Characterization of mTOR Activity and Metabolic Profile in Pediatric Rhabdomyosarcoma
Abstract
1. Introduction
2. Results
2.1. Patients and Samples
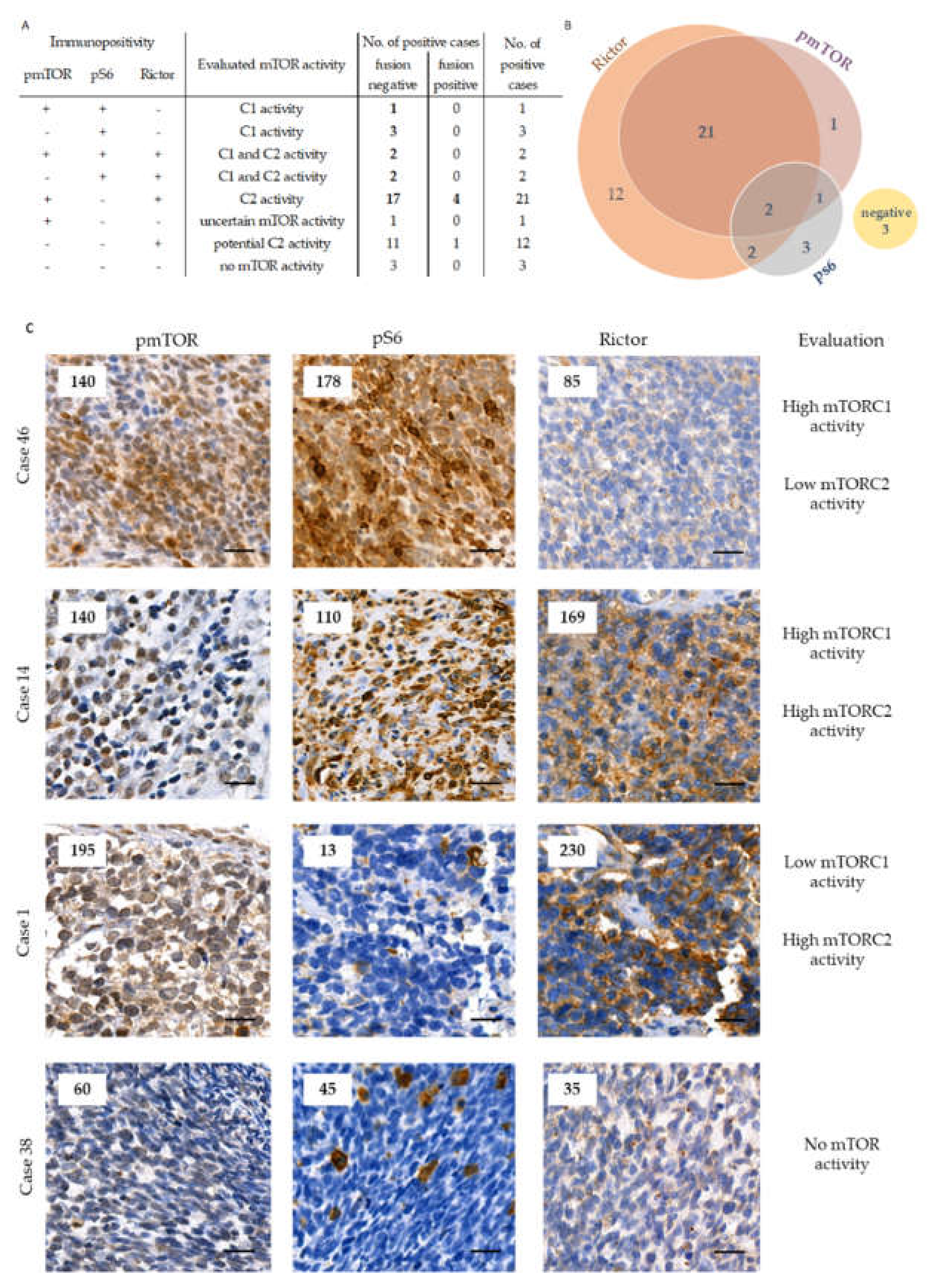
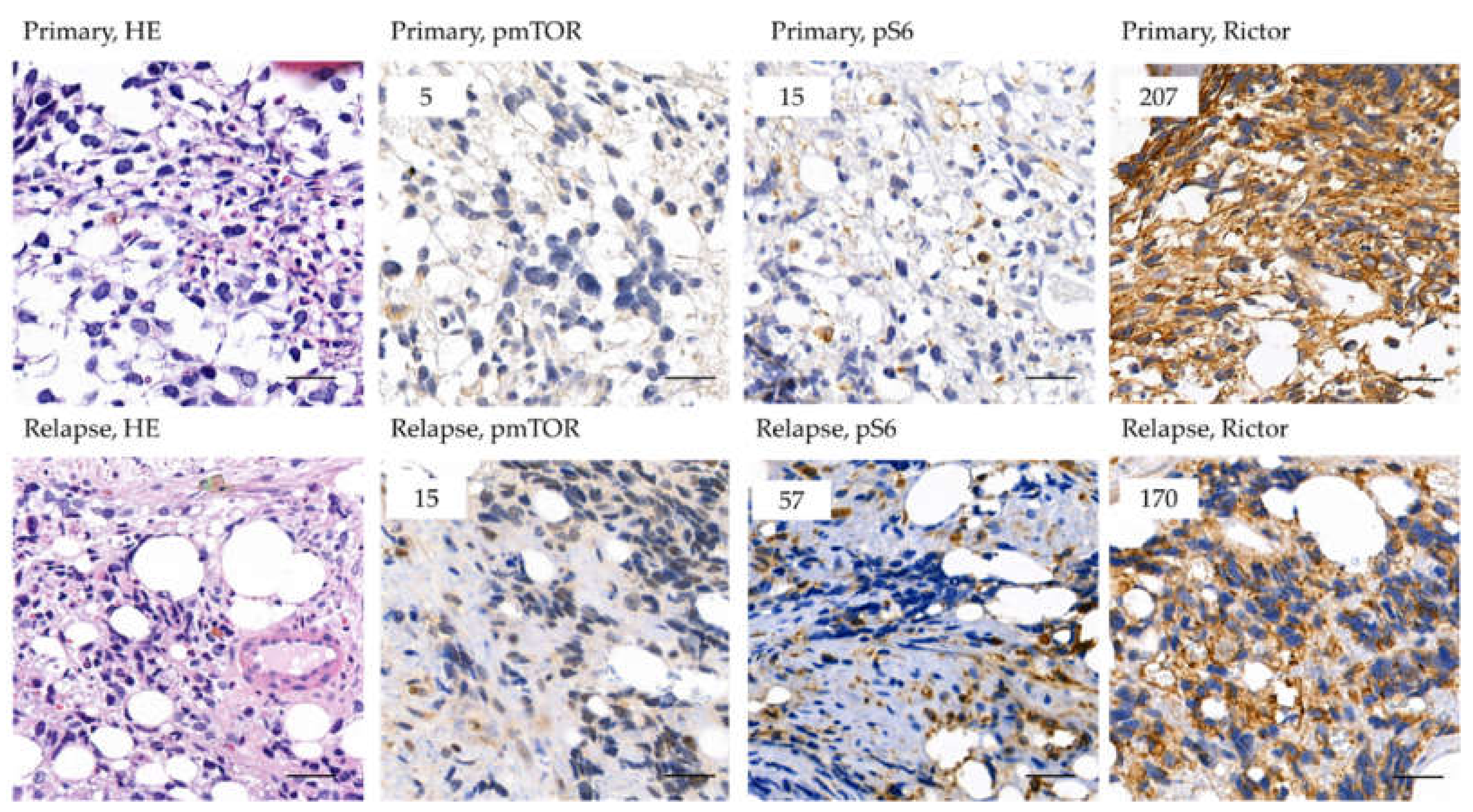
2.2. The Expression of mTOR Activity-Related Proteins in RMS
2.3. Analysis of Rictor Amplification in Rhabdomyosarcomas
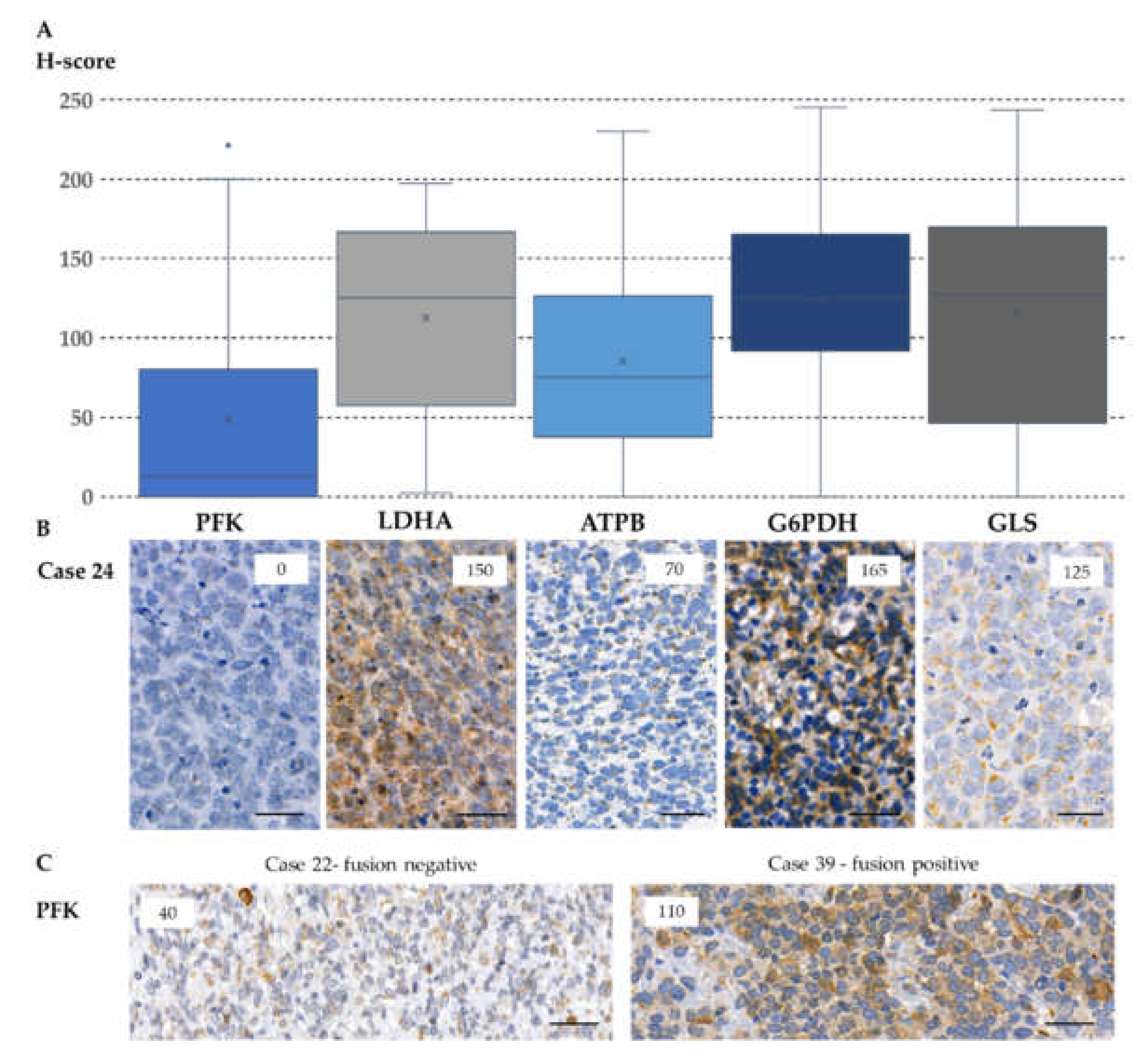
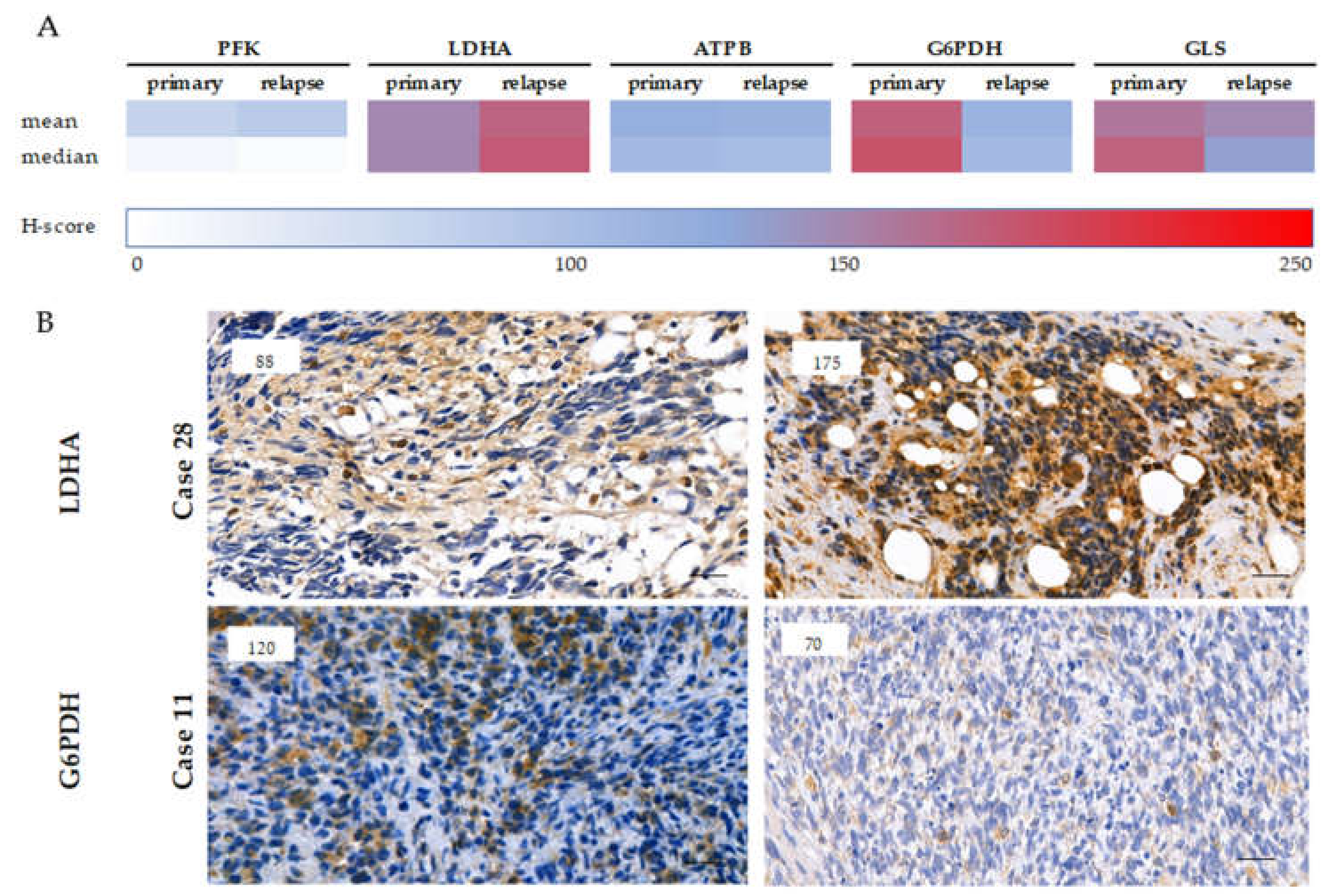
2.4. Expression of Metabolic Pathway-Related Proteins, Footprint of Warburg Phenotype, and Glutaminolysis in Rhabdomyosarcoma Cells
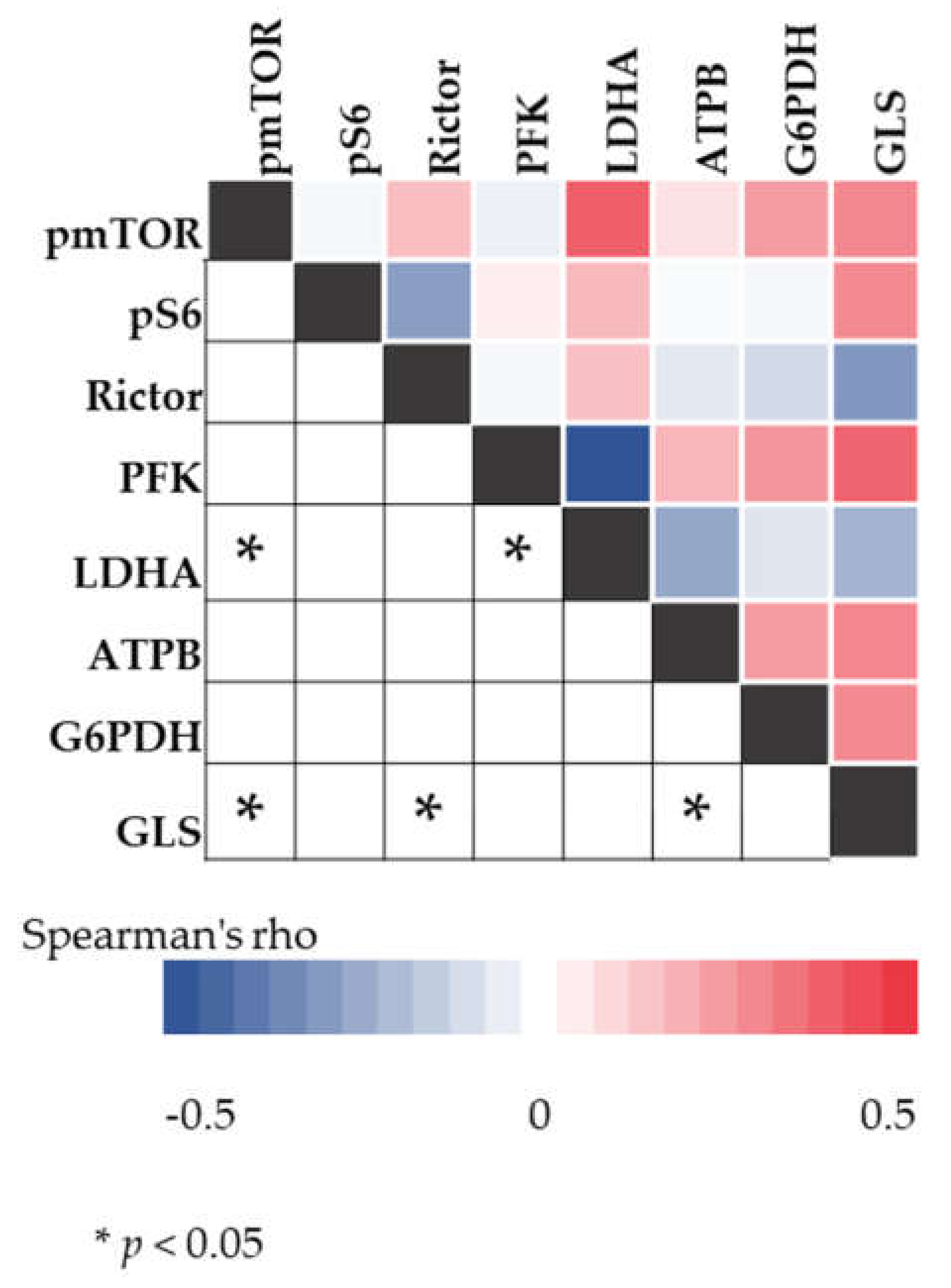
2.5. Correlation between mTOR and Metabolic Pathway-Related Proteins
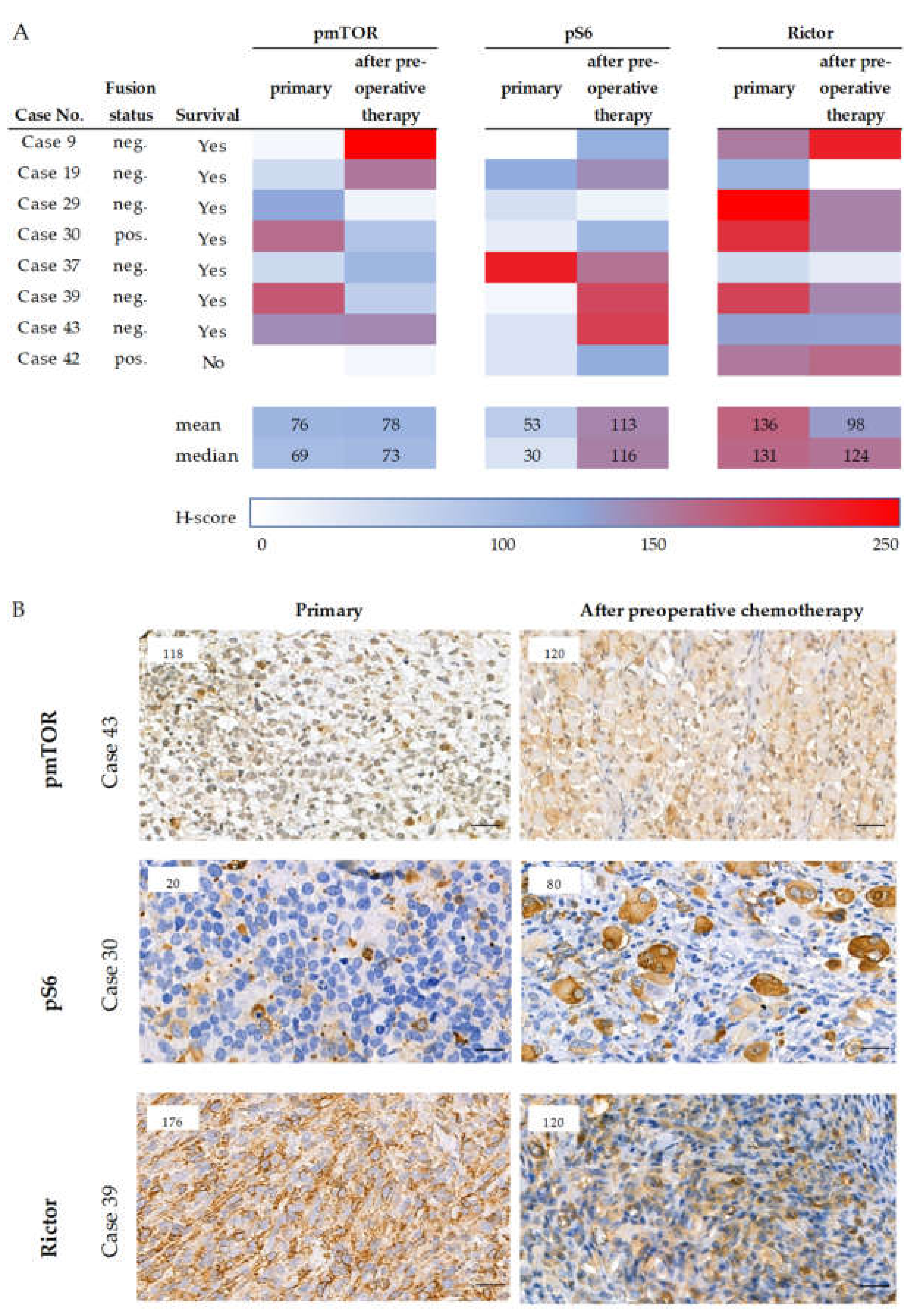
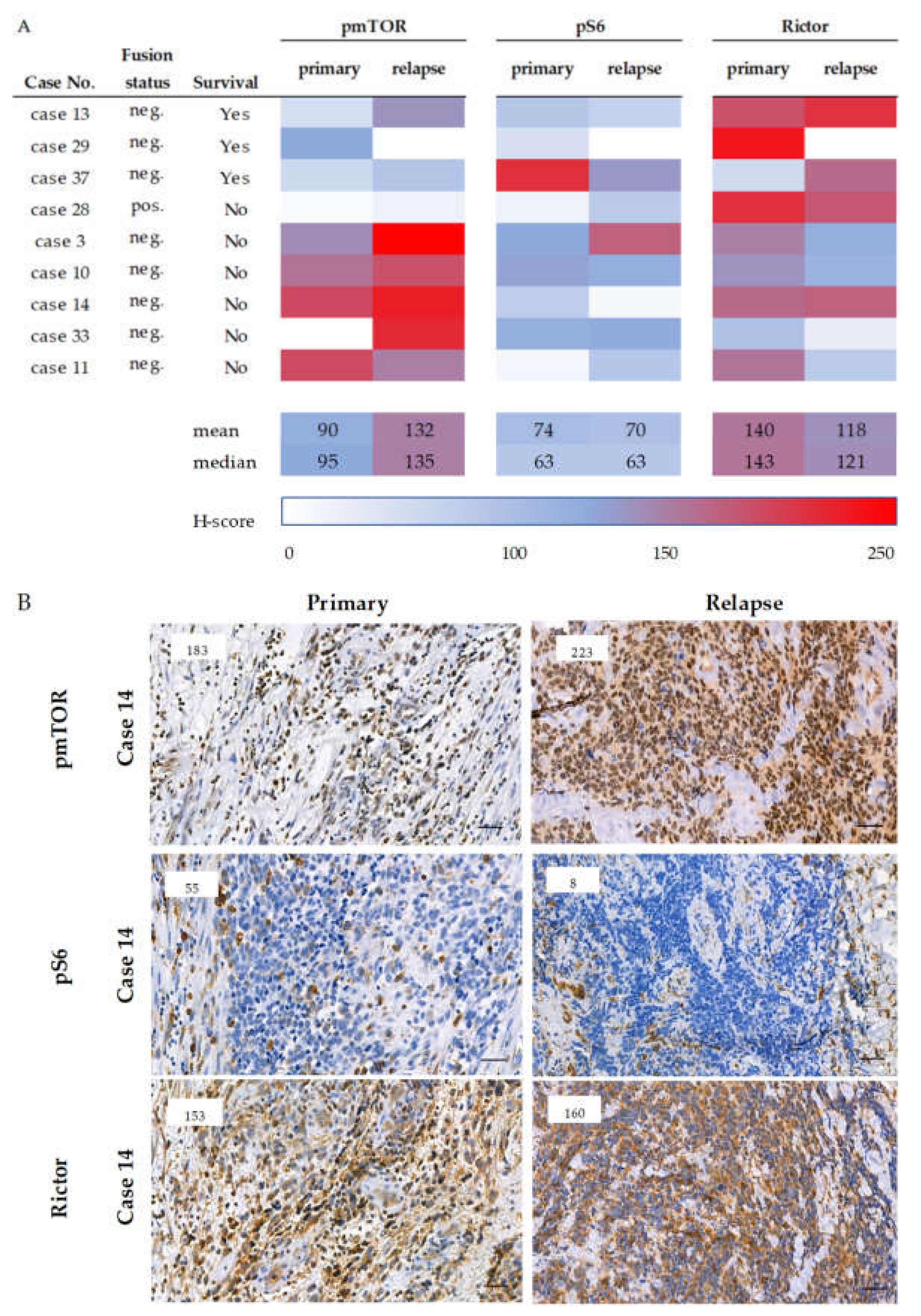
2.6. Survival and the Progression of a Studied Rhabdomyosarcoma Case
3. Discussion
4. Materials and Methods
4.1. Tissue Microarray Construction and Immunohistochemistry
4.2. RICTOR Fluorescence in Situ Hybridization and Droplet Digital PCR for RICTOR Copy Number Variation Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pizzo, P.A.; Poplack, D.G.; Adamson, P.C.; Blaney, S.M.; Helman, L.J. Principles and Practice of Pediatric Oncology, 7th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2015; p. 1266. [Google Scholar]
- Saletta, F.; Wadham, C.; Ziegler, D.S.; Marshall, G.M.; Haber, M.; McCowage, G.; Norris, M.D.; Byrne, J.A. Molecular profiling of childhood cancer: Biomarkers and novel therapies. BBA Clin. 2014, 1, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Helman, L.J.; Meltzer, P. Mechanisms of sarcoma development. Nat. Rev. Cancer 2003, 3, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Skapek, S.X.; Ferrari, A.; Gupta, A.A.; Lupo, P.J.; Butler, E.; Shipley, J.; Barr, F.G.; Hawkins, D.S. Rhabdomyosarcoma. Nat. Rev. Dis. Primers 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, T.; Venier, R.; Yohe, M.; Sindiri, S.; Gryder, B.E.; Shern, J.F.; Kabaroff, L.; Dickson, B.; Schleicher, K.; Chouinard-Pelletier, G.; et al. Functional screening of FGFR4-driven tumorigenesis identifies PI3K/mTOR inhibition as a therapeutic strategy in rhabdomyosarcoma. Oncogene 2018, 37, 2630–2644. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Helman, L.J. The biology behind mTOR inhibition in sarcoma. Oncologist 2007, 12, 1007–1018. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Gupta, S.; Roy, A.; Dwarakanath, B.S. Metabolic Cooperation and Competition in the Tumor Microenvironment: Implications for Therapy. Front. Oncol. 2017, 7, 68. [Google Scholar] [CrossRef]
- Pópulo, H.; Lopes, J.M.; Soares, P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Coller, H.A. Is cancer a metabolic disease? Am. J. Pathol. 2014, 184, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu. Rev. Biomed. Eng. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Arnoczky, K.J.; Hsieh, F.C.; Lin, H.J.; Qualman, S.J.; Yu, S.; Xiang, H.; Lin, J. Phosphorylation profiles of protein kinases in alveolar and embryonal rhabdomyosarcoma. Mod. Pathol. 2007, 20, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Kaylani, S.Z.; Xu, J.; Srivastava, R.K.; Kopelovich, L.; Pressey, J.G.; Athar, M. Rapamycin targeting mTOR and hedgehog signaling pathways blocks human rhabdomyosarcoma growth in xenograft murine model. Biochem. Biophys. Res. Commun. 2013, 435, 557–561. [Google Scholar] [CrossRef]
- Houghton, P.J.; Morton, C.L.; Kolb, E.A.; Gorlick, R.; Lock, R.; Carol, H.; Reynolds, C.P.; Maris, J.M.; Keir, S.T.; Billups, C.A.; et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr. Blood Cancer 2008, 50, 799–805. [Google Scholar] [CrossRef]
- Mascarenhas, L.; Chi, Y.Y.; Hingorani, P.; Anderson, J.R.; Lyden, E.R.; Rodeberg, D.A.; Indelicato, D.J.; Kao, S.C.; Dasgupta, R.; Spunt, S.L.; et al. Randomized Phase II Trial of Bevacizumab or Temsirolimus in Combination With Chemotherapy for First Relapse Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2019, JCO1900576. [Google Scholar] [CrossRef]
- Geoerger, B.; Kieran, M.W.; Grupp, S.; Perek, D.; Clancy, J.; Krygowski, M.; Ananthakrishnan, R.; Boni, J.P.; Berkenblit, A.; Spunt, S.L. Phase II trial of temsirolimus in children with high-grade glioma, neuroblastoma and rhabdomyosarcoma. Eur J. Cancer 2012, 48, 253–262. [Google Scholar] [CrossRef]
- Balogh, P.; Bánusz, R.; Csóka, M.; Váradi, Z.; Varga, E.; Sápi, Z. Primary alveolar rhabdomyosarcoma of the bone: Two cases and review of the literature. Diagn. Pathol. 2016, 11, 99. [Google Scholar] [CrossRef]
- Espina, V.; Edmiston, K.H.; Heiby, M.; Pierobon, M.; Sciro, M.; Merritt, B.; Banks, S.; Deng, J.; VanMeter, A.J.; Geho, D.H.; et al. A portrait of tissue phosphoprotein stability in the clinical tissue procurement process. Mol. Cell Proteom. 2008, 7, 1998–2018. [Google Scholar] [CrossRef]
- Theiss, A.P.; Chafin, D.; Bauer, D.R.; Grogan, T.M.; Baird, G.S. Immunohistochemistry of colorectal cancer biomarker phosphorylation requires controlled tissue fixation. PLoS ONE 2014, 9, e113608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sebestyén, A.; Sticz, T.B.; Márk, A.; Hajdu, M.; Timár, B.; Nemes, K.; Nagy, N.; Váradi, Z.; Kopper, L. Activity and complexes of mTOR in diffuse large B-cell lymphomas--a tissue microarray study. Mod. Pathol 2012, 25, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Krencz, I.; Sebestyen, A.; Papay, J.; Lou, Y.; Lutz, G.F.; Majewicz, T.L.; Khoor, A. Correlation between immunohistochemistry and RICTOR fluorescence in situ hybridization amplification in small cell lung carcinoma. Hum. Pathol 2019, 93, 74–80. [Google Scholar] [CrossRef]
- Gkountakos, A.; Pilotto, S.; Mafficini, A.; Vicentini, C.; Simbolo, M.; Milella, M.; Tortora, G.; Scarpa, A.; Bria, E.; Corbo, V. Unmasking the impact of Rictor in cancer: Novel insights of mTORC2 complex. Carcinogenesis 2018, 39, 971–980. [Google Scholar] [CrossRef]
- GPOH. CWS-Guidance for Risk Adapted Treatment of Soft Tissue Sarcoma and Soft Tissue Tumours in Children, Adolescents, and Young Adults; GPOH: Stuttgart, Germany, 2012. [Google Scholar]
- Hawkins, D.S.; Spunt, S.L.; Skapek, S.X.; Committee, C.S.T.S. Children’s Oncology Group’s 2013 blueprint for research: Soft tissue sarcomas. Pediatr. Blood Cancer 2013, 60, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Pugach, E.K.; Hettmer, S.; Storer, N.Y.; Liu, J.; Wills, A.A.; DiBiase, A.; Chen, E.Y.; Ignatius, M.S.; Poss, K.D.; et al. A novel chemical screening strategy in zebrafish identifies common pathways in embryogenesis and rhabdomyosarcoma development. Development 2013, 140, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, J.; Taylor, K.R.; Bishop, R.; Valenti, M.; De Haven Brandon, A.; Gowan, S.; Eccles, S.A.; Ruddle, R.R.; Johnson, L.D.; Raynaud, F.I.; et al. Dual blockade of the PI3K/AKT/mTOR (AZD8055) and RAS/MEK/ERK (AZD6244) pathways synergistically inhibits rhabdomyosarcoma cell growth in vitro and in vivo. Clin. Cancer Res. 2013, 19, 5940–5951. [Google Scholar] [CrossRef]
- Cen, L.; Hsieh, F.C.; Lin, H.J.; Chen, C.S.; Qualman, S.J.; Lin, J. PDK-1/AKT pathway as a novel therapeutic target in rhabdomyosarcoma cells using OSU-03012 compound. Br. J. Cancer 2007, 97, 785–791. [Google Scholar] [CrossRef]
- Petricoin, E.F.; Espina, V.; Araujo, R.P.; Midura, B.; Yeung, C.; Wan, X.; Eichler, G.S.; Johann, D.J.; Qualman, S.; Tsokos, M.; et al. Phosphoprotein pathway mapping: Akt/mammalian target of rapamycin activation is negatively associated with childhood rhabdomyosarcoma survival. Cancer Res. 2007, 67, 3431–3440. [Google Scholar] [CrossRef]
- Krześniak, M.; Zajkowicz, A.; Matuszczyk, I.; Rusin, M. Rapamycin prevents strong phosphorylation of p53 on serine 46 and attenuates activation of the p53 pathway in A549 lung cancer cells exposed to actinomycin D. Mech. Ageing Dev. 2014, 139, 11–21. [Google Scholar] [CrossRef]
- Hosoi, H.; Dilling, M.B.; Shikata, T.; Liu, L.N.; Shu, L.; Ashmun, R.A.; Germain, G.S.; Abraham, R.T.; Houghton, P.J. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 1999, 59, 886–894. [Google Scholar] [PubMed]
- Wagner, L.M.; Fouladi, M.; Ahmed, A.; Krailo, M.D.; Weigel, B.; DuBois, S.G.; Doyle, L.A.; Chen, H.; Blaney, S.M. Phase II study of cixutumumab in combination with temsirolimus in pediatric patients and young adults with recurrent or refractory sarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2015, 62, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Li, C.; Khan, J.; Banerjee, N.S.; Chow, L.T.; Athar, M. Combined mTORC1/mTORC2 inhibition blocks growth and induces catastrophic macropinocytosis in cancer cells. Proc. Natl. Acad. Sci. USA 2019, 116, 24583–24592. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Park, A.; Akiyama, R.; Tap, W.D.; Denny, C.T.; Federman, N. Evaluation of In Vitro Activity of the Class I PI3K Inhibitor Buparlisib (BKM120) in Pediatric Bone and Soft Tissue Sarcomas. PLoS ONE 2015, 10, e0133610. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, E.K.; Patwardhan, P.P.; Vasudeva, S.D.; de Stanchina, E.; Tap, W.D.; Schwartz, G.K. MLN0128, an ATP-competitive mTOR kinase inhibitor with potent in vitro and in vivo antitumor activity, as potential therapy for bone and soft-tissue sarcoma. Mol. Cancer Ther. 2015, 14, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Manara, M.C.; Nicoletti, G.; Zambelli, D.; Ventura, S.; Guerzoni, C.; Landuzzi, L.; Lollini, P.L.; Maira, S.M.; García-Echeverría, C.; Mercuri, M.; et al. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin. Cancer Res. 2010, 16, 530–540. [Google Scholar] [CrossRef]
- Hugle, M.; Fulda, S. Dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 synergizes with chloroquine to induce apoptosis in embryonal rhabdomyosarcoma. Cancer Lett. 2015, 360, 1–9. [Google Scholar] [CrossRef]
- Bavelloni, A.; Piazzi, M.; Ramazzotti, G.; Fiume, R.; Blalock, W.L.; Faenza, I. PI3N±-selective inhibitor alpelisib (BYL719), may be effective as anticancer agents in Rhabdomyosarcoma. Ital. J. Anat. Embryol. 2017, 122, 21. [Google Scholar]
- Kennedy, B.K.; Lamming, D.W. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016, 23, 990–1003. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Medvetz, D.; Priolo, C.; Henske, E.P. Therapeutic targeting of cellular metabolism in cells with hyperactive mTORC1: A paradigm shift. Mol. Cancer Res. 2015, 13, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.W.; Kucia, M.; Jankowski, K.; Higashi, R.M.; Ratajczak, J.; Ratajczak, M.Z.; Lane, A.N. Rhabdomyosarcoma cells show an energy producing anabolic metabolic phenotype compared with primary myocytes. Mol. Cancer 2008, 7, 79. [Google Scholar] [CrossRef]
- Tselios, C.; Lambrou, G. Signaling pathways that overactivate metabolism and drive neoplasia, in rhabdomyosarcoma. J. Res. Pract. Musculoskelet. Syst. 2019, 3, 17–25. [Google Scholar] [CrossRef]
- Jahnke, V.E.; Sabido, O.; Defour, A.; Castells, J.; Lefai, E.; Roussel, D.; Freyssenet, D. Evidence for mitochondrial respiratory deficiency in rat rhabdomyosarcoma cells. PLoS ONE 2010, 5, e8637. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y.D.; Wu, J.; Cui, J.; Chen, T. Carnitine palmitoyltransferase 1A (CPT1A): A transcriptional target of PAX3-FKHR and mediates PAX3-FKHR-dependent motility in alveolar rhabdomyosarcoma cells. BMC Cancer 2012, 12, 154. [Google Scholar] [CrossRef]
- Issaq, S.H.; Mendoza, A.; Fox, S.D.; Helman, L.J. Glutamine synthetase is necessary for sarcoma adaptation to glutamine deprivation and tumor growth. Oncogenesis 2019, 8, 20. [Google Scholar] [CrossRef]
- Krencz, I.; Sebestyén, A.; Fábián, K.; Márk, Á.; Moldvay, J.; Khoor, A.; Kopper, L.; Pápay, J. Expression of mTORC1/2-related proteins in primary and brain metastatic lung adenocarcinoma. Hum. Pathol. 2017, 62, 66–73. [Google Scholar] [CrossRef]
- Vicentini, C.; Cantù, C.; Antonello, D.; Simbolo, M.; Mafficini, A.; Luchini, C.; Rusev, B.; Porcaro, A.B.; Iacovelli, R.; Fassan, M.; et al. ERG alterations and mTOR pathway activation in primary prostate carcinomas developing castration-resistance. Pathol. Res. Pract. 2018, 214, 1675–1680. [Google Scholar] [CrossRef]
- Uhlyarik, A.; Piurko, V.; Vizkeleti, L.; Pápai, Z.; Rásó, E.; Lahm, E.; Kiss, E.; Sikter, M.; Vachaja, J.; Kenessey, I.; et al. EGFR Protein Expression of KRAS Wild-Type Colorectal Cancer: Predictive Value of the Sidedness for Efficacy of Anti-EGFR Therapy. Pathol. Oncol. Res. 2019. [Google Scholar] [CrossRef]
| Histology | Patients | |
|---|---|---|
| (N = 48) | ||
| Fusion-negative | 43 | |
| Embryonal | 39 | |
| Botryoid | 1 | |
| Spindle cell | 3 | |
| Fusion-positive | 5 | |
| Gender | (No.) | |
| Female | 20 | |
| Male | 28 | |
| Age | (years) | |
| Median (range) | 5.7 (0–17.3) | |
| Risk group | (No.) | |
| SR | 1 | |
| MR | 11 | |
| HR | 36 | |
| Overall survival | 68.8% | |
| Examined Cases | Rictor H-Score | RICTOR FISH (Average RICTOR/ Nucleus) | RICTOR PCR (RICTOR/AP3B1 Ratio) |
|---|---|---|---|
| Case 1 | 230 | equivocal (4.70) | negative (1.47) |
| Case 37 | 40 | negative (3.90) | negative (0.83) |
| Other 25 cases | median: 169.00 | median: 2.00 | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felkai, L.; Krencz, I.; Kiss, D.J.; Nagy, N.; Petővári, G.; Dankó, T.; Micsík, T.; Khoor, A.; Tornóczky, T.; Sápi, Z.; et al. Characterization of mTOR Activity and Metabolic Profile in Pediatric Rhabdomyosarcoma. Cancers 2020, 12, 1947. https://doi.org/10.3390/cancers12071947
Felkai L, Krencz I, Kiss DJ, Nagy N, Petővári G, Dankó T, Micsík T, Khoor A, Tornóczky T, Sápi Z, et al. Characterization of mTOR Activity and Metabolic Profile in Pediatric Rhabdomyosarcoma. Cancers. 2020; 12(7):1947. https://doi.org/10.3390/cancers12071947
Chicago/Turabian StyleFelkai, Luca, Ildikó Krencz, Dorottya Judit Kiss, Noémi Nagy, Gábor Petővári, Titanilla Dankó, Tamás Micsík, András Khoor, Tamás Tornóczky, Zoltán Sápi, and et al. 2020. "Characterization of mTOR Activity and Metabolic Profile in Pediatric Rhabdomyosarcoma" Cancers 12, no. 7: 1947. https://doi.org/10.3390/cancers12071947
APA StyleFelkai, L., Krencz, I., Kiss, D. J., Nagy, N., Petővári, G., Dankó, T., Micsík, T., Khoor, A., Tornóczky, T., Sápi, Z., Sebestyén, A., & Csóka, M. (2020). Characterization of mTOR Activity and Metabolic Profile in Pediatric Rhabdomyosarcoma. Cancers, 12(7), 1947. https://doi.org/10.3390/cancers12071947






