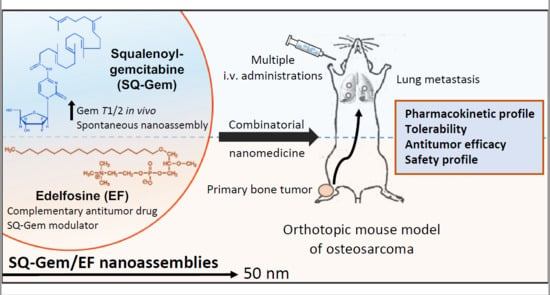
Combinatorial Nanomedicine Made of Squalenoyl-Gemcitabine and Edelfosine for the Treatment of Osteosarcoma
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterization of SQ–Gem and SQ–Gem/EF NAs
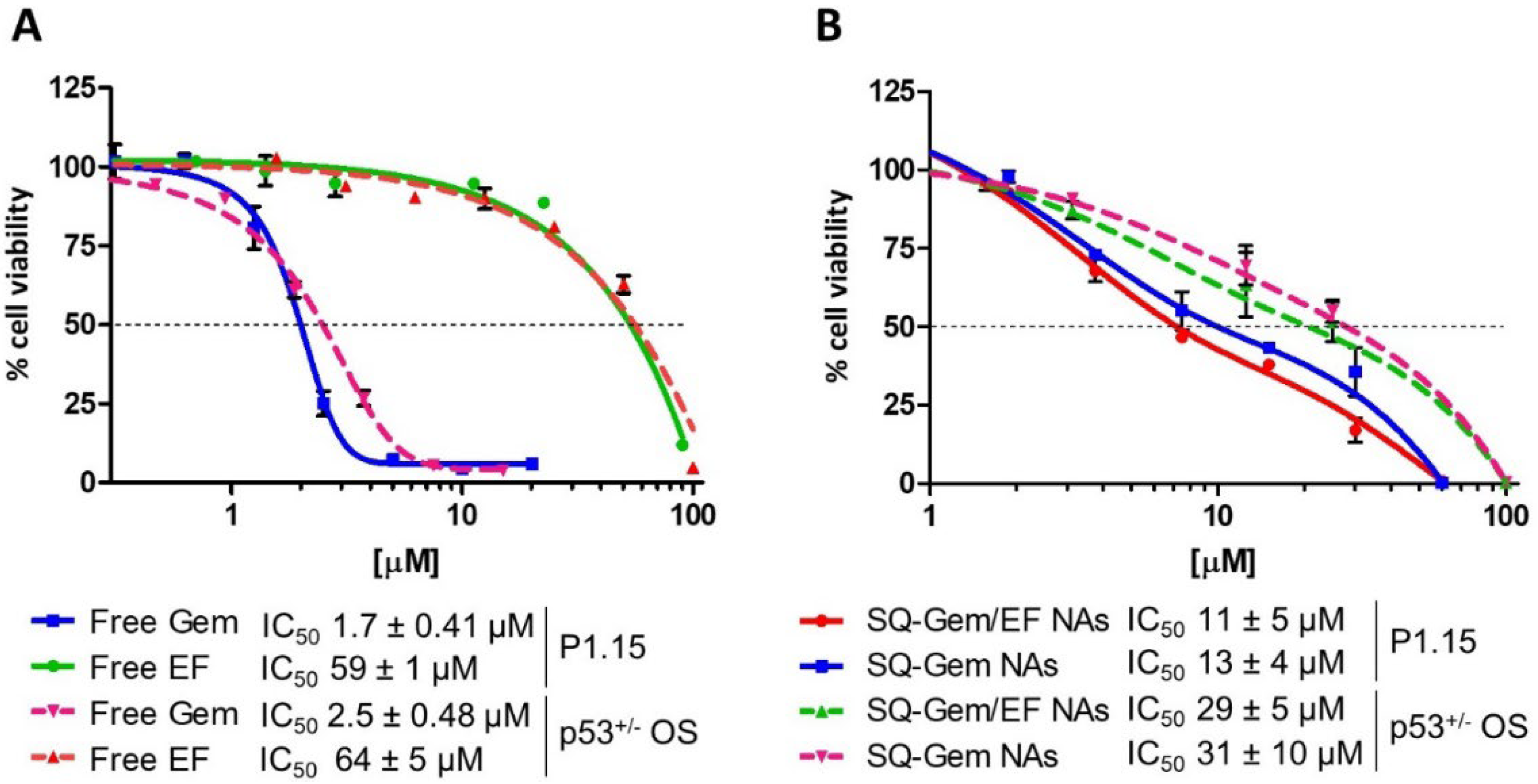
2.2. SQ–Gem and SQ–Gem/EF NAs Displayed a Similar Anticancer Activity In Vitro
2.3. SQ–Gem/EF NAs Drastically Diminished the Total Gem Pool Exposure in the Blood Stream in Comparison with SQ–Gem NAs
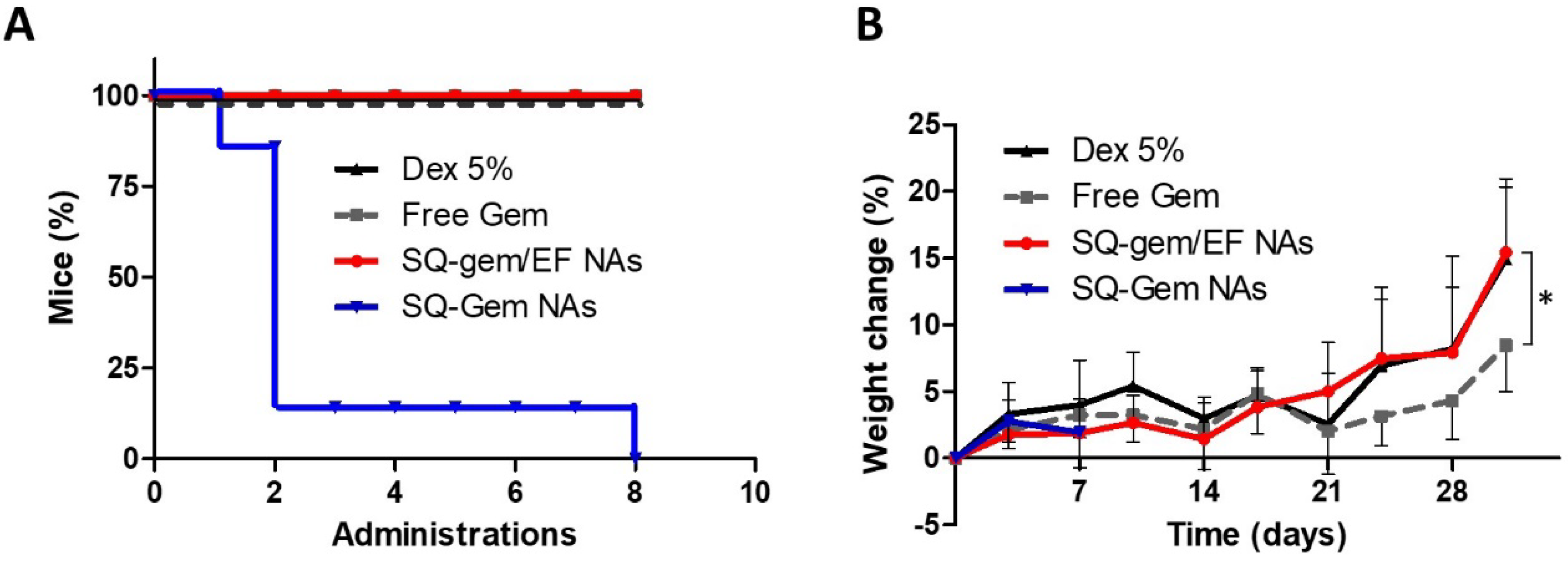
2.4. The Insertion of EF into SQ–Gem Increased NAs Tolerability
2.5. SQ–Gem/EF NAs Delayed the Progression of the Primary Tumor Growth and Lung Metastases in a P1.15 Orthotopic OS Model
2.6. Safety Profile
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Formulation and Characterization of Squalenoyl NAs
4.3. Cell Culture and Cytotoxicity Assays
4.4. Pharmacokinetic Studies
4.5. In Vivo Efficacy Studies
4.6. Clinical Chemistry and Hematology Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arndt, C.A.S.; Rose, P.S.; Folpe, A.L.; Laack, N.N. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin. Proc. 2012, 87, 475–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, A.; Yamamoto, N.; Hayashi, K.; Matsubara, H.; Miwa, S.; Igarashi, K. Joint-preservation surgery for pediatric osteosarcoma of the knee joint. Cancer Mestastasis Rev. 2019, 38, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Kager, L.; Tamamyan, G.; Bielack, S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017, 13, 357–368. [Google Scholar] [CrossRef]
- Botter, S.M.; Neri, D.; Fuchs, B. Recent advances in osteosarcoma. Curr. Opin. Pharmacol. 2014, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current treatment and a collaborative pathway to success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patiño-García, A.; Zalacain, M.; Folio, C.; Zandueta, C.; Sierrasesúmaga, L.; San Julián, M.; Toledo, G.; De Las Rivas, J.; Lecanda, F. Profiling of chemonaive osteosarcoma and paired-normal cells identifies EBF2 as a mediator of osteoprotegerin inhibition to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin. Cancer Res. 2009, 15, 5082–5091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, S.A.; Mirabello, L.; Wang, Z.; Gastier-Foster, J.M.; Gorlick, R.; Khanna, C.; Flanagan, A.M.; Tirabosco, R.; Andrulis, I.L.; Wunder, J.S.; et al. Genome-wide association study identifies two susceptibility loci for osteosarcoma. Nat. Genet. 2013, 45, 799–803. [Google Scholar] [CrossRef] [Green Version]
- Gianferante, D.M.; Mirabello, L.; Savage, S.A. Germline and somatic genetics of osteosarcoma—Connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017, 13, 480–491. [Google Scholar] [CrossRef]
- Lisa Mirabello, O.; Yeager, M.; Mai, P.L.; Gastier-Foster, J.M.; Gorlick, R.; Khanna, C.; Patiño-Garcia, A.; Sierrasesúmaga, L.; Lecanda, F.; Andrulis, I.L.; et al. Germline TP53 Variants and Susceptibility to. JNCI J. Natl. Cancer Inst. 2015, 107, 101. [Google Scholar]
- Liebner, D.A. The indications and efficacy of conventional chemotherapy in primary and recurrent sarcoma. J. Surg. Oncol. 2015, 111, 622–631. [Google Scholar] [CrossRef]
- Lim, J.W.; Yeap, F.S.H.; Chan, Y.H.; Yeoh, A.E.J.; Quah, T.C.; Tan, P.L. Second malignant neoplasms in childhood cancer survivors treated in a tertiary paediatric oncology centre. Ann. Acad. Med. Singap. 2017, 46, 11–19. [Google Scholar] [PubMed]
- Hattinger, C.M.; Patrizio, M.P.; Magagnoli, F.; Luppi, S.; Serra, M. An update on emerging drugs in osteosarcoma: Towards tailored therapies? Expert Opin. Emerg. Drugs 2019, 24, 153–171. [Google Scholar] [CrossRef]
- Grodzinski, P.; Kircher, M.; Goldberg, M.; Gabizon, A. Integrating nanotechnology into cancer care. ACS Nano 2019, 13, 7370–7376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Jones, R.L.; Marchesi, E.; Paioli, A.; Cesari, M.; Longhi, A.; Meazza, C.; Coccoli, L.; Fagioli, F.; Asaftei, S.; et al. Gemcitabine and docetaxel in relapsed and unresectable high-grade osteosarcoma and spindle cell sarcoma of bone. BMC Cancer 2016, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gesto, D.S.; Cerqueira, N.M.F.S.A.; Fernandes, P.A.; Ramos, M.J. Gemcitabine: A critical nucleoside for cancer therapy. Curr. Med. Chem. 2012, 19, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Reddy, L.H.; Othman, M.; Gillet, B.; Desmaële, D.; Zouhiri, F.; Dosio, F.; Gref, R.; Couvreur, P. Squalene based nanocomposites: A new platform for the design of multifunctional pharmaceutical theragnostics. ACS Nano 2011, 5, 1513–1521. [Google Scholar] [CrossRef]
- Couvreur, P.; Stella, B.; Reddy, L.H.; Hillaireau, H.; Dubernet, C.; Desmaële, D.; Lepêtre-Mouelhi, S.; Rocco, F.; Dereuddre-Bosquet, N.; Clayette, P.; et al. Squalenoyl nanomedicines as potential therapeutics. Nano Lett. 2006, 6, 2544–2548. [Google Scholar] [CrossRef]
- Reddy, L.H.; Marque, P.-E.; Dubernet, C.; Mouelhi, S.-L.; Desmaele, D.; Couvreur, P. Preclinical toxicology (subacute and acute) and efficacy of a new squalenoyl gemcitabine anticancer nanomedicine. J. Pharmacol. Exp. Ther. 2008, 325, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Nogales, C.; González-Fernández, Y.; Aldaz, A.; Couvreur, P.; Blanco-Prieto, M.J. Nanomedicines for pediatric cancers. ACS Nano 2018, 12, 7482–7496. [Google Scholar] [CrossRef]
- Teixeira, S.F.; Rodrigues, C.P.; Costa, C.J.S.; Pettinati, T.N.; de Azevedo, R.A.; Mambelli, L.I.; Jorge, S.D.; Ramos, R.N.; Ferro, E.S.; Barbuto, J.A.M.; et al. Edelfosine: An antitumor drug prototype. Anticancer. Agents Med. Chem. 2018, 18, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Gajate, C.; Mollinedo, F. Lipid Rafts, Endoplasmic reticulum and mitochondria in the antitumor action of the alkylphospholipid analog edelfosine. Anticancer Agents Med. Chem. 2014, 14, 509–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Nogales, C.; Sebastián, V.; Irusta, S.; Desmaële, D.; Couvreur, P.; Blanco-Prieto, M.J. A unique multidrug nanomedicine made of squalenoyl-gemcitabine and alkyl-lysophospholipid edelfosine. Eur. J. Pharm. Biopharm. 2019, 144, 165–173. [Google Scholar] [CrossRef]
- Kansara, M.; Teng, M.W.; Smyth, M.J.; Thomas, D.M. Translational biology of osteosarcoma. Nat. Rev. Cancer 2014, 14, 722–735. [Google Scholar] [CrossRef]
- Sobot, D.; Mura, S.; Yesylevskyy, S.O.; Dalbin, L.; Cayre, F.; Bort, G.; Mougin, J.; Desmaële, D.; Lepetre-Mouelhi, S.; Pieters, G.; et al. Conjugation of squalene to gemcitabine as unique approach exploiting endogenous lipoproteins for drug delivery. Nat. Commun. 2017, 8, 15678. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Dubernet, C.; Mouelhi, S.L.; Marque, P.E.; Desmaele, D.; Couvreur, P. A new nanomedicine of gemcitabine displays enhanced anticancer activity in sensitive and resistant leukemia types. J. Control. Release 2007, 124, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Khoury, H.; Paci, A.; Deroussent, A.; Ferreira, H.; Dubernet, C.; Declèves, X.; Besnard, M.; Chacun, H.; Lepêtre-Mouelhi, S.; et al. Squalenoylation favorably modifies the in vivo pharmacokinetics and biodistribution of gemcitabine in mice. Drug Metab. Dispos. 2008, 36, 1570–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksimenko, A.; Alami, M.; Zouhiri, F.; Brion, J.-D.; Pruvost, A.; Mougin, J.; Hamze, A.; Boissenot, T.; Provot, O.; Desmaële, D.; et al. Therapeutic modalities of squalenoyl nanocomposites in colon cancer: An ongoing search for improved efficacy. ACS Nano 2014, 8, 2018–2032. [Google Scholar] [CrossRef]
- Ando, T.; Ichikawa, J.; Okamoto, A.; Tasaka, K.; Nakao, A.; Hamada, Y. Gemcitabine inhibits viability, growth, and metastasis of osteosarcoma cell lines. J. Orthop. Res. 2005, 23, 964–969. [Google Scholar] [CrossRef]
- Zandueta, C.; Ormazábal, C.; Perurena, N.; Martínez-Canarias, S.; Zalacaín, M.; Julián, M.S.; Grigoriadis, A.E.; Valencia, K.; Campos-Laborie, F.J.; Rivas, J.D.L.; et al. Matrix-Gla protein promotes osteosarcoma lung metastasis and associates with poor prognosis. J. Pathol. 2016, 239, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Weekes, D.; Kashima, T.G.; Zandueta, C.; Perurena, N.; Thomas, D.P.; Sunters, A.; Vuillier, C.; Bozec, A.; El-Emir, E.; Miletich, I.; et al. Regulation of osteosarcoma cell lung metastasis by the c-Fos/AP-1 target FGFR1. Oncogene 2016, 35, 2852–2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, G.; Zamzam, M.; Kamel, A.; Ahmed, S.; Salama, A.; Zaki, I.; Kamal, N.; Elshafiey, M. Effect of timing of pulmonary metastasis occurrence on the outcome of metastasectomy in osteosarcoma patients. J. Pediatr. Surg. 2019, 54, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Briglia, M.; Fazio, A.; Signoretto, E.; Faggio, C.; Lang, F. Edelfosine induced suicidal death of human erythrocytes. Cell. Physiol. Biochem. 2015, 37, 2221–2230. [Google Scholar] [CrossRef] [PubMed]
- Berdel, W.E.; Fink, U.; Rastetter, J. Clinical phase I pilot study of the alkyl lysophospholipid derivative ET-18-OCH3 1. Lipids 1987, 22, 967–969. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, Y.; Brown, H.K.; Patiño-García, A.; Heymann, D.; Blanco-Prieto, M.J. Oral administration of edelfosine encapsulated lipid nanoparticles causes regression of lung metastases in pre-clinical models of osteosarcoma. Cancer Lett. 2018, 430, 193–200. [Google Scholar] [CrossRef]
- Fossella, F.V.; Lippman, S.M.; Shin, D.M.; Tarassoff, P.; Calayag-Jung, M.; Perez-Soler, R.; Lee, J.S.; Murphy, W.K.; Glisson, B.; Rivera, E.; et al. Maximum-tolerated dose defined for single-agent gemcitabine: A phase I dose-escalation study in chemotherapy-naive patients with advanced non- small-cell lung cancer. J. Clin. Oncol. 1997, 15, 310–316. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Hu, H.-Z.; Qing, X.-C.; Zhang, Z.-C.; Shao, Z.-W. Recent advances of drug delivery nanocarriers in osteosarcoma treatment. J. Cancer 2020, 11, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Popescu, R.C.; Andronescu, E.; Vasile, B.Ș.; Truşcă, R.; Boldeiu, A.; Mogoantă, L.; Mogoșanu, G.D.; Temelie, M.; Radu, M.; Grumezescu, A.M.; et al. Fabrication and cytotoxicity of gemcitabine-functionalized magnetite nanoparticles. Molecules 2017, 22, 1080. [Google Scholar] [CrossRef] [Green Version]
- Caliskan, Y.; Dalgic, A.D.; Gerekci, S.; Gulec, E.A.; Tezcaner, A.; Ozen, C.; Keskin, D. A new therapeutic combination for osteosarcoma: Gemcitabine and Clofazimine co-loaded liposomal formulation. Int. J. Pharm. 2019, 557, 97–104. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, L.; Bao, Y.; Zhong, X.; Chen, Y.; Wu, Q. Clinical evaluation of neoadjuvant chemotherapy for osteosarcoma. J. BUON. 2019, 24, 1181–1185. [Google Scholar]
- Grigoriadis, A.E.; Schellander, K.; Wang, Z.Q.; Wagner, E.F. Osteoblasts are target cells for transformation in c-fos transgenic mice. J. Cell Biol. 1993, 122, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nogales, C.; Mura, S.; Couvreur, P.; Blanco-Prieto, M.J. Squalenoyl–gemcitabine/edelfosine nanoassemblies: Anticancer activity in pediatric cancer cells and pharmacokinetic profile in mice. Int. J. Pharm. 2020, 582, 119345. [Google Scholar]



| NAs | Size (nm) | PDI | ζ (mV) 1 | % Gem 2 | % EF 2 | % Total 2 |
|---|---|---|---|---|---|---|
| SQ–Gem/EF | 50 ± 4 | 0.12 ± 0.03 | −12 ± 1 | 22.49 | 44.78 | 67.28 |
| SQ–Gem | 116 ± 10 | 0.09 ± 0.01 | −17 ±1 | 40.7 | 0 | 40.7 |
| Parameter | SQ–Gem NAs | SQ–Gem/EF NAs | |||
|---|---|---|---|---|---|
| SQ–Gem | Gem | SQ–Gem | Gem | EF | |
| t1/2 (h) | 2.12 ± 0.12 | 4.49 ± 0.47 | 2.37 ± 1.44 | 5 ± 0.6 | 30 ± 10 |
| Cmax (μg/mL) | 49 ± 13 | 3.3 ± 0.1 | 79 ± 10 | 3.65 ± 1.34 | 42 ± 6 |
| Cl_obs (mL/kg/h) | 462 ± 159 | 689 ± 47 | 948± 86 | 856 ± 18 | 86 ± 7 |
| Vss_obs (mL/kg) | 902 ± 280 | 4526 ± 165 | 1265 ± 694 | 7334 ± 1073 | 3534 ± 788 |
| AUC 0-inf_obs (μg·h/mL) | 88 ± 34 | 21 ± 1 | 39 ± 4 | 17 ± 0.38 | 346 ± 30 |
| Parameters | Units | Dex 5% | Free Gem | SQ–Gem/EF NAs |
|---|---|---|---|---|
| Plasma | ||||
| ALT | (UI/L) | 20.3 | 24.2 | 17.8 |
| AST | (UI/L) | 177.8 | 129.4 | 141.5 |
| Creatinine | (mg/100 mL) | 0.093 | 0.063 | 0.092 |
| Urea | (mg/100 mL) | 42.1 | 45.6 | 37.2 |
| Blood | ||||
| WBC | [103/µL] | 3.09 | 1.79 ** | 1.83 ** |
| Neutrophils | [103/µL] | 1.56 | 0.54 *** | 0.70 ** |
| Neutrophils | (%) | 50.59 | 31.60 ** | 39.54 |
| RBC | [106/µL] | 8.82 | 7.93 * | 7.94 * |
| HGB | [g/dL] | 14.41 | 12.93 * | 13.41 |
| Platelets | [103/µL] | 711 | 753.5 | 710.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Nogales, C.; Moreno, H.; Zandueta, C.; Desmaële, D.; Lecanda, F.; Couvreur, P.; Blanco-Prieto, M.J. Combinatorial Nanomedicine Made of Squalenoyl-Gemcitabine and Edelfosine for the Treatment of Osteosarcoma. Cancers 2020, 12, 1895. https://doi.org/10.3390/cancers12071895
Rodríguez-Nogales C, Moreno H, Zandueta C, Desmaële D, Lecanda F, Couvreur P, Blanco-Prieto MJ. Combinatorial Nanomedicine Made of Squalenoyl-Gemcitabine and Edelfosine for the Treatment of Osteosarcoma. Cancers. 2020; 12(7):1895. https://doi.org/10.3390/cancers12071895
Chicago/Turabian StyleRodríguez-Nogales, Carlos, Haritz Moreno, Carolina Zandueta, Didier Desmaële, Fernando Lecanda, Patrick Couvreur, and María J. Blanco-Prieto. 2020. "Combinatorial Nanomedicine Made of Squalenoyl-Gemcitabine and Edelfosine for the Treatment of Osteosarcoma" Cancers 12, no. 7: 1895. https://doi.org/10.3390/cancers12071895