Immunophenotypes Based on the Tumor Immune Microenvironment Allow for Unsupervised Penile Cancer Patient Stratification
Abstract
:1. Introduction
2. Results
2.1. Clinicopathological Characteristics
2.2. Tumor Immune Microenvironmental Characteristics Associated with hrHPV
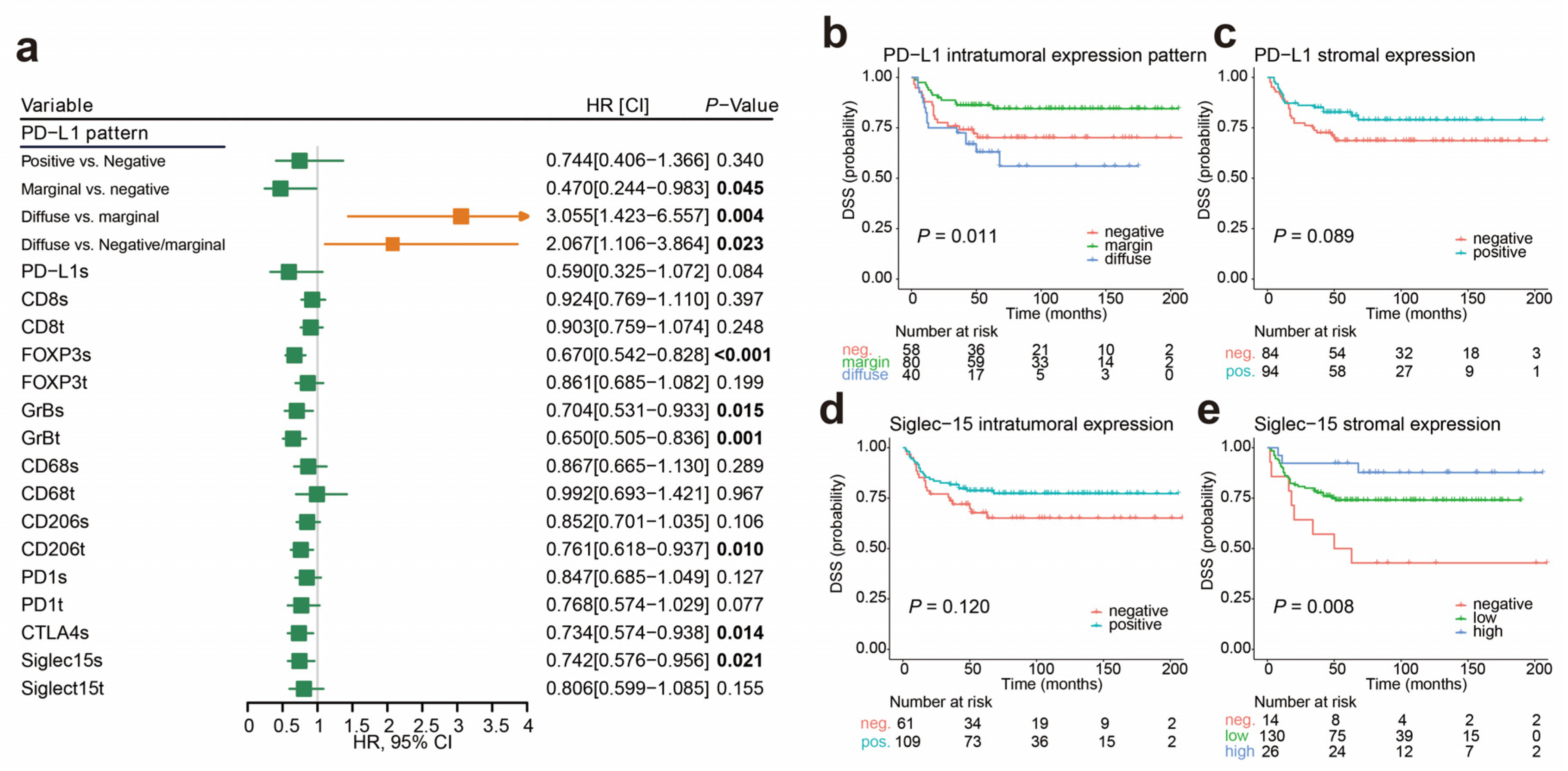
2.3. Cutoff Values for the Immune Markers Associated with Prognosis and LNM
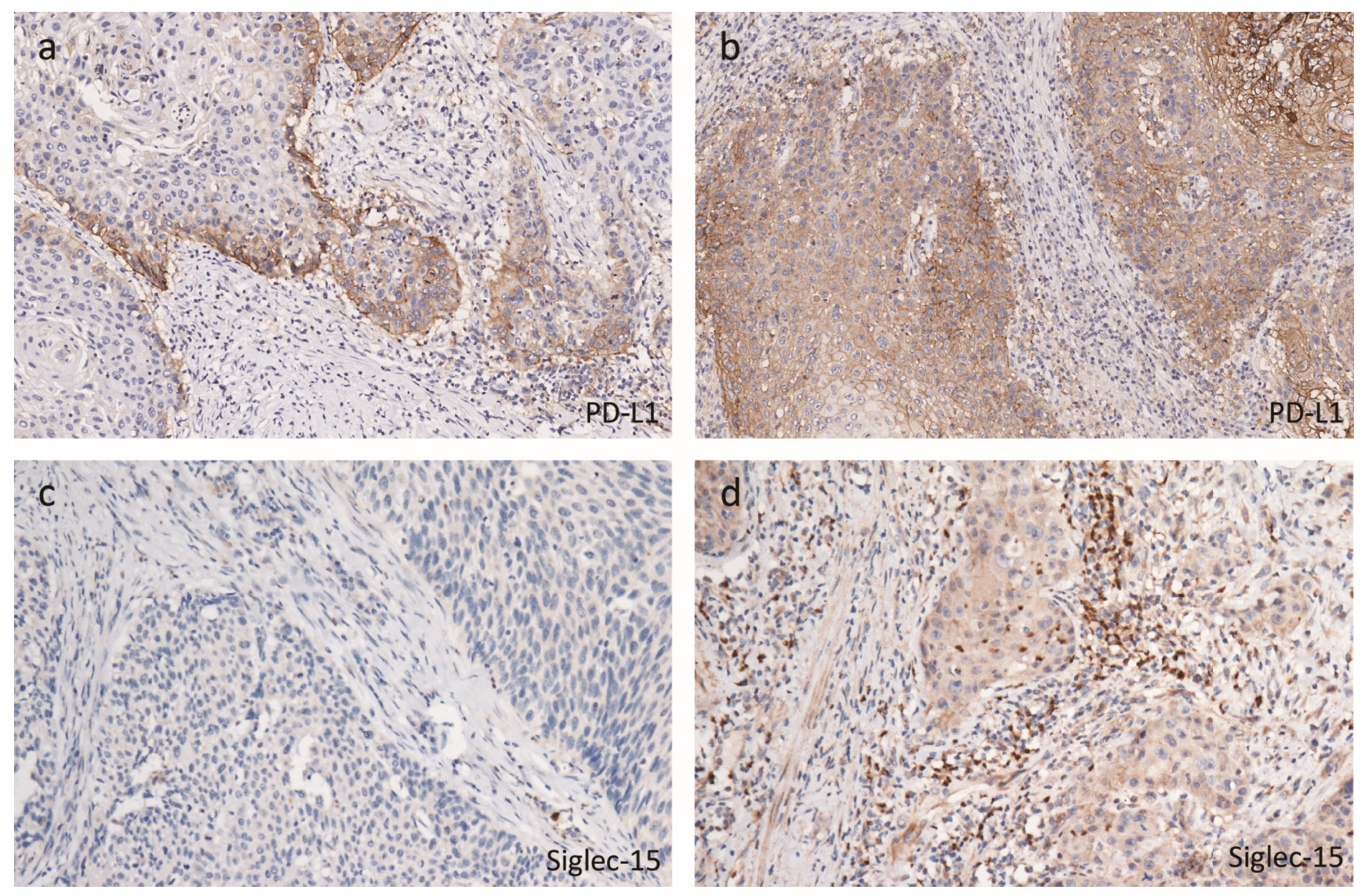
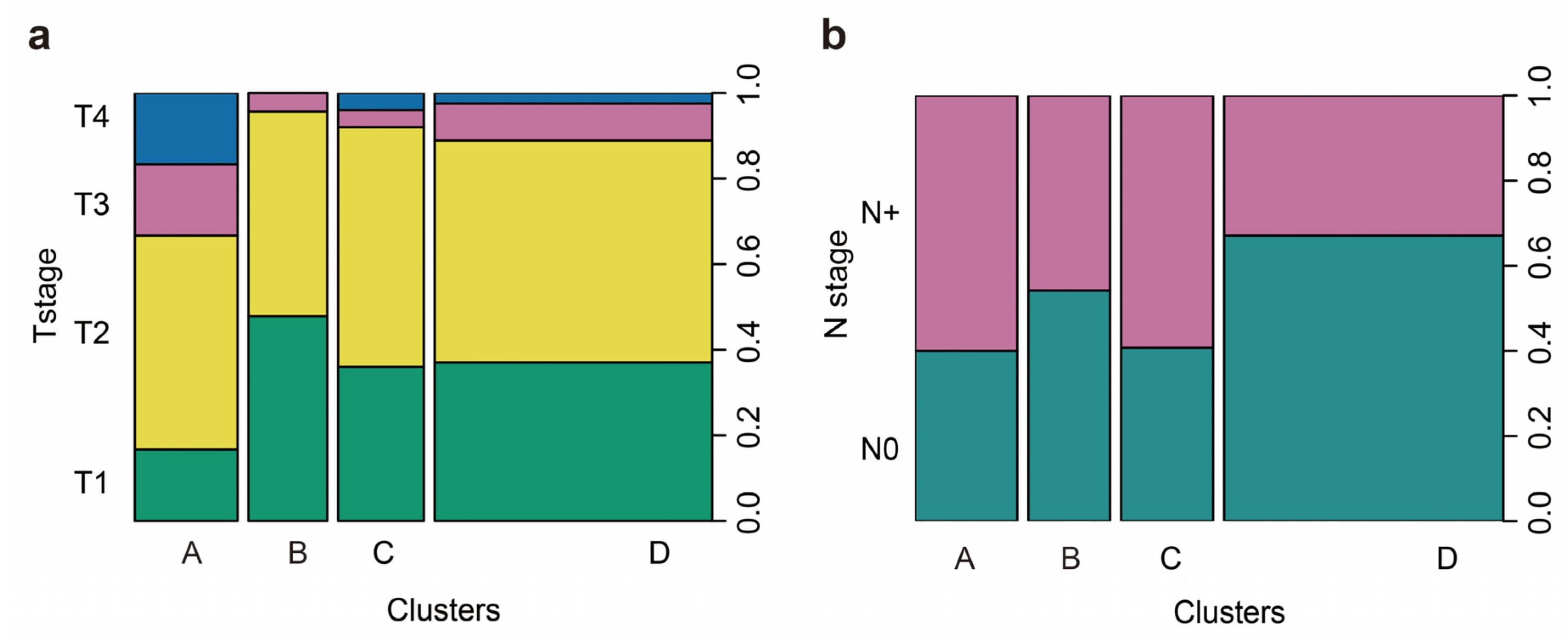
2.4. Immunophenotypes in peSCC
2.5. Immunophenotypes Associated with Prognosis
2.6. Association of Immunophenotypes with LNM
3. Discussion
4. Materials and Methods
4.1. Patient Selection and Clinical Data Collection
4.2. hrHPV Detection and Typing
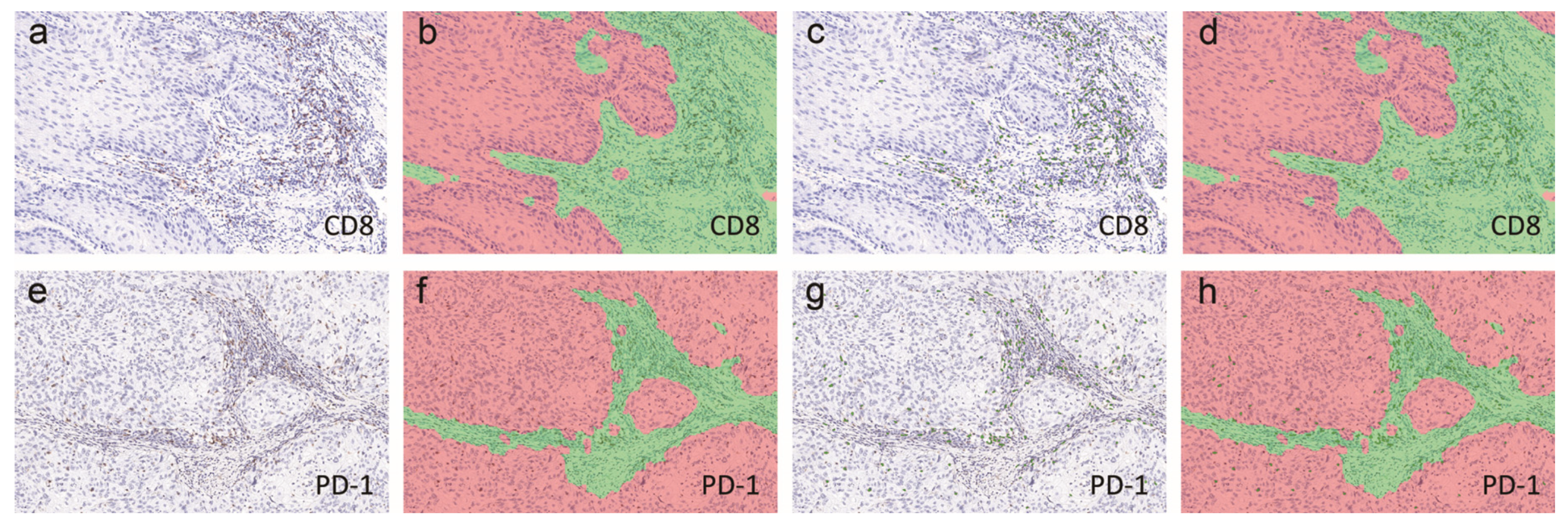
4.3. Immunohistochemistry and Scoring
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Pang, C.; Guan, Y.; Li, H.; Chen, W.; Zhu, G. Urologic cancer in China. Jpn. J. Clin. Oncol. 2016, 46, 497–501. [Google Scholar] [CrossRef] [Green Version]
- Sand, F.L.; Rasmussen, C.L.; Frederiksen, M.H.; Andersen, K.K.; Kjaer, S.K. Prognostic Significance of HPV and p16 Status in Men Diagnosed with Penile Cancer: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2018, 27, 1123–1132. [Google Scholar] [CrossRef] [Green Version]
- Hui, G.; Ghafouri, S.N.; Shen, J.; Liu, S.; Drakaki, A. Treating Penile Cancer in the Immunotherapy and Targeted Therapy Era. Case Rep. Oncol. Med. 2019, 2019, 8349793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marliot, F.; Lafontaine, L.; Galon, J. Immunoscore assay for the immune classification of solid tumors: Technical aspects, improvements and clinical perspectives. Methods Enzymol. 2020, 636, 109–128. [Google Scholar] [CrossRef]
- Djajadiningrat, R.S.; Horenblas, S.; Heideman, D.A.; Sanders, J.; de Jong, J.; Jordanova, E.S. Classic and nonclassic HLA class I expression in penile cancer and relation to HPV status and clinical outcome. J. Urol. 2015, 193, 1245–1251. [Google Scholar] [CrossRef]
- Ottenhof, S.R.; Djajadiningrat, R.S.; Thygesen, H.H.; Jakobs, P.J.; Jozwiak, K.; Heeren, A.M.; de Jong, J.; Sanders, J.; Horenblas, S.; Jordanova, E.S. The Prognostic Value of Immune Factors in the Tumor Microenvironment of Penile Squamous Cell Carcinoma. Front. Immunol. 2018, 9, 1253. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.H.; Han, H.; Lu, J.B.; Liu, T.Y.; Huang, K.B.; Deng, C.Z.; Li, Z.S.; Chen, J.P.; Yao, K.; Qin, Z.K.; et al. Up-regulation of indoleamine 2,3-dioxygenase 1 (IDO1) expression and catalytic activity is associated with immunosuppression and poor prognosis in penile squamous cell carcinoma patients. Cancer Commun. (Lond. Engl.) 2020, 40, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Li, Z.; Guo, S.; Chen, P.; Chen, X.; Zhou, Q.; Chen, J.; Yu, X.; Wu, X.; Ma, W.; et al. Tumor PD-L1 expression is correlated with increased TILs and poor prognosis in penile squamous cell carcinoma. Oncoimmunology 2017, 6, e1269047. [Google Scholar] [CrossRef] [Green Version]
- Cocks, M.; Taheri, D.; Ball, M.W.; Bezerra, S.M.; Del Carmen Rodriguez, M.; Ricardo, B.F.P.; Bivalacqua, T.J.; Sharma, R.B.; Meeker, A.; Chaux, A.; et al. Immune-checkpoint status in penile squamous cell carcinoma: A North American cohort. Hum. Pathol. 2017, 59, 55–61. [Google Scholar] [CrossRef]
- Lohneis, P.; Boral, S.; Kaufmann, A.M.; Lehmann, A.; Schewe, C.; Dietel, M.; Anagnostopoulos, I.; Johrens, K. Human papilloma virus status of penile squamous cell carcinoma is associated with differences in tumour-infiltrating T lymphocytes. Virchows Arch. 2015, 466, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Udager, A.M.; Liu, T.Y.; Skala, S.L.; Magers, M.J.; McDaniel, A.S.; Spratt, D.E.; Feng, F.Y.; Siddiqui, J.; Cao, X.; Fields, K.L.; et al. Frequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: Potential opportunities for immunotherapeutic approaches. Ann. Oncol. 2016, 27, 1706–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassallo, J.; Rodrigues, A.F.; Campos, A.H.; Rocha, R.M.; da Cunha, I.W.; Zequi, S.C.; Guimaraes, G.C.; da Fonseca, F.P.; Lopes, A.; Cubilla, A.; et al. Pathologic and imunohistochemical characterization of tumoral inflammatory cell infiltrate in invasive penile squamous cell carcinomas: Fox-P3 expression is an independent predictor of recurrence. Tumour Biol. 2015, 36, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Ottenhof, S.R.; Djajadiningrat, R.S.; de Jong, J.; Thygesen, H.H.; Horenblas, S.; Jordanova, E.S. Expression of Programmed Death Ligand 1 in Penile Cancer is of Prognostic Value and Associated with HPV Status. J. Urol. 2017, 197, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Wittekindt, C.; Reuschenbach, M.; Hennig, B.; Thevarajah, M.; Würdemann, N.; Prigge, E.S.; von Knebel Doeberitz, M.; Dreyer, T.; Gattenlöhner, S.; et al. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int. J. Cancer 2016, 138, 2263–2273. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, W.; Hao, D.; He, B.; Kong, L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int. Immunopharmacol. 2019, 70, 459–466. [Google Scholar] [CrossRef]
- Van Esch, E.M.; van Poelgeest, M.I.; Trimbos, J.B.; Fleuren, G.J.; Jordanova, E.S.; van der Burg, S.H. Intraepithelial macrophage infiltration is related to a high number of regulatory T cells and promotes a progressive course of HPV-induced vulvar neoplasia. Int. J. Cancer 2015, 136, E85–E94. [Google Scholar] [CrossRef]
- Seminerio, I.; Kindt, N.; Descamps, G.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Journe, F.; Saussez, S. High infiltration of CD68+ macrophages is associated with poor prognoses of head and neck squamous cell carcinoma patients and is influenced by human papillomavirus. Oncotarget 2018, 9, 11046–11059. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.; Kadletz, L.; Wiebringhaus, R.; Kenner, L.; Selzer, E.; Füreder, T.; Rajky, O.; Berghoff, A.S.; Preusser, M.; Heiduschka, G. PD-1 and PD-L1 expression in HNSCC primary cancer and related lymph node metastasis–impact on clinical outcome. Histopathology 2018, 73, 573–584. [Google Scholar] [CrossRef]
- Karpathiou, G.; Casteillo, F.; Giroult, J.B.; Forest, F.; Fournel, P.; Monaya, A.; Froudarakis, M.; Dumollard, J.M.; Prades, J.M.; Peoc’h, M. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: Immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget 2017, 8, 19310–19322. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, J.W.; Liu, L.N.; Flies, D.B.; Nie, X.X.; Toki, M.; Zhang, J.P.; Song, C.; Zarr, M.; Zhou, X.; et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. CR 2019, 38, 255. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Lee, J.E.; Sung, K.J.; Sung, Y.H.; Pack, C.G.; Jung, M.K.; Han, B.; et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Heeren, A.M.; Punt, S.; Bleeker, M.C.; Gaarenstroom, K.N.; van der Velden, J.; Kenter, G.G.; de Gruijl, T.D.; Jordanova, E.S. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod. Pathol. 2016, 29, 753–763. [Google Scholar] [CrossRef] [Green Version]
- Takashima, Y.; Sakakibara-Konishi, J.; Hatanaka, Y.; Hatanaka, K.C.; Ohhara, Y.; Oizumi, S.; Hida, Y.; Kaga, K.; Kinoshita, I.; Dosaka-Akita, H.; et al. Clinicopathologic Features and Immune Microenvironment of Non-Small-cell Lung Cancer With Primary Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. Clin. Lung Cancer 2018, 19, 352–359.e351. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.X.; Leng, R.X.; Fan, Y.G.; Pan, H.F.; Li, B.Z.; Wu, C.H.; Wu, Q.; Wang, N.N.; Xiong, Q.R.; Geng, X.P.; et al. Intratumoral and peritumoral expression of CD68 and CD206 in hepatocellular carcinoma and their prognostic value. Oncol. Rep. 2017, 38, 886–898. [Google Scholar] [CrossRef] [Green Version]
- Haque, A.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206(+) tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci. Rep. 2019, 9, 14611. [Google Scholar] [CrossRef] [PubMed]
- Jaynes, J.M.; Sable, R.; Ronzetti, M.; Bautista, W.; Knotts, Z.; Abisoye-Ogunniyan, A.; Li, D.; Calvo, R.; Dashnyam, M.; Singh, A.; et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Siglec-15: An Attractive Immunotherapy Target. Cancer Discov. 2020, 10, 7–8. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.-W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Wylie, K.M.; Wyczalkowski, M.A.; Karpova, A.; Ley, J.; Sun, S.; Mashl, R.J.; Liang, W.W.; Wang, X.; Johnson, K.; et al. Dynamic host immune response in virus-associated cancers. Commun. Biol. 2019, 2, 109. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO classification of tumours of the urinary system and male genital organs—Part A: Renal, penile, and testicular tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Chu, C.; Chen, K.; Tan, X.; Lu, J.; Yang, Y.; Zhang, Y.; Yao, K.; Cao, Y. Prevalence of human papillomavirus and implication on survival in Chinese penile cancer. Virchows Arch. 2020. [Google Scholar] [CrossRef]


| Variable | Total, n = 178 (%) | HrHPV−, n = 120 (%) | HrHPV+, n = 58 (%) | p |
|---|---|---|---|---|
| Age | ||||
| Median (range) | 52.0 (24.0–86.0) | 52.5 (26.0–79.0) | 51.5 (24.0–86.0) | 0.871 |
| Grade of differentiation | 0.081 | |||
| G1 | 106 (61.3) | 77 (67.0) | 29 (50.0) | |
| G2 | 53 (30.6) | 31 (27.0) | 22 (37.9) | |
| G3 | 14 (8.1) | 7 (6.1) | 7 (12.1) | |
| T stage | 0.255 | |||
| T1 | 66 (37.9) | 46 (39.3) | 20 (35.1) | |
| T2 | 85 (48.9) | 56 (47.9)) | 29 (50.9) | |
| T3 | 14 (8.0) | 7 (6.0) | 7 (12.3) | |
| T4 | 9 (5.2) | 8 (6.8) | 1 (1.8) | |
| N stage | 0.856 | |||
| N0 | 103 (57.9) | 70 (58.3) | 33 (56.9) | |
| N+ | 75 (42.1) | 50 (41.7) | 25 (43.1) | |
| Histological subtype | 0.143 | |||
| HPV-related | 16 (9.2) | 8 (7.0) | 8 (13.8) | |
| Non HPV-related | 157 (90.8) | 107 (93.0) | 50 (86.2) | |
| M stage | 0.817 | |||
| M0 | 171 (96.1) | 115 (95.8) | 56 (96.6) | |
| M1 | 7 (3.9) | 5 (4.2) | 2 (3.4) | |
| LVI | 0.256 | |||
| No | 152 (88.4) | 103 (90.4) | 49 (84.5) | |
| Yes | 20 (11.6) | 11 (9.6) | 9 (15.5) | |
| NI | 0.351 | |||
| No | 145 (84.3) | 94 (82.5) | 51 (87.9) | |
| Yes | 27 (15.7) | 20 (17.5) | 7 (12.1) | |
| Necrosis | 0.527 | |||
| No | 152 (88.4) | 102 (89.5) | 50 (86.2) | |
| Yes | 20 (11.6) | 12 (10.5) | 8 (13.8) | |
| Death by penile cancer | 0.019 | |||
| No | 134 (75.3) | 84 (70.0) | 50 (86.2) | |
| Yes | 44 (24,7) | 36 (30.0) | 8 (13.8) |
| Variable | Contrast | Multivariable Analysis | |
|---|---|---|---|
| HR [95% CI] | p | ||
| T stage | Per T stage | 1.709 [1.181–2.472] | 0.004 |
| N stage | N+ vs N0 | 30.403 [7.212–128.174] | <0.001 |
| hrHPV status | Positive vs. Negative | 0.359 [0.162-0.797] | 0.024 |
| Immune clusters | A and C vs. B and D | 2.349 [1.191-4.633] | 0.014 |
| Variable | Total, n (%) | Cluster A, n (%) | Cluster B, n (%) | Cluster C, n (%) | Cluster D, n (%) | p |
|---|---|---|---|---|---|---|
| Age | 51.9 ± 13.61 | 48.37 ± 14.77 | 51.13 ± 13.52 | 53.93 ± 16.31 | 52.76 ± 12.16 | 0.391 |
| Grade of differentiation | 0.066 | |||||
| G1 | 95 (60.1) | 19 (65.5) | 12 (52.2) | 12 (46.2) | 52 (65.0) | |
| G2 | 50 (31.6) | 8 (27.6) | 7 (30.4) | 9 (34.6) | 26 (32.5) | |
| G3 | 13 (8.2) | 2 (6.9) | 4 (17.4) | 5 (19.2) | 2 (2.5) | |
| T stage | 0.013 | |||||
| T1 and T2 | 137 (86.2) | 20 a (66.7) | 22 a,b (95.7) | 23 a,b (92.0) | 72 b (88.9) | |
| T3 and T4 | 22 (13.8) | 10 a (33.3) | 1 a,b (4.3) | 2 a,b (8.1) | 9 b (11.1) | |
| N stage | 0.020 | |||||
| N0 | 91 (55.8) | 12 a (40.0) | 13 b (54.2) | 11 a (40.7) | 55 b (67.1) | |
| N+ | 72 (44.2) | 18 a (60.0) | 11 b (45.8) | 16 a (59.3) | 27 b (32.9) | |
| Histological subtype | 0.421 | |||||
| HPV-related | 16 (10.1) | 1 (3.4) | 4 (17.4) | 3 (11.5) | 8 (10.0) | |
| Non HPV-related | 142 (89.9) | 28 (96.6) | 19 (82.6) | 23 (88.5) | 72 (90.0) | |
| M stage | 0.557 | |||||
| M0 | 156 (95.7) | 28 (93.3) | 22 (91.7) | 26 (96.3) | 80 (97.6) | |
| M1 | 7 (4.3) | 2 (6.7) | 2 (8.3) | 1 (3.7) | 2 (2.4) | |
| LVI | 0.282 | |||||
| No | 138 (87.9) | 24 (82.8) | 22 (95.7) | 20 (80.0) | 72 (90.0 | |
| Yes | 19 (11.6) | 5 (17.2) | 1 (4.3) | 5 (20.0) | 8 (10.0) | |
| NI | 0.563 | |||||
| No | 131 (83.4) | 24 (82.8) | 21 (91.3) | 19 (76.0) | 67 (83.8) | |
| Yes | 26 (16.6) | 5 (17.2) | 2 (8.7) | 6 (24.0) | 13 (16.2) | |
| Necrosis | 0.597 | |||||
| No | 138 (87.9) | 25 (86.2) | 19 (82.6) | 21 (84.0) | 73 (91.2) | |
| Yes | 19 (12.1) | 4 (13.8) | 4 (17.4) | 4 (16.0) | 7 (8.8) | |
| Death by penile cancer | <0.001 | |||||
| No | 114 (73.0) | 14 a (46.7) | 20 b (83.3) | 15 a (55.6) | 70 b (85.4) | |
| Yes | 44 (27.0) | 16 a (53.3) | 4 b (16.7) | 12 a (44.4) | 12 b (14.6) | |
| hrHPV | 0.538 | |||||
| Negative | 110 (67.5) | 22 (73.3) | 17 (70.8) | 20 (74.1) | 51 (62.2) | |
| Positive | 53 (32.5) | 8 (26.7) | 7 (29.2) | 7 (25.9) | 31 (37.8) |
| Variable | Contrast | Multivariable Analysis | |
|---|---|---|---|
| HR [95% CI] | p | ||
| Tumor grade | Per grade | 3.610 [1.815–7.181] | <0.001 |
| T stage | Per T stage | 1.908 [1.082–3.364] | 0.026 |
| LVI | Present vs. absent | 41.248 [3.657–465.225] | 0.003 |
| Histological Subtypes | Related vs. Non-related | 0.105 [0.016–0.664] | 0.017 |
| Immune clusters | A and C vs. B and D | 2.482 [1.057–5.833] | 0.037 |
| Marker | Principal Role | Catalog Number | Company | Dilution | Positive Control | Cellular Localization |
|---|---|---|---|---|---|---|
| CD8 | Cytotoxic T cell | ZA-0508 | ZSJQ-BIO | 1:200 | Tonsil | Membrane/cytoplasm |
| FOXP3 | Regulatory T cell | YT6169 | Immunoway | 1:100 | Tonsil | Nucleus |
| Granzyme B | Cytotoxic T cell granules | ZA-0599 | ZSJQ-BIO | 1:200 | Tonsil | Cytoplasm |
| CD68 | Macrophage | ZM-0060 | ZSJQ-BIO | 1:200 | Tonsil | Cytoplasm |
| CD206 | M2 macrophage | Ab64693 | Abcam | 1:100 | Lung | Membrane/cytoplasm |
| PD-1 | Immune checkpoint | ZM-0381 | ZSJQ-BIO | 1:200 | Tonsil | Cytoplasm/membrane |
| CTLA-4 | Immune checkpoint | Ab227709 | Abcam | 1:100 | Tonsil | Membrane/cytoplasm |
| PD-L1 | Immune checkpoint | 13684(E1L3N) | Cell Signaling | 1:400 | Lung carcinoma | Membrane/cytoplasm |
| Siglec-15 | Immune checkpoint | Ab198684 | Abcam | 1:100 | Tonsil | Cytoplasm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.; Yao, K.; Lu, J.; Zhang, Y.; Chen, K.; Lu, J.; Zhang, C.Z.; Cao, Y. Immunophenotypes Based on the Tumor Immune Microenvironment Allow for Unsupervised Penile Cancer Patient Stratification. Cancers 2020, 12, 1796. https://doi.org/10.3390/cancers12071796
Chu C, Yao K, Lu J, Zhang Y, Chen K, Lu J, Zhang CZ, Cao Y. Immunophenotypes Based on the Tumor Immune Microenvironment Allow for Unsupervised Penile Cancer Patient Stratification. Cancers. 2020; 12(7):1796. https://doi.org/10.3390/cancers12071796
Chicago/Turabian StyleChu, Chengbiao, Kai Yao, Jiangli Lu, Yijun Zhang, Keming Chen, Jiabin Lu, Chris Zhiyi Zhang, and Yun Cao. 2020. "Immunophenotypes Based on the Tumor Immune Microenvironment Allow for Unsupervised Penile Cancer Patient Stratification" Cancers 12, no. 7: 1796. https://doi.org/10.3390/cancers12071796
APA StyleChu, C., Yao, K., Lu, J., Zhang, Y., Chen, K., Lu, J., Zhang, C. Z., & Cao, Y. (2020). Immunophenotypes Based on the Tumor Immune Microenvironment Allow for Unsupervised Penile Cancer Patient Stratification. Cancers, 12(7), 1796. https://doi.org/10.3390/cancers12071796





