, V. Rapamycin induces the fusion-type independent downregulation of the EWS/FLI-1 proteins and inhibits Ewing’s sarcoma cell proliferation. Oncogene 2003, 22, 9282–9287. [Google Scholar] [CrossRef]
- Wan, X.; Helman, L.J. The biology behind mTOR inhibition in sarcoma. Oncologist 2007, 12, 1007–1018. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hawk, N.; Yue, P.; Kauh, J.; Ramalingam, S.S.; Fu, H.; Khuri, F.R.; Sun, S.-Y. Overcoming mTOR inhibition-induced paradoxical activation of survival signaling pathways enhances mTOR inhibitors’ anticancer efficacy. Cancer Biol. Ther. 2008, 7, 1952–1958. [Google Scholar] [CrossRef] [Green Version]
- Naing, A.; LoRusso, P.; Fu, S.; Hong, D.S.; Anderson, P.; Benjamin, R.S.; Ludwig, J.; Chen, H.X.; Doyle, L.A.; Kurzrock, R. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clin. Cancer Res. 2012, 18, 2625–2631. [Google Scholar] [CrossRef] [Green Version]
- Demetri, G.D.; Chawla, S.P.; Ray-Coquard, I.; Le Cesne, A.; Staddon, A.P.; Milhem, M.M.; Penel, N.; Riedel, R.F.; Bui-Nguyen, B.; Cranmer, L.D.; et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J. Clin. Oncol. 2013, 31, 2485–2492. [Google Scholar] [CrossRef]
- Mita, M.M.; Mita, A.C.; Chu, Q.S.; Rowinsky, E.K.; Fetterly, G.J.; Goldston, M.; Patnaik, A.; Mathews, L.; Ricart, A.D.; Mays, T. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J. Clin. Oncol. 2008, 26, 361–367. [Google Scholar] [CrossRef]
- Schwartz, G.K.; Tap, W.D.; Qin, L.X.; Livingston, M.B.; Undevia, S.D.; Chmielowski, B.; Agulnik, M.; Schuetze, S.M.; Reed, D.R.; Okuno, S.H. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: A multicentre, open-label, phase 2 trial. Lancet Oncol 2013, 14, 371–382. [Google Scholar] [CrossRef] [Green Version]
- Wagner, L.M.; Fouladi, M.; Ahmed, A.; Krailo, M.D.; Weigel, B.; DuBois, S.G.; Doyle, L.A.; Chen, H.; Blaney, S.M. Phase II study of cixutumumab in combination with temsirolimus in pediatric patients and young adults with recurrent or refractory sarcoma: A report from the children’s oncology group. Pediatr. Blood Cancer 2014, 62, 440–444. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, M.A.; Martins, M.F.E.; Wang, Q.; Sonis, S.; Demetri, G.; George, S.; Butrynski, J.; Treister, N.S. Clinical presentation and management of mTOR inhibitor-associated stomatitis. Oral Oncol. 2011, 47, 998–1003. [Google Scholar] [CrossRef]
- Koshkin, V.S.; Bolejack, V.; Schwartz, L.H.; Wahl, R.L.; Chugh, R.; Reinke, D.K.; Zhao, B.; O, J.H.; Patel, S.R.; Schuetze, S.M. Assessment of Imaging Modalities and Response Metrics in Ewing Sarcoma: Correlation With Survival. J. Clin. Oncol. 2016, 34, 3680. [Google Scholar] [CrossRef]
- Pappo, A.S.; Patel, S.R.; Crowley, J.; Reinke, D.K.; Kuenkele, K.-P.; Chawla, S.P.; Toner, G.C.; Maki, R.G.; Meyers, P.A.; Chugh, R.; et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: Results of a phase II Sarcoma Alliance for Research through Collaboration study. J. Clin. Oncol. 2011, 29, 4541–4547. [Google Scholar] [CrossRef]
- Anderson, P.M.; Bielack, S.S.; Gorlick, R.G.; Skubitz, K.; Daw, N.C.; Herzog, C.E.; Monge, O.R.; Lassaletta, A.; Boldrini, E.; Papai, Z.; et al. A phase II study of clinical activity of SCH 717454 (robatumumab) in patients with relapsed osteosarcoma and Ewing sarcoma. Pediatr. Blood Cancer 2016, 63, 1761–1770. [Google Scholar] [CrossRef]
- Hyun, O.J.; Luber, B.S.; Leal, J.P.; Wang, H.; Bolejack, V.; Schuetze, S.M.; Schwartz, L.H.; Helman, L.J.; Reinke, D.; Baker, L.H.; et al. Response to Early Treatment Evaluated with 18F-FDG PET and PERCIST 1.0 Predicts Survival in Patients with Ewing Sarcoma Family of Tumors Treated with a Monoclonal Antibody to the Insulinlike Growth Factor 1 Receptor. J. Nucl. Med. 2016, 57, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Carden, C.P.; Molife, L.R.; de Bono, J.S. Predictive biomarkers for targeting insulin-like growth factor-I (IGF-I) receptor. Mol. Cancer Ther. 2009, 8, 2077–2078. [Google Scholar] [CrossRef] [Green Version]
- Osher, E.; Macaulay, V.M. Therapeutic Targeting of the IGF Axis. Cells 2019, 8, 895. [Google Scholar] [CrossRef] [Green Version]
- Haluska, P.; Menefee, M.; Plimack, E.R.; Rosenberg, J.; Northfelt, D.; LaVallee, T.; Shi, L.; Yu, X.-Q.; Burke, P.; Huang, J.; et al. Phase I dose-escalation study of MEDI-573, a bispecific, antiligand monoclonal antibody against IGFI and IGFII, in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 4747–4757. [Google Scholar] [CrossRef] [Green Version]
- Lodhia, K.A.; Tienchaiananda, P.; Haluska, P. Understanding the Key to Targeting the IGF Axis in Cancer: A Biomarker Assessment. Front. Oncol. 2015, 5, 142. [Google Scholar] [CrossRef] [Green Version]
- Asmane, I.; Watkin, E.; Alberti, L.; Duc, A.; Marec-Berard, P.; Ray-Coquard, I.; Cassier, P.; Decouvelaere, A.-V.; Ranchère, D.; Kurtz, J.-E.; et al. Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: A predictive biomarker for IGF-1R monoclonal antibody (Ab) therapy in sarcomas. Eur. J. Cancer 2012, 48, 3027–3035. [Google Scholar] [CrossRef]
- Ziai, P.; Hayeri, M.R.; Salei, A.; Salavati, A.; Houshmand, S.; Alavi, A.; Teytelboym, O.M. Role of Optimal Quantification of FDG PET Imaging in the Clinical Practice of Radiology. Radiographics 2016, 36, 481–496. [Google Scholar] [CrossRef] [Green Version]


| PI | IRB Number | IGF-1R Ab | mTOR Inhibitor | Sponsor | N (U.S.) | N | Age (Years) | PFS (Months) | OS (Months) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Kurzrock | 2005-0806 | Teprotumumab (R1507) | N/A | Roche | 9 | 5 | 23.7 | 2.36 | 9.68 | [9] |
| Pappo | 2007-0515 | Teprotumumab (R1507) | N/A | Roche | 115 | 13 | 31.3 | 2.02 | 9.13 | [32] |
| Anderson | 2007-0881 | Robatumumab (SCH717454) | N/A | Schering-Plough | 144 | 17 | 20.1 | 3.48 | 17 | [33] |
| Naing | 2007-0595 | Cixutumumab (IMC-A12) | Temsirolimus | ImClone | 17 | 11 | 19.7 | 5.94 | 21.5 | [25] |
| Schwartz | 2010-0191 | Cixutumumab (IMC-A12) | Temsirolimus | ImClone | 26 | 5 | 32.8 | 3.94 | 17.1 | [28] |
| Early Imaging Response (Day 7–26) | Best Response | Clinical Benefit | ||
|---|---|---|---|---|
| p-Value | ROC | p-Value | ROC | |
| WHO (CT imaging) | 0.0086 | 0.84 | 0.0048 | 0.85 |
| RECIST (CT imaging) | 0.0104 | 0.85 | 0.0039 | 0.86 |
| EORTC (PET imaging) | 0.0092 | 0.82 | 0.0071 | 0.80 |
| PERCIST (PET imaging) | 0.0063 | 0.82 | 0.0068 | 0.78 |
| TLG (PET imaging) | 0.0053 | 0.92 | 0.0028 | 0.88 |
| IHC pIGF-1R Status | ||||
|---|---|---|---|---|
| Gender | pIGF-1R Negative | pIGF-1R Positive | All | |
| Female | 2 | 3 | 5 | |
| Male | 4 | 9 | 13 | |
| All | 6 | 12 | 18 | |
| Age at Diagnosis | Mean | 20.8 | 22.6 | 22 |
| Median | 22.5 | 21 | 21 | |
| Min | 10.2 | 12.3 | 10.2 | |
| Max | 31.3 | 46.8 | 46.8 | |
| Tissue Type | ||||
| Bone | 5 | 12 | 17 | |
| Soft tissue | 1 | 0 | 1 | |
| All | 6 | 12 | 18 | |
| PFS | Mean | 12.7 | 4.88 | 7.48 |
| Median | 9.7 | 1.45 | 3.65 | |
| OS | Mean | 46.2 | 20.2 | 28.9 |
| Median | 47.3 | 6.5 | 16.8 | |
| IGF-1R best response | ||||
| Progression | 0 | 7 | 7 | |
| Stable Disease | 0 | 1 | 1 | |
| Partial response | 5 | 4 | 9 | |
| Complete Response | 1 | 0 | 1 | |
| All | 6 | 12 | 18 | |
| Clinical Benefit | ||||
| Progression | 0 | 7 | 7 | |
| Benefit | 6 | 5 | 11 | |
| All | 6 | 12 | 18 | |
| Week 6 Response (WHO) | Mean | 39.3 | 114 | 88.9 |
| Median | 25.4 | 120 | 76.9 | |
| Week 6 Response (RECIST) | Mean | 66.2 | 93.4 | 84.4 |
| Median | 63.5 | 105 | 84 | |
| Week 6 Response (SUV-Max) | Mean | 62.2 | 108 | 98.6 |
| Median | 62.2 | 102 | 74.8 | |
| Week 6 Response (SUV-Peak) | Mean | 46.4 | 106 | 94.1 |
| Median | 46.4 | 99.9 | 77.6 | |
| Biomarker (WHO Percent) | Mean | 67.5 | 102 | 89.7 |
| Median | 65.9 | 91 | 81.8 | |
| Biomarker (RECIST Percent) | Mean | 86.1 | 96.9 | 93 |
| Median | 86.9 | 100 | 98.4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, H.M.; Morani, A.C.; Daw, N.C.; Lamhamedi-Cherradi, S.-E.; Subbiah, V.; Menegaz, B.A.; Vishwamitra, D.; Eskandari, G.; George, B.; Benjamin, R.S.; et al. IGF-1R/mTOR Targeted Therapy for Ewing Sarcoma: A Meta-Analysis of Five IGF-1R-Related Trials Matched to Proteomic and Radiologic Predictive Biomarkers. Cancers 2020, 12, 1768. https://doi.org/10.3390/cancers12071768
Amin HM, Morani AC, Daw NC, Lamhamedi-Cherradi S-E, Subbiah V, Menegaz BA, Vishwamitra D, Eskandari G, George B, Benjamin RS, et al. IGF-1R/mTOR Targeted Therapy for Ewing Sarcoma: A Meta-Analysis of Five IGF-1R-Related Trials Matched to Proteomic and Radiologic Predictive Biomarkers. Cancers. 2020; 12(7):1768. https://doi.org/10.3390/cancers12071768
Chicago/Turabian StyleAmin, Hesham M., Ajaykumar C. Morani, Najat C. Daw, Salah-Eddine Lamhamedi-Cherradi, Vivek Subbiah, Brian A. Menegaz, Deeksha Vishwamitra, Ghazaleh Eskandari, Bhawana George, Robert S. Benjamin, and et al. 2020. "IGF-1R/mTOR Targeted Therapy for Ewing Sarcoma: A Meta-Analysis of Five IGF-1R-Related Trials Matched to Proteomic and Radiologic Predictive Biomarkers" Cancers 12, no. 7: 1768. https://doi.org/10.3390/cancers12071768
APA StyleAmin, H. M., Morani, A. C., Daw, N. C., Lamhamedi-Cherradi, S.-E., Subbiah, V., Menegaz, B. A., Vishwamitra, D., Eskandari, G., George, B., Benjamin, R. S., Patel, S., Song, J., Lazar, A. J., Wang, W.-L., Kurzrock, R., Pappo, A., Anderson, P. M., Schwartz, G. K., Araujo, D., ... Ludwig, J. A. (2020). IGF-1R/mTOR Targeted Therapy for Ewing Sarcoma: A Meta-Analysis of Five IGF-1R-Related Trials Matched to Proteomic and Radiologic Predictive Biomarkers. Cancers, 12(7), 1768. https://doi.org/10.3390/cancers12071768





