Head and Neck Cancer Metastasis and the Effect of the Local Soluble Factors, from the Microenvironment, on Signalling Pathways: Is It All about the Akt?
Abstract
1. Introduction
2. Results
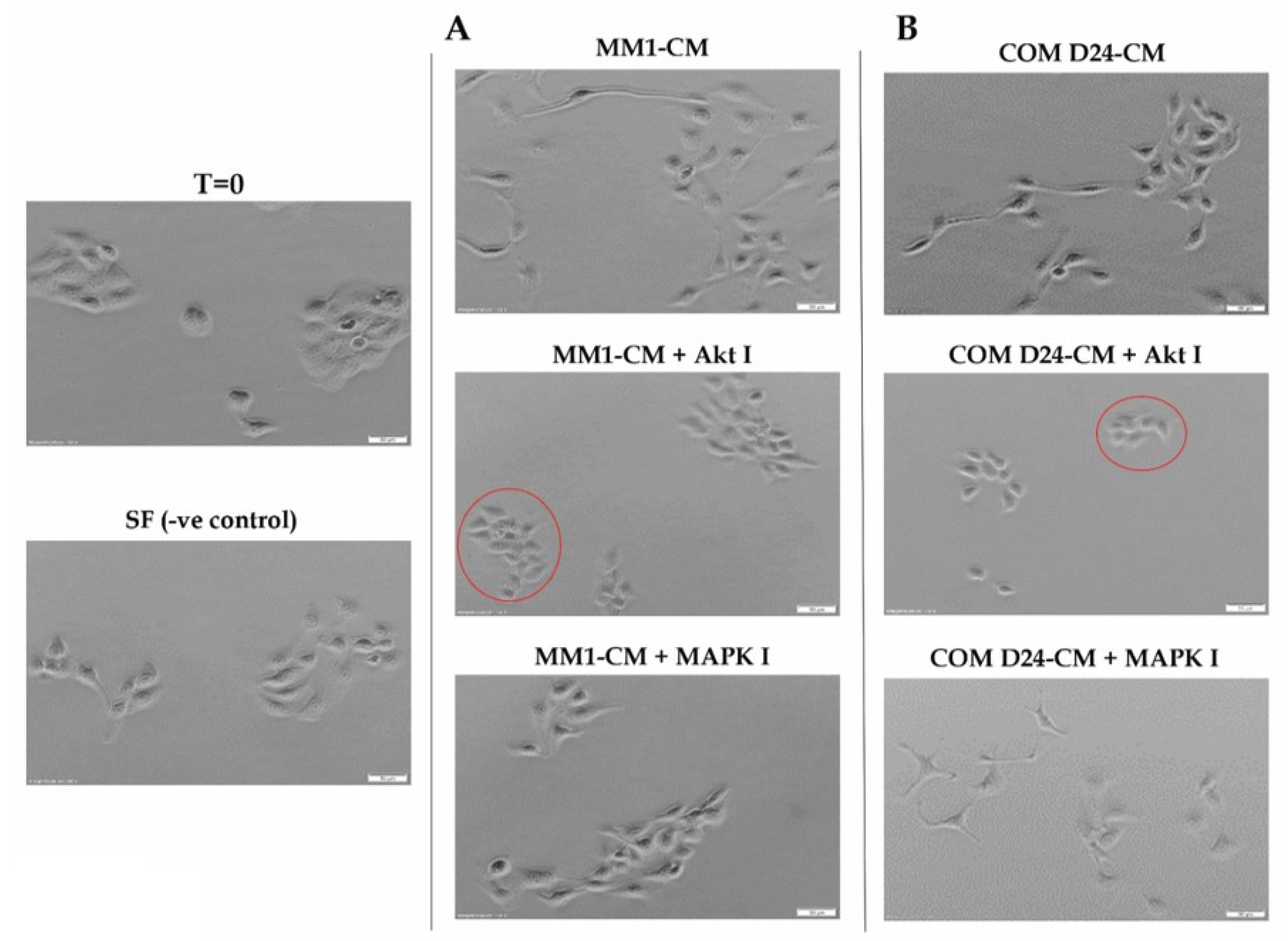
2.1. Conditioned Medium Collected from Fibroblasts Induced Cancer Cell Scattering in an Akt-Dependent Manner
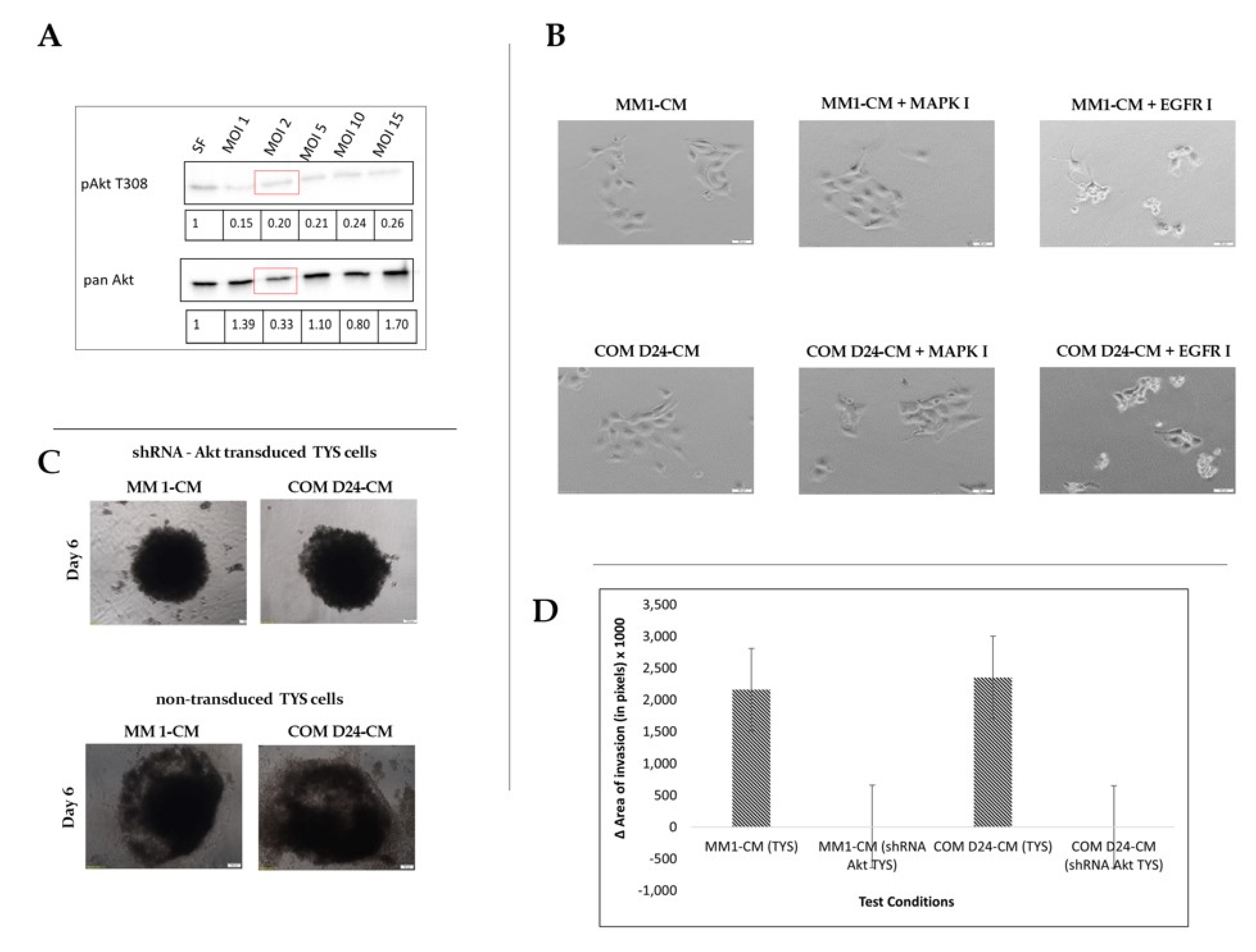
2.2. Conditioned Medium from Oral Cancer-Associated Fibroblasts (COM D24) Activated Akt More Than the Negative Control in Scattered Cancer Cells
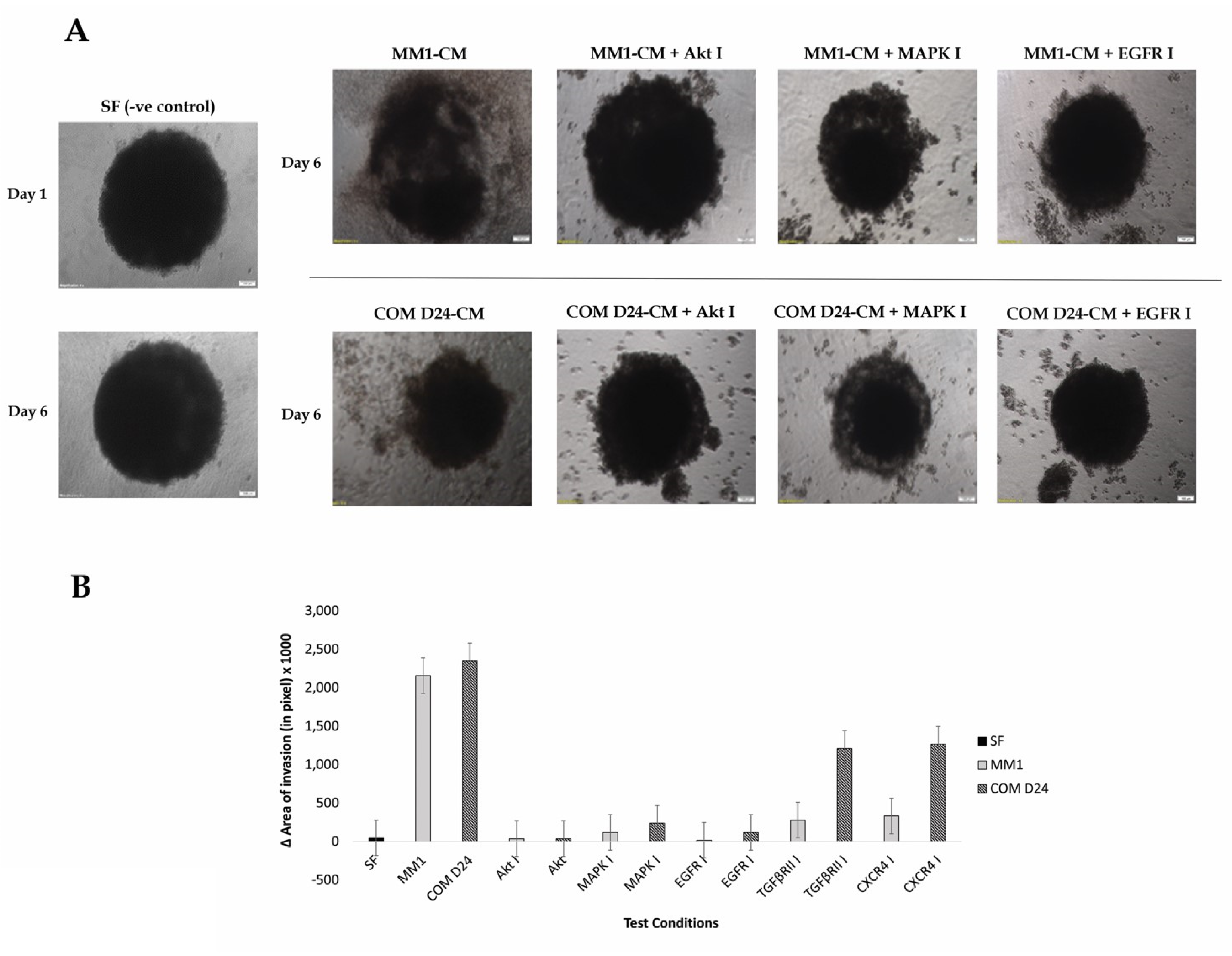
2.3. COM D24-CM and MM1-CM Stimulated Cancer Cell Invasion into the Collagen Matrix
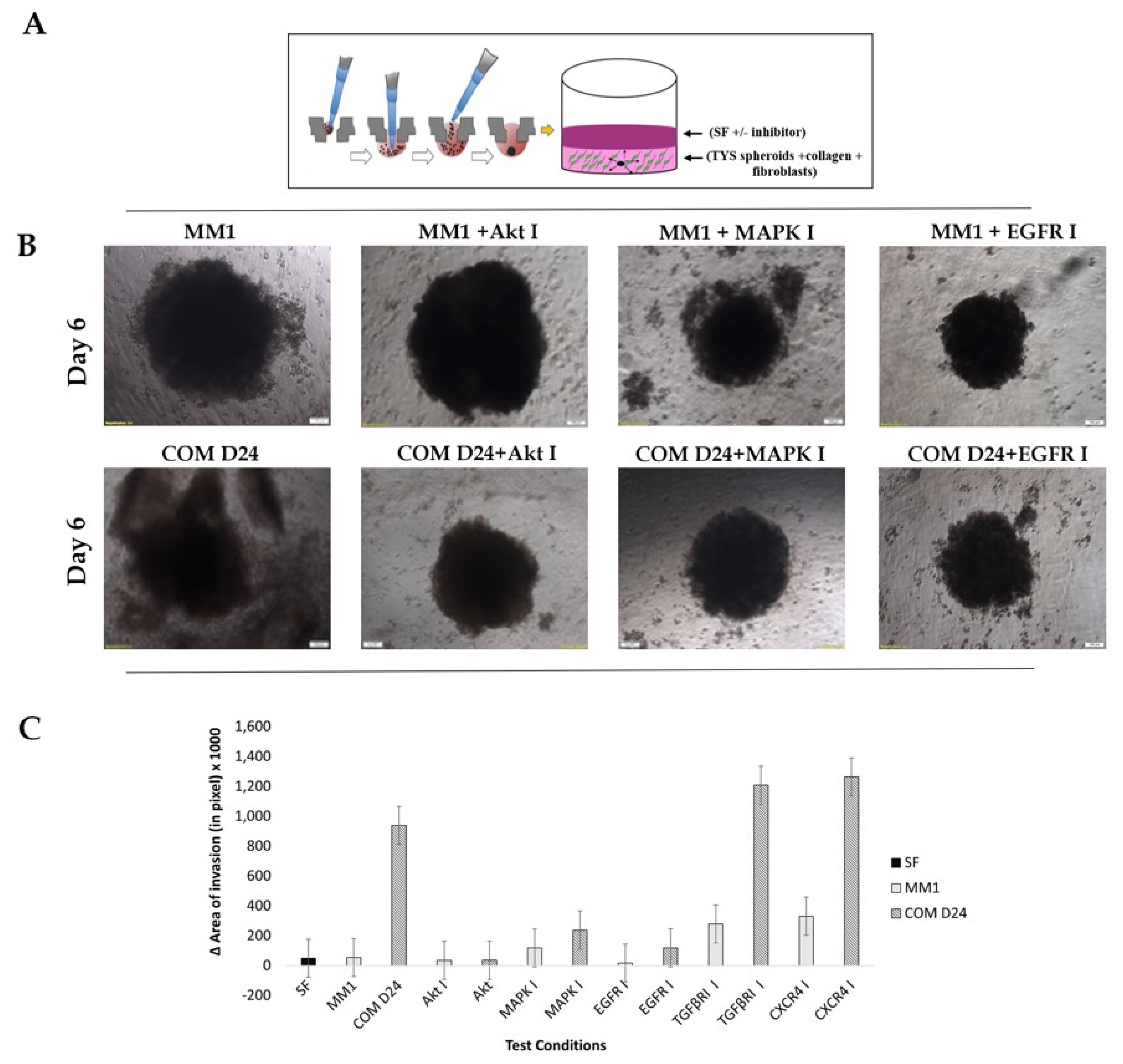
2.4. Fibroblast Conditioned Medium Stimulated Cancer Cell Evasion from the Spheroids into the Collagen Matrix More Than the Control
2.5. COM D24 Cells Stimulated Cancer Cells to Invade from the Spheroids into the Collagen Matrix and the Akt Inhibitor Completely Blocked This Invasion
2.6. EGFR Inhibitor Was Toxic to Akt-Silenced Oral Adenoid Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Antibodies and Inhibitors
4.2. Cell Culture
4.3. Conditioned Medium Preparation
4.4. Cell Scatter Assay
4.5. Cell Lysis, SDS-PAGE, and Western Blot
4.6. 3-D-Collagen Gel Assay
4.7. 3-D Spheroid Invasion Assay
4.8. Gene Silencing Using Akt 1 shRNA (Human) Lentivirus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| CAF | Cancer Associated Fibroblasts |
| TME | Tumour Microenvironment |
| CM | Conditioned Medium |
| MAPK | Mitogen Activated Protein Kinase |
| EGFR | Epidermal Growth Factor Receptor |
| TGFβR | Transforming Growth Factor beta Receptor |
| CXCR4 | C-X-C Chemokine Receptor 4 |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sasahira, T.; Kirita, T.; Kuniyasu, H. Update of molecular pathobiology in oral cancer: A review. Int. J. Clin. Oncol. 2014, 19, 431–436. [Google Scholar] [CrossRef] [PubMed]
- De Wever, O.; Mareel, M. Role of tissue stroma in cancer cell invasion. J. Pathol. 2003, 200, 429–447. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Koontongkaew, S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J. Cancer 2013, 4, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-associated fibroblasts: Their characteristics and their roles in tumor growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef] [PubMed]
- Kidacki, M.; Lehman, H.L.; Warrick, J.I.; Stairs, D.B. Signaling pathways supporting tumor invasion in head and neck squamous cell carcinoma. J. Clin. Exp. Pathol. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, C.H.; Tan, L.D.; Wang, Q.S.; Li, X.Q.; Feng, Y.M. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 2014, 110, 724–732. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.; Chen, S.-W.; Liu, L.-l.; Li, L.; Gao, F.; Zhuang, S.-M.; Wang, L.-p.; Li, Y.; Song, M. Cancer-associated fibroblasts provide a suitable microenvironment for tumor development and progression in oral tongue squamous cancer. J. Transl. Med. 2015, 13, 198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Chen, W.L.; Wang, Y.Y.; Lin, Z.Y.; Zhang, D.M.; Fan, S.; Li, J.S. A role for cancer-associated fibroblasts in inducing the epithelial-to-mesenchymal transition in human tongue squamous cell carcinoma. J. Oral Pathol. Med. 2014, 43, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Sakakura, K.; Kudo, T.; Toyoda, M.; Kaira, K.; Oyama, T.; Chikamatsu, K. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget 2017, 8, 8633–8647. [Google Scholar] [CrossRef]
- Bello, I.O.; Vered, M.; Dayan, D.; Dobriyan, A.; Yahalom, R.; Alanen, K.; Nieminen, P.; Kantola, S.; Läärä, E.; Salo, T. Cancer-associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011, 47, 33–38. [Google Scholar] [CrossRef]
- Cirri, P.; Chiarugi, P. Cancer associated fibroblasts: The dark side of the coin. Am. J. Cancer Res. 2011, 1, 482–497. [Google Scholar]
- De Wever, O.; Demetter, P.; Mareel, M.; Bracke, M. Stromal myofibroblasts are drivers of invasive cancer growth. Int. J. Cancer 2008, 123, 2229–2238. [Google Scholar] [CrossRef]
- Braig, F.; Kriegs, M.; Voigtlaender, M.; Habel, B.; Grob, T.; Biskup, K.; Blanchard, V.; Sack, M.; Thalhammer, A.; Batalla, I.B.; et al. Cetuximab resistance in head and neck cancer is mediated by EGFR-K(521) polymorphism. Cancer Res. 2017, 77, 1188–1199. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Trigo, J.; Hitt, R.; Koralewski, P.; Diaz-Rubio, E.; Rolland, F.; Knecht, R.; Amellal, N.; Schueler, A.; Baselga, J. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J. Clin. Oncol. 2007, 25, 2171–2177. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Psyrri, A.; Seiwert, T.Y.; Jimeno, A. Molecular pathways in head and neck cancer: EGFR, PI3K, and more. Am. Soc. Clin. Oncol. Educ. Book 2013, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Patel, M.; Couch, M.; Hayes, D.N. Molecular biology of head & neck cancer: Risks and pathways. Hematol. Oncol. Clin. North Am. 2008, 22, 1099–1124. [Google Scholar] [CrossRef] [PubMed]
- Rebucci, M.; Peixoto, P.; Dewitte, A.; Wattez, N.; de Nuncques, M.A.; Rezvoy, N.; Vautravers-Dewas, C.; Buisine, M.P.; Guerin, E.; Peyrat, J.P.; et al. Mechanisms underlying resistance to cetuximab in the HNSCC cell line: Role of AKT inhibition in bypassing this resistance. Int. J. Oncol. 2011, 38, 189–200. [Google Scholar] [PubMed]
- Ellis, I.R.; Jones, S.J.; Lindsay, Y.; Ohe, G.; Schor, A.M.; Schor, S.L.; Leslie, N.R. Migration Stimulating Factor (MSF) promotes fibroblast migration by inhibiting AKT. Cell. Signal. 2010, 22, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef]
- Alessi, D.R.; Cohen, P. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 1998, 8, 55–62. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR complex. Science 2005, 307, 1098. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Grille, S.J.; Bellacosa, A.; Upson, J.; Klein-Szanto, A.J.; van Roy, F.; Lee-Kwon, W.; Donowitz, M.; Tsichlis, P.N.; Larue, L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003, 63, 2172–2178. [Google Scholar]
- Xu, W.; Yang, Z.; Lu, N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef]
- Li, Y.Y.; Tao, Y.W.; Gao, S.; Li, P.; Zheng, J.M.; Zhang, S.E.; Liang, J.; Zhang, Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 2018, 36, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Webb, D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and cancer associated fibroblasts in the reactive stroma and its relation to cancer biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef]
- Pankova, D.; Chen, Y.; Terajima, M.; Schliekelman, M.J.; Baird, B.N.; Fahrenholtz, M.; Sun, L.; Gill, B.J.; Vadakkan, T.J.; Kim, M.P.; et al. Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol. Cancer Res. 2016, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Nurmenniemi, S.; Koivula, M.-K.; Nyberg, P.; Tervahartiala, T.; Sorsa, T.; Mattila, P.S.; Salo, T.; Risteli, J. Type I and III collagen degradation products in serum predict patient survival in head and neck squamous cell carcinoma. Oral Oncol. 2012, 48, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Sok, J.C.; Lee, J.A.; Dasari, S.; Joyce, S.; Contrucci, S.C.; Egloff, A.M.; Trevelline, B.K.; Joshi, R.; Kumari, N.; Grandis, J.R.; et al. Collagen type XI α1 facilitates head and neck squamous cell cancer growth and invasion. Br. J. Cancer 2013, 109, 3049–3056. [Google Scholar] [CrossRef]
- Menke, A.; Philippi, C.; Vogelmann, R.; Seidel, B.; Lutz, M.P.; Adler, G.; Wedlich, D. Down-regulation of E-cadherin gene expression by collagen type I and type III in pancreatic cancer cell lines. Cancer Res. 2001, 61, 3508–3517. [Google Scholar]
- Wu, Y.H.; Chang, T.H.; Huang, Y.F.; Huang, H.D.; Chou, C.Y. COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene 2014, 33, 3432–3440. [Google Scholar] [CrossRef]
- Lim, J.; Kim, J.H.; Paeng, J.Y.; Kim, M.J.; Hong, S.D.; Lee, J.I.; Hong, S.P. Prognostic value of activated Akt expression in oral squamous cell carcinoma. J. Clin. Pathol. 2005, 58, 1199–1205. [Google Scholar] [CrossRef]
- Knowles, J.A.; Golden, B.; Yan, L.; Carroll, W.R.; Helman, E.E.; Rosenthal, E.L. Disruption of the AKT pathway inhibits metastasis in an orthotopic model of head and neck squamous cell carcinoma. Laryngoscope 2011, 121, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Molife, L.R.; Yan, L.; Vitfell-Rasmussen, J.; Zernhelt, A.M.; Sullivan, D.M.; Cassier, P.A.; Chen, E.; Biondo, A.; Tetteh, E.; Siu, L.L.; et al. Phase 1 trial of the oral AKT inhibitor MK-2206 plus carboplatin/paclitaxel, docetaxel, or erlotinib in patients with advanced solid tumors. J. Hematol. Oncol. 2014, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Betka, J. Distant metastases from lip and oral cavity cancer. ORL 2001, 63, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, A.; Song, J.; Thakur, N.; Itoh, S.; Marcusson, A.; Bergh, A.; Heldin, C.H.; Landström, M. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci. Signal. 2017. [Google Scholar] [CrossRef]
- Islam, M.; Alghamdi, A.; Sriramula, P.; Shalgm, B.; Jones, S.; Ellis, I. Is it all just an Akt—You’d be SMAD to believe it! Role of TGFβ1 in oral cancer metastasis. Dent. Oral Biol. Craniofacial Res. 2018, 1. [Google Scholar] [CrossRef]
- Conley-LaComb, M.; Saliganan, A.; Kandagatla, P.; Chen, Y.; Cher, M.; Chinni, S. PTEN loss mediated Akt activation promotes prostate tumor growth and metastasis via CXCL12/CXCR4 signaling. Mol. Cancer Res. 2013, 12, 85. [Google Scholar] [CrossRef]
- Guo, J.; Yu, X.; Gu, J.; Lin, Z.; Zhao, G.; Xu, F.; Lu, C.; Ge, D. Regulation of CXCR4/AKT-signaling-induced cell invasion and tumor metastasis by RhoA, Rac-1, and Cdc42 in human esophageal cancer. Tumor Biol. 2016, 37, 6371–6378. [Google Scholar] [CrossRef]
- Xue, L.; Mao, X.; Ren, L.; Chu, X. Inhibition of CXCL12/CXCR4 axis as a potential targeted therapy of advanced gastric carcinoma. Cancer Med. 2017, 6, 1424–1436. [Google Scholar]

| Name | Catalogue/Ref No. | Company and Address | Dilution/ Conc. Used |
|---|---|---|---|
| Primary antibodies | |||
| Phospho-Akt (Thr308) (C31E5E) Rabbit mAb | 2965 | Cell Signaling Tech., Denver, MI, USA | 1:1000 |
| Phospho-Akt (Ser473) (D9E) XP Rabbit mAb | 4060 | Cell Signaling Tech., Denver, MI, USA | 1:2000 |
| Akt (pan) (C67E7) Rabbit mAb | 4691 | Cell Signaling Tech., Denver, MI, USA | 1:1000 |
| Phospho- MAPK 42/44 Rabbit mAb | 9101 | Cell Signaling Tech., Denver, MI, USA | 1:2000 |
| Secondary antibodies | |||
| Goat anti-rabbit IgG, HRP-linked | 7074 | Cell Signaling Tech., Denver, MI, USA | 1:2000 |
| Chemical inhibitors | |||
| MK2206 (Akt inhibitor) | S1078 | Selleckchem, Houston, TX, USA | 5 µM |
| Gefitinib (EGFR inhibitor) | 4765S | Cell Signaling Tech., Denver, MI, USA | 10 µM |
| PD98059 (MAPK inhibitor) | 9900L | Cell Signaling Tech., Denver, MI, USA | 50 µM |
| TGFβRI kinase inhibitor VII | 616458 | Calbiochem, San Diego, CA, USA | 5 µM |
| AMD3100 Octahydrochloride hydrate (CXCR4 Inhibitor) | A5602 | Cell Signaling Tech., Denver, MI, USA | 1 µM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.; Ghoshal, A.; Jones, S.; Ellis, I.; Islam, M. Head and Neck Cancer Metastasis and the Effect of the Local Soluble Factors, from the Microenvironment, on Signalling Pathways: Is It All about the Akt? Cancers 2020, 12, 2093. https://doi.org/10.3390/cancers12082093
Ahmed H, Ghoshal A, Jones S, Ellis I, Islam M. Head and Neck Cancer Metastasis and the Effect of the Local Soluble Factors, from the Microenvironment, on Signalling Pathways: Is It All about the Akt? Cancers. 2020; 12(8):2093. https://doi.org/10.3390/cancers12082093
Chicago/Turabian StyleAhmed, Hanan, Arpa Ghoshal, Sarah Jones, Ian Ellis, and Mohammad Islam. 2020. "Head and Neck Cancer Metastasis and the Effect of the Local Soluble Factors, from the Microenvironment, on Signalling Pathways: Is It All about the Akt?" Cancers 12, no. 8: 2093. https://doi.org/10.3390/cancers12082093
APA StyleAhmed, H., Ghoshal, A., Jones, S., Ellis, I., & Islam, M. (2020). Head and Neck Cancer Metastasis and the Effect of the Local Soluble Factors, from the Microenvironment, on Signalling Pathways: Is It All about the Akt? Cancers, 12(8), 2093. https://doi.org/10.3390/cancers12082093







