Characteristics of PSA Bounce after Radiotherapy for Prostate Cancer: A Meta-Analysis
Abstract
1. Introduction
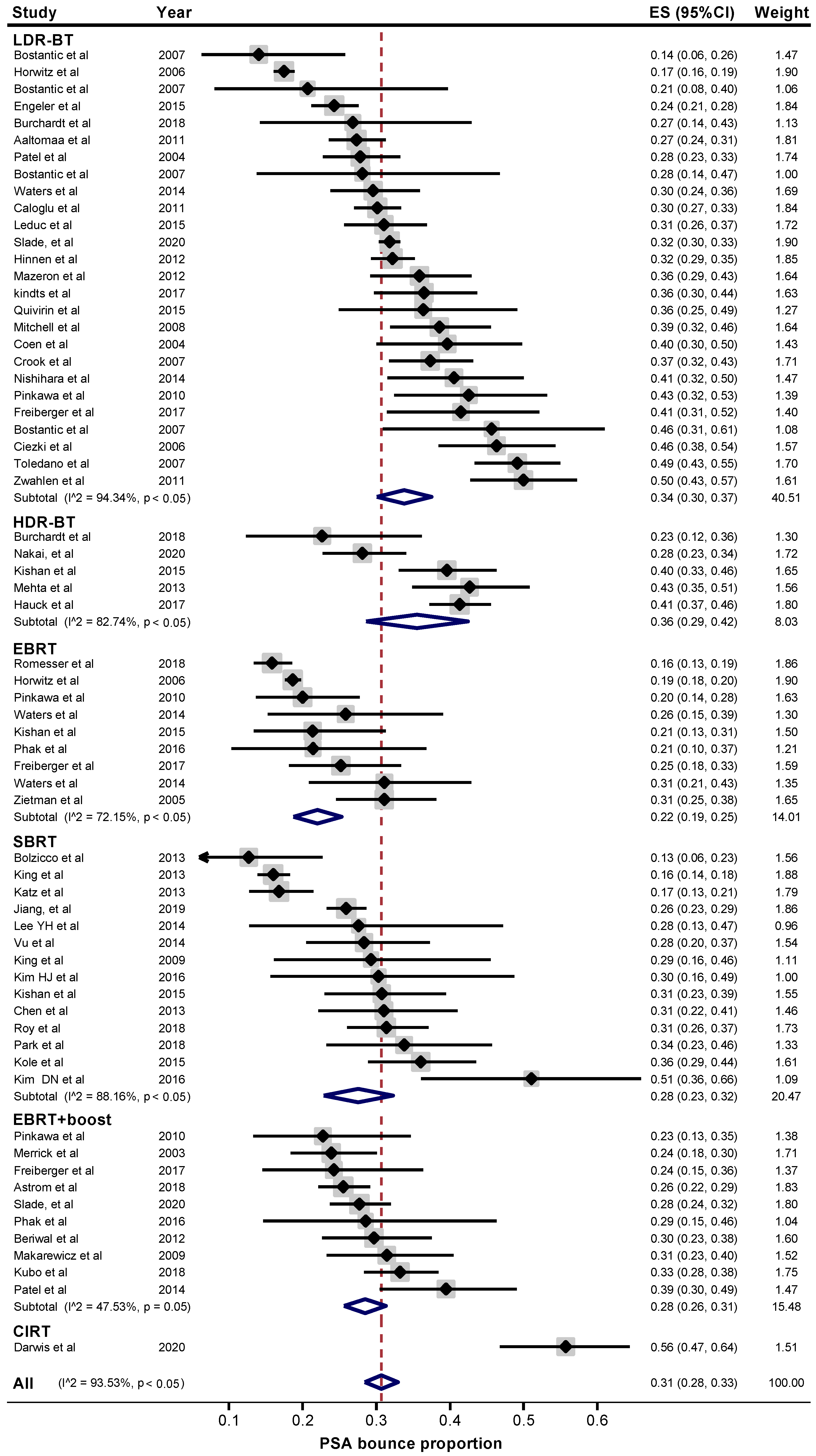
2. Results
3. Discussion
4. Materials and Methods
4.1. Endpoint Definition
4.2. Inclusion and Exclusion Criteria
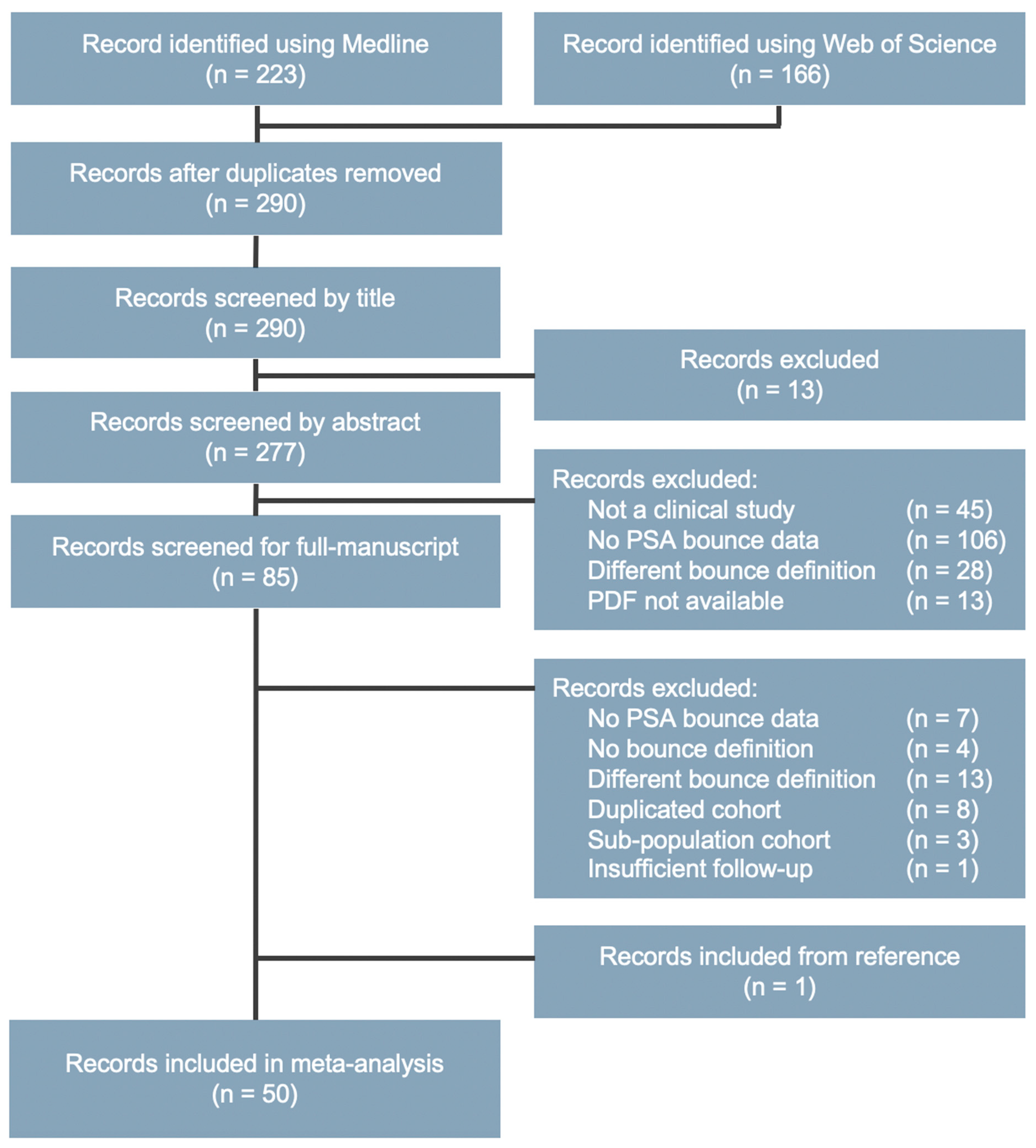
4.3. Study Selection
4.4. Data Extraction
4.5. Quality Assessment
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Parker, C.; Gillessen, S.; Heidenreich, A.; Horwich, A. Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v69–v77. [Google Scholar] [CrossRef]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef]
- Wallner, K.; Blasko, J.; Dattolli, M. Prostate Brachytherapy Made Complicated; SmartMedicine Press: Seattle, WA, USA, 1997; pp. 14.11–14.15. [Google Scholar]
- Caloglu, M.; Ciezki, J. Prostate-specific antigen bounce after prostate brachytherapy: Review of a confusing phenomenon. Urology 2009, 74, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Pickles, T. Prostate-specific antigen (PSA) bounce and other fluctuations: Which biochemical relapse definition is least prone to psa false calls? An analysis of 2030 men treated for prostate cancer with external beam or brachytherapy with or without adjuvant androgen deprivation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Merrick, G.S.; Butler, W.M.; Wallner, K.E.; Galbreath, R.W.; Anderson, R.L. Prostate-specific antigen spikes after permanent prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 450–456. [Google Scholar] [CrossRef]
- Patel, C.; Elshaikh, M.A.; Angermeier, K.; Ulchaker, J. PSA bounce predicts early success in patients with permanent Iodine-125 prostate implant. Urology 2004, 63, 110–113. [Google Scholar] [CrossRef]
- Coen, J.; Zietman, A.; Grocela, J.; Heney, N.; Babayan, R. Definitions of biochemical control after permanent interstitial brachytherapy as sole treatment for localized prostate cancer: Interpreting the PSA bounce. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, S444. [Google Scholar] [CrossRef]
- Zietman, A.L.; Christodouleas, J.P.; Shipley, W.U. PSA bounces after neoadjuvant androgen deprivation and external beam radiation: Impact on definitions of failure. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 714–718. [Google Scholar] [CrossRef]
- Ciezki, J.P.; Reddy, C.A.; Garcia, J.; Angermeier, K.; Ulchaker, J.; Mahadevan, A.; Chehade, N.; Altman, A.; Klein, E.A. PSA kinetics after prostate brachytherapy: PSA bounce phenomenon and its implications for PSA doubling time. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 512–517. [Google Scholar] [CrossRef]
- Horwitz, E.; Levy, L.; Martinez, A.; Potters, L.; Beyer, D.; Blasko, J.; Sandler, H.; Buskirk, S.; Zietman, A.; Kuban, D. The post-treatment PSA bounce for prostate cancer patients treated with external beam RT or permanent brachytherapy alone is not biochemically or clinically significant: A multi-institutional pooled analysis of more than 7500 patients. Int. J. Radiat. Oncol. 2006, 66, S205. [Google Scholar] [CrossRef]
- Toledano, A.; Chauveinc, L.; Flam, T.; Thiounn, N.; Solignac, S.; Timbert, M.; Rosenwald, J.C.; Cosset, J.M. PSA bounce after permanent implant prostate brachytherapy may mimic a biochemical failure. Cancer/Radiother. 2007, 11, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Bostancic, C.H.; Merrick, G.S.; Butler, W.M.; Wallner, K.E.; Allen, Z.; Glbreath, R.; Lief, J.; Gutman, S.E. Isotope and patient age predict for PSA spikes after permanent prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.; Gillian, C.; Yeung, I.; Austen, L.; Mclean, M. PSA kinetics and PSA bounce following permanent seed prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 426–433. [Google Scholar] [CrossRef]
- Mitchell, D.M.; Swindell, R.; Elliott, T.; Wylie, J.P.; Taylor, C.M.; Logue, J.P. Analysis of prostate-specific antigen bounce after I125 permanent seed implant for localised prostate cancer. Radiother. Oncol. 2008, 88, 102–107. [Google Scholar] [CrossRef]
- Makarewicz, R.; Lebioda, A.; Terlikiewicz, J.; Biedka, M. PSA bouncing after brachytherapy HDR and external beam radiation therapy: A study of 121 patients with minimum 5-years follow-up. J. Contemp. Brachyther. 2009, 1, 92–96. [Google Scholar]
- King, C.R.; Brooks, J.D.; Gill, H.; Pawlicki, T.; Cotruz, C. Stereotactic body radiotherapy for localized prostate cancer: Interim results of a prospective phase II clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1043–1048. [Google Scholar] [CrossRef]
- Pinkawa, M.; Piroth, M.D.; Holy, R.; Fischedick, K.; Schaar, S.; Borchers, H.; Heidenreich, A.; Eble, M.J. Prostate-specific antigen kinetics following external-beam radiotherapy and temporary (Ir-192) or permanent (I-125) brachytherapy for prostate cancer. Radiother. Oncol. 2010, 96, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Caloglu, M.; Ciezki, J.P.; Reddy, C.A.; Angermeier, K.; Ulchaker, J.; Chehade, N.; Altman, A.; Magi-Galuzzi, C.; Klein, E.A. PSA bounce and biochemical failure after brachytherapy for prostate cancer: A study of 820 patients with a minimum of 3 years of follow-up. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Zwahlen, D.R.; Smith, R.; Andrianopoulos, N.; Matheson, B.; Royce, P.; Millar, J.L. Prostate-specific antigen bounce after permanent Iodine-125 prostate brachytherapy—An Australian analysis. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Aaltomaa, S.H.; Kataja, V.V.; Raty, A.; Palmgren, J.-E.; Lahtinen, T. Does the outcome of prostate cancer patients with large prostates differ from small prostate size in permanent seed, low dose-rate brachytherapy? Scand. J. Urol. Nephrol. 2011, 45, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Beriwal, S.; Smith, R.P.; Houser, C.; Benoit, R.M. Prostate-specific antigen spikes with 131Cs brachytherapy: Is there a difference with other radioisotopes? Brachytherapy 2012, 11, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, K.A.; Monninkhof, E.M.; Battermann, J.J.; Van Roermund, J.G.H.; Frank, S.J.; Van Vulpen, M. Prostate specific antigen bounce is related to overall survival in prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Mazeron, R.; Bajard, A.; Montbarbon, X.; Gassa, F.; Malet, C.; Rocher, F.; Clippe, S.; Bringeon, G.; Desmettre, O.; Pommier, P. Permanent 125I-seed prostate brachytherapy: Early prostate specific antigen value as a predictor of PSA bounce occurrence. Radiat. Oncol. 2012, 7, 46. [Google Scholar] [CrossRef]
- Bolzicco, G.; Favretto, M.S.; Satariano, N.; Scremin, E.; Tambone, C.; Tasca, A. A single-center study of 100 consecutive patients with localized prostate cancer treated with stereotactic body radiotherapy. BMC Urol. 2013, 13, 49. [Google Scholar] [CrossRef]
- Chen, L.N.; Suy, S.; Uhm, S.; Oermann, E.K.; Ju, A.W.; Chen, V.; Hanscom, H.N.; Laing, S.; Kim, J.S.; Batipps, G.P. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: The Georgetown University experience. Radiat. Oncol. 2013, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.J.; Santoro, M.; Diblasio, F.; Ashley, R. Stereotactic body radiotherapy for localized prostate cancer: Disease control and quality of life at 6 years. Radiat. Oncol. 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- King, C.R.; Freeman, D.; Kaplan, I.; Fuller, D.; Bolzicco, G.; Collins, S.; Meier, R.; Wang, J.; Kupelian, P.; Steinberg, M.; et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother. Oncol. 2013, 109, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.H.; Kamrava, M.; Wang, P.C.; Steinberg, M.; Demanes, J. Prostate-specific antigen bounce after high-dose-rate monotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 729–733. [Google Scholar] [CrossRef]
- Lee, Y.H.; Son, S.H.; Yoon, S.C.; Yu, M.; Choi, B.O.; Kim, Y.S.; Jang, H.S.; Lee, S.N.; Jang, J.S.; Hwang, T.K. Stereotactic body radiotherapy for prostate cancer: A preliminary report. Asia. Pac. J. Clin. Oncol. 2014, 10, 46–53. [Google Scholar] [CrossRef]
- Nishihara, K.; Nakiri, M.; Chikui, K.; Suekane, S.; Matsuoka, K.; Hattori, C.; Ogo, E.; Abe, T.; Matsumoto, Y.; Ishitake, T. Relationship between sexual function and prostate-specific antigen bounce after Iodine-125 permanent implant brachytherapy for localized prostate cancer. Int. J. Urol. 2014, 21, 658–663. [Google Scholar] [CrossRef][Green Version]
- Vu, C.C.; Haas, J.A.; Katz, A.E.; Witten, M.R. Prostate-specific antigen bounce following stereotactic body radiation therapy for prostate cancer. Front. Oncol. 2014, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Souhami, L.; Mansure, J.J.; Duclos, M.; Aprikian, A.; Faria, S.; David, M.; Cury, F.L. Prostate-specific antigen bounce after high-dose-rate prostate brachytherapy and hypofractionated external beam radiotherapy. Brachytherapy 2014, 13, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.; Delouya, G.; Donath, D.; Lambert, C.; Larrivée, S.; Zorn, K.C.; Taussky, D. Risk factors for PSA bounce following radiotherapy: Outcomes from a multi-modal therapy analysis. Can. J. Urol. 2014, 21, 7548–7553. [Google Scholar] [PubMed]
- Kole, T.P.; Chen, L.N.; Obayomi-Davies, O.; Kim, J.S.; Lei, S.; Suy, S.; Dritschilo, A.; Collins, S.P. Prostate specific antigen kinetics following robotic stereotactic body radiotherapy for localized prostate cancer. Acta Oncol. 2015, 54, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.U.; Wang, P.; Upadhyaya, S.K.; Hauswald, H.; Demanes, D.J.; Nickols, N.G.; Kamrava, M.; Sadeghi, A.; Kupelian, P.A.; Steinberg, M.L.; et al. SBRT and HDR brachytherapy produce lower PSA nadirs and different PSA decay patterns than conventionally fractionated IMRT in patients with low- or intermediate-risk prostate cancer. Pract. Radiat. Oncol. 2015, 6, 268–275. [Google Scholar] [CrossRef]
- Leduc, N.; Atallah, V.; Creoff, M.; Rabia, N.; Taouil, T.; Escarmant, P.; Vinh-Hung, V. Prostate-specific antigen bounce after curative brachytherapy for early-stage prostate cancer: A study of 274 african-caribbean patients. Brachytherapy 2015, 14, 826–833. [Google Scholar] [CrossRef]
- Quivrin, M.; Loffroy, R.; Cormier, L.; Mazoyer, F.; Bertaut, A.; Chambade, D.; Martin, E.; Maingon, P.; Walker, P.; Créhange, G. Multiparametric MRI and post implant CT-based dosimetry after prostate brachytherapy with iodine seeds: The higher the dose to the dominant index lesion, the lower the PSA bounce. Radiother. Oncol. 2015, 117, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Engeler, D.S.; Schwab, C.; Thöni, A.F.; Hochreiter, W.; Prikler, L.; Suter, S.; Stucki, P.; Schiefer, J.; Plasswilm, L.; Schmid, H.-P.; et al. PSA bounce after 125I-brachytherapy for prostate cancer as a favorable prognosticator. Strahlenther. Onkol. 2015, 191, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Phak, J.H.; Kim, W.C. Hypofractionated stereotactic body radiotherapy in low- and intermediate-risk prostate carcinoma. Radiat. Oncol. J. 2016, 34, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.N.; Straka, C.; Cho, L.C.; Lotan, Y.; Yan, J.; Kavanagh, B.; Raben, D.; Cooley, S.; Brindle, J.; Xie, X.J.; et al. Early and multiple PSA bounces can occur following high-dose prostate stereotactic body radiation therapy: Subset analysis of a phase 1/2 trial. Pract. Radiat. Oncol. 2016, 7, e43–e49. [Google Scholar] [CrossRef] [PubMed]
- Phak, J.H.; Kim, H.J.; Kim, W.C. Prostate-specific antigen kinetics following hypofractionated stereotactic body radiotherapy boost as post-external beam radiotherapy versus conventionally fractionated external beam radiotherapy for localized prostate cancer. Prostate Int. 2016, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Freiberger, C.; Berneking, V.; Vögeli, T.A.; Kirschner-Hermanns, R.; Eble, M.J.; Pinkawa, M. Long-term prognostic significance of rising PSA levels following radiotherapy for localized prostate cancer—Focus on overall survival. Radiat. Oncol. 2017, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hauck, C.R.; Ye, H.; Chen, P.Y.; Gustafson, G.S.; Limbacher, A.; Krauss, D.J. Increasing fractional doses increases the probability of benign PSA bounce in patients undergoing definitive HDR brachytherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kindts, I.; Stellamans, K.; Billiet, I.; Pottel, H.; Lambrecht, A. I-125 brachytherapy in younger prostate cancer patients: Outcomes in low- and intermediate-risk disease. Strahlenther. Onkol. 2017, 1–7. [Google Scholar] [CrossRef]
- Romesser, P.B.; Pei, X.; Shi, W.; Zhang, Z.; Kollmeier, M.; McBride, S.M.; Zelefsky, M.J. Prostate-specific antigen (PSA) bounce after dose-escalated external beam radiation therapy is an independent predictor of PSA recurrence, metastasis, and survival in prostate adenocarcinoma patients. Int. J. Radiat. Oncol. 2017, 100, 59–67. [Google Scholar] [CrossRef]
- Park, Y.; Park, H.J.; Jang, W.I.; Jeong, B.K.; Kim, H.-J.; Chang, A.R. Long-term results and PSA kinetics after robotic SBRT for prostate cancer: Multicenter retrospective study in korea (korean radiation oncology group study 15–01). Radiat. Oncol. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Åström, L.; Sandin, F.; Holmberg, L. Good prognosis following a PSA bounce after high dose rate brachytherapy and external radiotherapy in prostate cancer. Radiother. Oncol. 2018, 129, 561–566. [Google Scholar] [CrossRef]
- Burchardt, W.; Skowronek, J. Time to PSA rise differentiates the PSA bounce after HDR and LDR brachytherapy of prostate cancer. J. Contemp. Brachyther. 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Kubo, K.; Wadasaki, K.; Kimura, T.; Murakami, Y.; Kajiwara, M.; Teishima, J.; Matsubara, A.; Nagata, Y. Clinical features of prostate-specific antigen bounce after 125I brachytherapy for prostate cancer. J. Radiat. Res. 2018, 59, 649–655. [Google Scholar] [CrossRef]
- Roy, S.; Loblaw, A.; Cheung, P.; Chu, W.; Chung, H.T.; Vesprini, D.; Ong, A.; Chowdhury, A.; Panjwani, D.; Pang, G.; et al. Prostate-specific antigen bounce after stereotactic body radiotherapy for prostate cancer: A pooled analysis of four prospective trials. Clin. Oncol. 2019, 31, 621–629. [Google Scholar] [CrossRef]
- Jiang, N.Y.; Dang, A.T.; Yuan, Y.; Chu, F.-I.; Shabsovich, D.; King, C.R.; Collins, S.P.; Aghdam, N.; Suy, S.; Mantz, C.A.; et al. Multi-institutional analysis of prostate-specific antigen kinetics after stereotactic body radiation therapy. Int. J. Radiat. Oncol. 2019, 105, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Darwis, N.D.; Oike, T.; Kawamura, H. Kinetics of prostate-specific antigen after carbon ion radiotherapy for prostate cancer. Cancers 2020, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Tanaka, N.; Asakawa, I.; Anai, S.; Miyake, M.; Morizawa, Y.; Hori, S.; Owari, T.; Fujii, T.; Yamaki, K.; et al. Prostate-specific antigen bounce after 125I-brachytherapy for prostate cancer is a favorable prognosticator in patients who are biochemical recurrence-free at 4 years and correlates with testosterone. Jpn. J. Clin. Oncol. 2020, 50, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Slade, A.N.; Dahman, B.; Chang, M.G. Racial differences in the PSA bounce in predicting prostate cancer outcomes after brachytherapy: Evidence from the department of veterans affairs. Brachytherapy 2020, 19, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Offord, C.P.; Kimura, G.; Kuribayashi, S.; Takeda, H.; Tsuchiya, S.; Shimojo, H.; Kanno, H.; Bozic, I.; Nowak, M.A.; et al. Tumour and immune cell dynamics explain the PSA bounce after prostate cancer brachytherapy. Br. J. Cancer 2016, 115, 195–202. [Google Scholar] [CrossRef]
- Helm, A.; Ebner, D.K.; Tinganelli, W.; Simoniello, P.; Bisio, A.; Marchesano, V.; Durante, M.; Yamada, S.; Shimokawa, T. Combining heavy-ion therapy with immunotherapy: An update on recent developments. Int. J. Part. Ther. 2019, 5, 84–93. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Onishi, M.; Okonogi, N.; Oike, T.; Yoshimoto, Y.; Sato, H.; Suzuki, Y.; Kamada, T.; Nakano, T. High linear energy transfer carbon-ion irradiation increases the release of the immune mediator high mobility group box 1 from human cancer cells. J. Radiat. Res. 2018, 59, 541–546. [Google Scholar] [CrossRef]
- Mahadevan, L.S.K.; Sahoo, N.; Aliru, M.L.; Krishnan, S. Dependence of immunogenic modulation of tumor cells by proton radiation on the linear energy transfer. Int. J. Radiat. Oncol. 2017, 99, E607. [Google Scholar] [CrossRef]
- Kirilova, A.; Damyanovich, A.; Crook, J.; Jezioranski, J.; Wallace, K.; Pintilie, M. 3D MR-spectroscopic imaging assessment of metabolic activity in the prostate during the PSA “bounce” following 125Iodine brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 371–378. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Academia and clinic annals of internal medicine preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Roever, L. PICO: Model for clinical questions. Evid. Based Med. Pr. 2018, 3, 1–2. [Google Scholar] [CrossRef]
- Amico, A.V.D.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. Study Quality Assessment Tools for Cases Series Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 1 April 2020).
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A stata command to perform meta-analysis of binomial data. Arch. Publ. Health 2014, 72, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; Higgins, J.P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; Cochrane: London, UK, 2019. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Meta-regression. In Introduction to Meta-Analysis; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Sanchez, J.; Dohoo, I.R.; Christensen, J.; Rajic, A. Factors influencing the prevalence of salmonella spp. in swine farms: A meta-analysis approach. Prev. Vet. Med. 2007, 81, 148–177. [Google Scholar] [CrossRef]
- Keithlin, J.; Sargeant, J.; Thomas, M.K.; Fazil, A. Systematic review and meta-analysis of the proportion of campylobacter cases that develop chronic sequelae. BMC Publ. Health 2014, 14, 1203. [Google Scholar] [CrossRef]
- Harbord, R.M.; Higgins, J.P.T. Meta-regression in stata. Stata J. 2008, 8, 493–519. [Google Scholar] [CrossRef]

| Author | Year | n | Modality | Age | Risk Group | ADT | Follow Up (M) | Bounce (%) | Amplitude (ng/mL) | Time to Bounce (M) | Nadir (ng/mL) | Time to Nadir (M) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Merrick et al. | 2002 | 218 | EBRT+LDR-BT | 66 ± 7 | L, I | No | 46 ± 14 | 23.9 | 0.9 (0.3–3.0) | 19 ± 9 | NA | NA | [6] |
| Patel et al. | 2004 | 295 | LDR-BT | NA | L, I | Yes, partly | 38 (24–68) | 28.0 | 0.5 (0.2–4.1) | 19 (8–40) | NA | NA | [7] |
| Coen et al. | 2004 | 101 | LDR-BT | NA | L, I | No | 54 (38–86) | 39.6 | 0.6 (0.2–7.5) | 18 (7–71) | NA | NA | [8] |
| Zietman et al. | 2005 | 190 | EBRT | NA | L, I, H | Yes, all | 60 (40–75) | 39.0 | 0.9 (0.5–1.8) | 28 (17–42) | NA | NA | [9] |
| Ciezki et al. | 2006 | 162 | LDR-BT | 68 (45–83) | L, I, H | Yes, partly | 73 | 46.3 | NA | 15 (2–57) | NA | NA | [10] |
| Horwitz et al. | 2006 | 4839 | EBRT | NA | L, I, H | No | 75 | 18.6 | NA | NA | NA | NA | [11] |
| 2693 | LDR-BT | NA | L, I, H | No | 60 | 17.5 | NA | NA | NA | NA | |||
| Toledano et al. | 2007 | 295 | LDR-BT | 60–65 | L, I | Yes, partly | 40 (9–66) | 49.0 | 0.8, mean (0.1–4.1) | 19, mean (6–58) | NA | NA | [12] |
| Bostantic et al. | 2007 | 57 | LDR-BT | 65 ± 6 | L | No | 62 ± 10 | 14.0 | 0.4 | 18 ± 9 | NA | NA | [13] |
| 46 | LDR-BT | 63 ± 7 | L | No | 64 ± 12 | 45.7 | 0.4 | 22 ± 11 | NA | NA | |||
| 29 | LDR-BT | 66 ± 6 | L | Yes, all | 67 ± 12 | 20.7 | 0.4 | 6 ± 6 | NA | NA | |||
| 32 | LDR-BT | 67 ± 5 | L | Yes, all | 62 ± 12 | 28.1 | 0.4 | 18 ± 8 | NA | NA | |||
| Crook et al. | 2007 | 292 | LDR-BT | 64 (45–80) | L, I | No | 44 (8–81) | 40.0 | 0.7 (0.2–11.7) | 15 (3–29) | 0.05 (0.01–0.20) | 40 | [14] |
| Mitchell et al. | 2008 | 205 | LDR-BT | 62, mean (43–75) | L, I | No | 45 (24–85) | 37.0 | 0.9 (0.2–5.8) | 14 (1–40) | NA | NA | [15] |
| Makarewicz et al. | 2009 | 121 | EBRT+HDR-BT | 68 (47–78) | L, I | No | 81 (60–106) | 31.0 | 0.2, mean (0.2–0.7) | 14 (7–26) | 0.8 (0.01–2.1) | NA | [16] |
| King et al. | 2009 | 41 | SBRT | 66 (48–83) | L | No | 33 (6–45) | 29.0 | 0.3 (0.2–2.4) | 18 (12–33) | 0.3 (0.03–2.6) | NA | [17] |
| Pinkawa et al. | 2010 | 135 | EBRT | 71 (52–83) | L, I, H | Yes, partly | 67 (9–97) | 20.0 | NA | NA | NA | NA | [18] |
| 66 | EBRT+HDR-BT | 72 (63–81) | L, I, H | Yes, partly | 75 (7–98) | 23.0 | NA | NA | NA | NA | |||
| 94 | LDR-BT | 69 (49–81) | L, I | Yes, partly | 76 (8–101) | 42.0 | NA | NA | NA | NA | |||
| Caloglu et al. | 2011 | 820 | LDR-BT | 68 (45–87) | L, I, H | Yes, partly | 58 (36–123) | 30.1 | NA | 17 (2–68) | NA | NA | [19] |
| Zwahlen et al. | 2011 | 194 | LDR-BT | 61 (47–75) | L | No | 60 (23–109) | 50.0 | 0.5 (0.2–8.3) | 14 (0–70) | 0.1 (0.0–3.5) | NA | [20] |
| Aaltomaa et al. | 2011 | 535 | LDR-BT | 64 (42–80) | L, I, H | Yes, partly | 69 (15–131) | 27.4 | NA | NA | NA | NA | [21] |
| Beriwal et al. | 2012 | 155 | EBRT+LDR-BT | 65 ± 7 | L, I, H | Yes, partly | 36 (24–60) | 29.7 | 0.6, mean (0.2–2.3) | 12, mean (6–36) | NA | NA | [22] |
| Hinnen et al. | 2012 | 975 | LDR-BT | 66 ± 6 | L, I, H | Yes, partly | 78 (27–215) | 32.0 | 1.7 (1.0–2.8, IQR) | 19 (12–24, IQR) | NA | 12 (6–15) | [23] |
| Mazeron et al. | 2012 | 198 | LDR-BT | 67 (49–80) | L, I | No | 63 (36–119) | 35.9 | 1.0 ± 1.0 | 18 ± 9 | NA | NA | [24] |
| Bolzicco et al. | 2013 | 71 | SBRT | 72 (52–82) | L, I, H | Yes, partly | 36 (6–76) | 12.6 | NA | 23 (18–30) | 0.4 | 36 | [25] |
| Chen et al. | 2013 | 100 | SBRT | 69 (48–90) | L, I, H | Yes, partly | 27 (16–42) | 31.0 | 0.5 (0.2–2.2) | 15 (3–21) | 0.4 (0.1–1.9) | 24 | [26] |
| Katz et al. | 2013 | 304 | SBRT | 69, mean (45–88) | L, I, H | Yes, partly | 60 (8–78) | 17.0 | 0.5 | 30 | 0.1 | 60 | [27] |
| King et al. | 2013 | 1100 | SBRT | 70 (44–91) | L, I, H | Yes, partly | 36 | 16.0 | 0.5 (0.2–5.29) | NA | NA | NA | [28] |
| Mehta et al. | 2013 | 157 | HDR-BT | 63 (42–90) | L, I | Yes, partly | 55 | 43.0 | 0.6 (0.2–4.5) | 13 (0.6–64) | NA | NA | [29] |
| Lee et al. | 2014 | 29 | SBRT | 72 (50-86) | L, I, H | Yes, partly | 41 (12–69) | 28.0 | 0.6 (0.3–1.5) | 9 | 0.3 (0.003–1.7) | 23 | [30] |
| Nishihara et al. | 2014 | 116 | LDR-BT | 66 (51–80) | L, I | No | 42 (18–77) | 40.5 | 0.4 (0.2–5.6) | 17 (8–36) | NA | NA | [31] |
| Vu et al. | 2014 | 120 | SBRT | 68 (47–88) | L, I, H | Yes, partly | 24 (18–78) | 28.0 | 0.5 | 9 | NA | NA | [32] |
| Patel et al. | 2014 | 114 | EBRT+HDR-BT | 68 (48–79) | L, I | No | 66 (24–124) | 39.0 | 0.4 (0.2–6.6) | 16 (3–76) | 0.1 (0.01–1.7) | 53 (8–118) | [33] |
| Waters et al. | 2014 | 74 | EBRT, hopo | 68 ± 5 | L | No | 36, min | 31.1 | 0.6 | NA | NA | NA | [34] |
| 58 | EBRT | 66 ± 5 | L | No | 36, min | 20.7 | 0.3 | NA | NA | NA | |||
| 230 | LDR-BT | 64 ± 6 | L | No | 36, min | 29.6 | 0.6 | NA | NA | NA | |||
| Kole et al. | 2015 | 175 | SBRT | 69 (48–85) | L, I, H | No | 36 | 36.2 | NA | 15 (1–42) | 0.3 (0.02–1.8) | 30 (3–48) | [35] |
| Kishan et al. | 2015 | 130 | SBRT | 69 (44–87) | L, I | No | 40 (12–93) | 30.8 | 0.5 (0.2–3.6) | 14 (3–43) | NA | NA | [36] |
| 2015 | 220 | HDR-BT | 64 (43–84) | L, I | No | 49 (12–94) | 39.5 | 0.6 (0.2–7.1) | 10 (3–63) | NA | NA | ||
| 2015 | 89 | EBRT | 66 (52–85) | L, I | No | 27 (12–90) | 21.3 | 0.5 (0.2–7.6) | 13 (3–66) | NA | NA | ||
| Leduc et al. | 2015 | 274 | LDR-BT | 62 (45–76) | L | Yes, partly | 50 (24–126) | 31.0 | 1.0 (0.2–12.4) | 12 (6–37) | NA | NA | [37] |
| Quivirin et al. | 2015 | 66 | LDR-BT | 64 ± 5 | L | No | 35 (13–72) | 36.4 | 1.8 ± 1.6 | 12 ± 6 | NA | NA | [38] |
| Engeler et al. | 2015 | 713 | LDR-BT | 63 (42–82) | L, I, H | Yes, partly | 41 (24–132) | 24.3 | 0.7 (0.2–6.1) | 12 (6–33) | NA | NA | [39] |
| Kim et al. | 2016 | 33 | SBRT | 67 (56–72) | L, I | No | 51 (6–71) | 30.3 | 0.2 (0.2–1.3) | 10 (6–12) | 0.2 | 33 | [40] |
| Kim et al. | 2016 | 47 | SBRT | 64 (52–82) | L, I | No | 42 (36–78) | 51.0 | 0.5 (0.2–6.2) | 9 (3–36) | NA | 36 (11, SD) | [41] |
| Phak et al. | 2016 | 35 | EBRT+SBRT | 69, mean (60–78) | L, I | No | 52 (14–74) | 28.6 | 0.2 (0.2–0.5) | 11 (6–25) | 0.2 (0.04–1.4) | 32 (12–51) | [42] |
| 42 | EBRT | 71, mean (61–79) | L, I | No | 52 (14–74) | 21.4 | 0.3 (0.2–1.2) | 15 (6–30) | 0.3 (0.04–1.8) | 25 (9–58) | |||
| Freiberger et al. | 2017 | 94 | LDR-BT | 69 (49–81) | L, I | Yes, partly | 108 | 42.0 | NA | NA | 0.05, mean | 32, mean | [43] |
| 66 | EBRT+HDR-BT | 72 (63–81) | L, I, H | Yes, partly | 108 | 24.0 | NA | NA | 0.1, mean | 31, mean | |||
| 135 | EBRT | 71 (52–83) | L, I, H | Yes, partly | 108 | 25.0 | NA | NA | 0.5, mean | 19, mean | |||
| Hauck et al. | 2017 | 554 | HDR-BT | 63 (40–83) | L, I, H | Yes, partly | 44 (12–162) | 43.2 | NA | 11, mean | 0.2 | NA | [44] |
| Kindts et al. | 2017 | 192 | LDR-BT | 60 (50–65) | L, I | Yes, partly | 66 | 36.0 | 0.6, mean | 18, mean | NA | NA | [45] |
| Romesser et al. | 2017 | 776 | EBRT | 61-72, IQR | L, I, H | Yes, partly | 110 (83–134) | 15.9 | 0.3 (0.2–0.7, IQR) | 24 (16–38, IQR) | NA | NA | [46] |
| Park et al. | 2018 | 74 | SBRT | 69 (47–81) | L, I, H | No | 63 (12–109) | 35.2 | 0.5 (0.2–2.6) | 11 (2–38) | 0.1 (0.01–2.6) | 47 (1–85) | [47] |
| Astrom et al. | 2018 | 623 | EBRT+HDR-BT | 66 (47–79) | L, I, H | Yes, partly | 132 (2–266) | 26.0 | 1.5 (0.3–12.0) | 15 (3–103) | NA | NA | [48] |
| Burchardt et al. | 2018 | 41 | LDR-BT | 64 ± 7 | L, I | Yes, partly | 37 ± 8 | 26.8 | 0.7 ± 1.1 | 18 ± 6 | 0.5 ± 1.1 | 23 ± 14 | [49] |
| 53 | HDR-BT | 67 ± 7 | L, I | Yes, partly | 33 ± 9 | 22.6 | 0.8 ± 0.5 | 10 ± 4 | 0.2 ± 0.4 | 19 ± 14 | |||
| Kubo et al. | 2018 | 352 | EBRT+LDR-BT | 69 (49–82) | L, I, H | Yes, partly | 82 (12–157) | 33.2 | NA | 20 (3–55) | NA | NA | [50] |
| Roy et al. | 2019 | 287 | SBRT | 69 (49–82, IQR) | L, I | Yes, partly | 60 (46–106) | 31.1 | 0.6 (0.35–1.61, IQR) | 17 (11–25, IQR) | NA | NA | [51] |
| Jiang et al. | 2019 | 1062 | SBRT | 68 (63–73, IQR) | L, I | No | 66 (36–60, IQR) | 26.0 | 0.5 (0.3–1.0, IQR) | 18 (12–31, IQR) | 0.2 (0.1–0.3, IQR) | 40 (24–66, IQR) | [52] |
| Darwis et al. | 2020 | 131 | Carbon ions | 64, mean (48–80) | L, I | No | 60 (39–60) | 55.7 | 0.7 ± 1.0 | 15 ± 11 | 0.5 ± 0.3 | 42 (9–60) | [53] |
| Nakai et al. | 2020 | 256 | HDR-BT | 67 ± 6 | L, I | No | 91 ± 23 | 32.3 | NA | 19 ± 23 | NA | NA | [54] |
| Slade et al. | 2020 | 4004 | LDR-BT | 64 ± 6 | L, I | No | 120 | 31.8 | NA | NA | NA | NA | [55] |
| 473 | EBRT | 64 ± 6 | L, I | No | 120 | 27.7 | NA | NA | NA | NA |
| Modality | Rate of Bounce (%) | Amplitude (ng/mL) | Time to Occurrence (M) | Nadir (ng/mL) | Time to Nadir (M) |
|---|---|---|---|---|---|
| LDR-BT | 34 (30–37) | 1.7 (1.3–2.0) | 18 (17–20) | 0.5 (−0.1–1.1) | 23 (19–28) |
| HDR-BT | 36 (29–42) | 1.4 (0.7–2.2) | 18 (12–25) | 0.2 (0.09–0.3) | 19 (15–23) |
| EBRT | 22 (19–25) | 0.8 (0.4–1.2) | 24 (20–29) | 0.6 (0.5–0.7) | 29 (25–32) |
| SBRT | 28 (23–32) | 1.0 (0.7–1.2) | 17 (14–20) | 0.6 (0.3–0.8) | 38 (26–51) |
| EBRT + boost | 28 (26–31) | 1.0 (0.7–1.4) | 18 (14–22) | 0.6 (0.4–0.8) | 44 (19–70) |
| CIRT | 56 (47–64) | 0.7 (0.5–1.0) | 15 (12–17) | 0.5 (0.4–0.6) | 42 (40–44) |
| Pooled ES | 31 (28–33) | 1.3 (1.1–1.4) | 18 (17–20) | 0.5 (0.4–0.6) | 35 (28–42) |
| I2, p values | 93.5% (p < 0.05) | 98.3% (p < 0.05) | 95.7% (p < 0.05) | 99.5% (p < 0.05) | 98.4% (p < 0.05) |
| Modality | Nadir (ng/mL) | Time to Nadir (M) | ||
|---|---|---|---|---|
| Bounce | No bounce | Bounce | No bounce | |
| LDR-BT | NA | NA | NA | NA |
| HDR-BT | NA | NA | NA | NA |
| EBRT | 0.7 (0.7–0.8) | 0.5 (0.5–0.5) | 42 (40–43) | 29 (28–29) |
| SBRT | 0.6 (0.5–0.7) | 0.3 (0.3–0.4) | NA | NA |
| EBRT + Boost | 0.3 (0.2–0.4) | 0.5 (0.4–0.6) | 64 (58–70) | 54 (48–60) |
| CIRT | 0.6 (0.5–0.7) | 0.4 (0.3–0.5) | 48 (45–50) | 36 (33–40) |
| Pooled effect size | 0.6 (0.3–0.8) | 0.5 (0.4–0.6) | 50 (42–59) | 39 (27–51) |
| I2, p values | 98.5% (p < 0.05) | 95.1% (p < 0.05) | 96.6% (p < 0.05) | 97.6% (p < 0.05) |
| Covariates | Rate of Bounce (n = 65) | Amplitude (n = 37) | Time to Occurrence (n = 45) | Nadir (n = 15) | Time to Nadir (n = 9) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p | R2 (%) | Coefficient | p | R2 (%) | Coefficient | p | R2 (%) | Coefficient | p | R2 (%) | Coefficient | p | R2 (%) | |
| Age | −0.07 (−0.10 to −0.03) | <0.01 | 20.2 | −0.14 (−0.22 to −0.06) | <0.01 | 28.1 | 0.30 (−0.27 to 0.87) | 0.33 | 1.1 | −0.01 (−0.06 to 0.05) | 0.78 | 0.0 | −0.32 (−4.82 to 4.18) | 0.87 | 0.0 |
| Modality | |||||||||||||||
| LDR-BT | −0.08 (−0.46 to 0.30) | 0.66 | 29.7 | 0.25 (−0.61 to 1.10) | 0.56 | 15.4 | −5.57 (−11.32 to 0.18) | 0.05 | 5.0 | −0.05 (−0.79 to 0.68) | 0.88 | 0.0 | −18.96 (−78.66 to 40.74) | 0.38 | 0.0 |
| HDR-BT | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |||||
| EBRT | −0.63 (−1.07 to −0.19) | <0.01 | −0.53 (−1.56 to 0.50) | 0.30 | NA | NA | 0.11 (−0.79 to 1.01) | 0.78 | −13.31 (−72.86 to 46.24) | 0.52 | |||||
| SBRT | −0.38 (−0.79 to 0.03) | 0.07 | −0.48 (−1.36 to 0.40) | 0.27 | −7.24 (−13.36 to –1.11) | 0.02 | 0.07 (−0.61 to 0.75) | 0.82 | −3.90 (−52.43 to 44.62) | 0.81 | |||||
| EBRT + boost | −0.32 (−0.75 to 0.11) | 0.14 | −0.41 (−1.35 to 0.53) | 0.38 | −5.92 (−12.39 to 0.56) | 0.07 | 0.07 (−0.65 to 0.80) | 0.82 | 2.23 (−49.31 to 53.77) | 0.89 | |||||
| CIRT | 0.83 (−0.26 to 1.68) | 0.05 | −0.70 (−2.24 to 0.83) | 0.35 | −9.50 (−20.68 to 1.67) | 0.09 | NA | NA | NA | NA | |||||
| ADT | −0.14 (−0.34 to 0.06) | 0.17 | −0.20 (−0.63 to 0.22) | 0.34 | 0.8 | 0.32 (−2.18 to 2.82) | 0.79 | 0.0 | −0.07 (−0.41 to 0.27) | 0.66 | 0.0 | −18.10 (−37.28 to 1.08) | 0.06 | 33.8 | |
| Risk group | −0.20 (−0.35 to −0.04) | 0.01 | 12.2 | −0.23 (−0.62 to 0.15) | 0.22 | 1.3 | 1.42 (−0.78 to 3.61) | 0.20 | 0.0 | 0.02 (−0.21 to 0.24) | 0.88 | 0.0 | 0.94 (−24.00 to 25.89) | 0.93 | 0.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwis, N.D.M.; Oike, T.; Kubo, N.; Gondhowiardjo, S.A.; Ohno, T. Characteristics of PSA Bounce after Radiotherapy for Prostate Cancer: A Meta-Analysis. Cancers 2020, 12, 2180. https://doi.org/10.3390/cancers12082180
Darwis NDM, Oike T, Kubo N, Gondhowiardjo SA, Ohno T. Characteristics of PSA Bounce after Radiotherapy for Prostate Cancer: A Meta-Analysis. Cancers. 2020; 12(8):2180. https://doi.org/10.3390/cancers12082180
Chicago/Turabian StyleDarwis, Narisa Dewi Maulany, Takahiro Oike, Nobuteru Kubo, Soehartati A Gondhowiardjo, and Tatsuya Ohno. 2020. "Characteristics of PSA Bounce after Radiotherapy for Prostate Cancer: A Meta-Analysis" Cancers 12, no. 8: 2180. https://doi.org/10.3390/cancers12082180
APA StyleDarwis, N. D. M., Oike, T., Kubo, N., Gondhowiardjo, S. A., & Ohno, T. (2020). Characteristics of PSA Bounce after Radiotherapy for Prostate Cancer: A Meta-Analysis. Cancers, 12(8), 2180. https://doi.org/10.3390/cancers12082180





