First-Line Treatment for Primary Breast Diffuse Large B-Cell Lymphoma Using Immunochemotherapy and Central Nervous System Prophylaxis: A Multicenter Phase 2 Trial
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
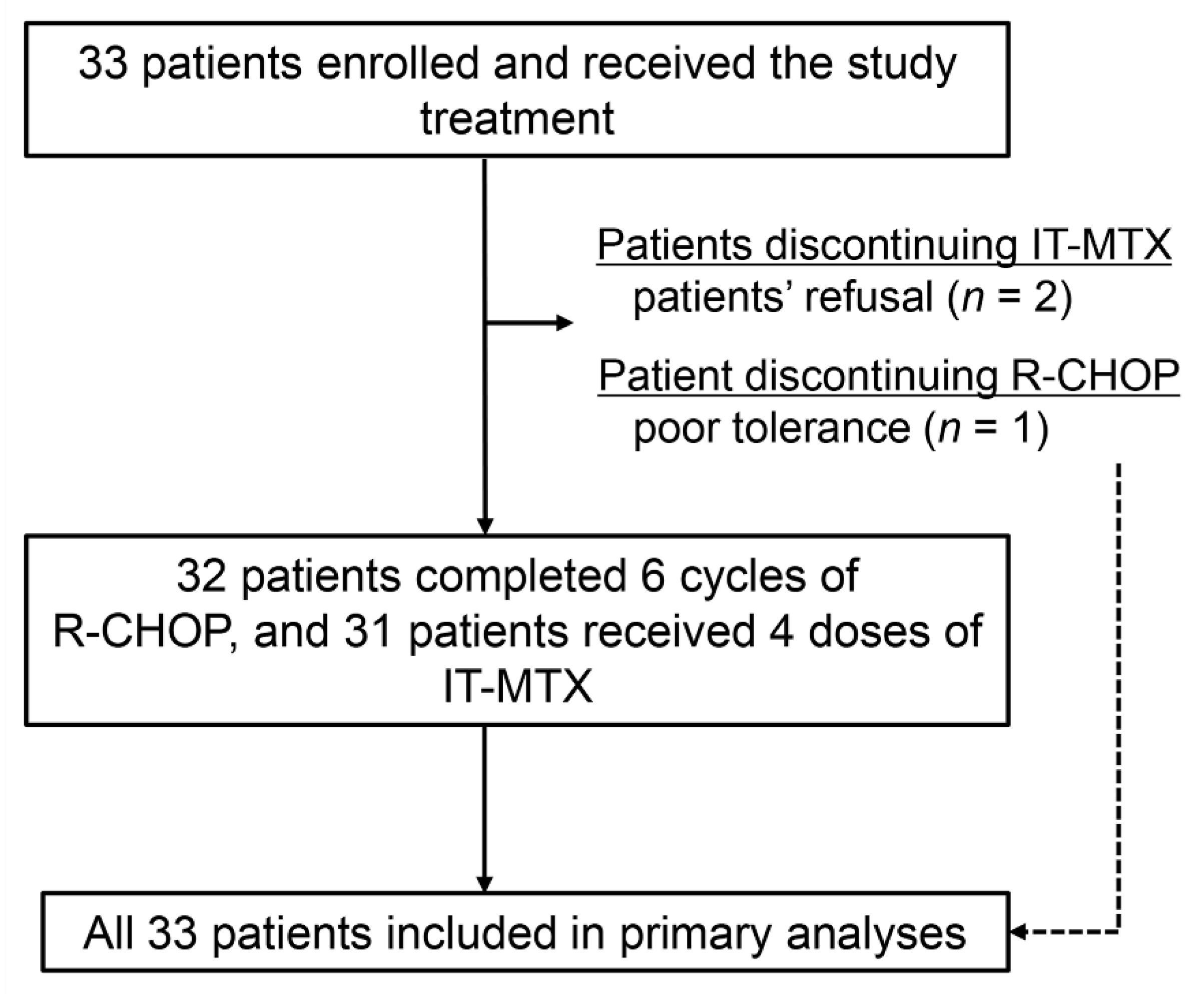
2.2. Feasibility and Treatment Response
2.3. Survival, Patterns of Relapse, and CNS Outcomes
2.4. Safety and Long-Term Survivorship
3. Discussion
4. Materials and Methods
4.1. Study Design and Patients
4.2. Treatments
4.3. End Points and Assessments
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brogi, E.; Harris, N.L. Lymphomas of the breast: Pathology and clinical behavior. Semin. Oncol. 1999, 26, 357–364. [Google Scholar] [PubMed]
- Yhim, H.Y.; Kim, J.S.; Kang, H.J.; Kim, S.J.; Kim, W.S.; Choi, C.W.; Eom, H.S.; Kim, J.A.; Lee, J.H.; Won, J.H.; et al. Matched-pair analysis comparing the outcomes of primary breast and nodal diffuse large B-cell lymphoma in patients treated with rituximab plus chemotherapy. Int. J. Cancer 2012, 131, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Hosein, P.J.; Maragulia, J.C.; Salzberg, M.P.; Press, O.W.; Habermann, T.M.; Vose, J.M.; Bast, M.; Advani, R.H.; Tibshirani, R.; Evens, A.M.; et al. A multicentre study of primary breast diffuse large B-cell lymphoma in the rituximab era. Br. J. Haematol. 2014, 165, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Song, Y.; Sun, X.; Su, L.; Zhang, W.; Jia, J.; Bai, O.; Yang, S.; Liang, R.; Li, X.; et al. Primary breast diffuse large B-cell lymphoma in the rituximab era: Therapeutic strategies and patterns of failure. Cancer Sci. 2018, 109, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.; Martinelli, G.; Kuper-Hommel, M.; Tsang, R.; Pruneri, G.; Yuen, K.; Roos, D.; Lennard, A.; Devizzi, L.; Crabb, S.; et al. Primary diffuse large B-cell lymphoma of the breast: Prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann. Oncol. 2008, 19, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Watanabe, T.; Munakata, W.; Mori, M.; Maruyama, D.; Kim, S.W.; Kobayashi, Y.; Taniguchi, H.; Maeshima, A.M.; Tanosaki, R.; et al. Bulky disease has an impact on outcomes in primary diffuse large B-cell lymphoma of the breast: A retrospective analysis at a single institution. Eur. J. Haematol. 2011, 87, 434–440. [Google Scholar] [CrossRef]
- Yhim, H.Y.; Kang, H.J.; Choi, Y.H.; Kim, S.J.; Kim, W.S.; Chae, Y.S.; Kim, J.S.; Choi, C.W.; Oh, S.Y.; Eom, H.S.; et al. Clinical outcomes and prognostic factors in patients with breast diffuse large B cell lymphoma; Consortium for Improving Survival of Lymphoma (CISL) study. BMC Cancer 2010, 10, e321. [Google Scholar] [CrossRef]
- Aviles, A.; Delgado, S.; Nambo, M.J.; Neri, N.; Murillo, E.; Cleto, S. Primary breast lymphoma: Results of a controlled clinical trial. Oncology 2005, 69, 256–260. [Google Scholar] [CrossRef]
- Aviv, A.; Tadmor, T.; Polliack, A. Primary diffuse large B-cell lymphoma of the breast: Looking at pathogenesis, clinical issues and therapeutic options. Ann. Oncol. 2013, 24, 2236–2244. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef]
- Kwak, J.Y. Treatment of diffuse large B cell lymphoma. Korean J. Intern. Med. 2012, 27, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Boehme, V.; Schmitz, N.; Zeynalova, S.; Loeffler, M.; Pfreundschuh, M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: An analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood 2009, 113, 3896–3902. [Google Scholar] [CrossRef]
- Tai, W.M.; Chung, J.; Tang, P.L.; Koo, Y.X.; Hou, X.; Tay, K.W.; Quek, R.; Tao, M.; Lim, S.T. Central nervous system (CNS) relapse in diffuse large B cell lymphoma (DLBCL): Pre- and post-rituximab. Ann. Hematol. 2011, 90, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, U.; Chiappella, A.; Ferreri, A.J.; Martelli, M.; Baldi, I.; Balzarotti, M.; Bottelli, C.; Conconi, A.; Gomez, H.; Lopez-Guillermo, A.; et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: Final results of an international phase II trial. J. Clin. Oncol. 2011, 29, 2766–2772. [Google Scholar] [CrossRef]
- Leppa, S.; Jorgensen, J.; Tierens, A.; Meriranta, L.; Ostlie, I.; de Nully Brown, P.; Fagerli, U.M.; Larsen, T.S.; Mannisto, S.; Munksgaard, L.; et al. Patients with high-risk DLBCL benefit from dose-dense immunochemotherapy combined with early systemic CNS prophylaxis. Blood Adv. 2020, 4, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Eyre, T.A.; Djebbari, F.; Kirkwood, A.A.; Collins, G.P. Efficacy of central nervous system prophylaxis with stand-alone intrathecal chemotherapy in diffuse large B-cell lymphoma patients treated with anthracycline-based chemotherapy in the rituximab era: A systematic review. Haematologica 2020, 105, 1914–1924. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Bruno-Ventre, M.; Donadoni, G.; Ponzoni, M.; Citterio, G.; Foppoli, M.; Vignati, A.; Scarfo, L.; Sassone, M.; Govi, S.; et al. Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. Br. J. Haematol. 2015, 168, 654–662. [Google Scholar] [CrossRef]
- Holte, H.; Leppa, S.; Bjorkholm, M.; Fluge, O.; Jyrkkio, S.; Delabie, J.; Sundstrom, C.; Karjalainen-Lindsberg, M.L.; Erlanson, M.; Kolstad, A.; et al. Dose-densified chemoimmunotherapy followed by systemic central nervous system prophylaxis for younger high-risk diffuse large B-cell/follicular grade 3 lymphoma patients: Results of a phase II Nordic Lymphoma Group study. Ann. Oncol. 2013, 24, 1385–1392. [Google Scholar] [CrossRef]
- Petrich, A.M.; Gandhi, M.; Jovanovic, B.; Castillo, J.J.; Rajguru, S.; Yang, D.T.; Shah, K.A.; Whyman, J.D.; Lansigan, F.; Hernandez-Ilizaliturri, F.J.; et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: A multicenter retrospective analysis. Blood 2014, 124, 2354–2361. [Google Scholar] [CrossRef]
- Ennishi, D.; Jiang, A.; Boyle, M.; Collinge, B.; Grande, B.M.; Ben-Neriah, S.; Rushton, C.; Tang, J.; Thomas, N.; Slack, G.W.; et al. Double-Hit Gene Expression Signature Defines a Distinct Subgroup of Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 190–201. [Google Scholar] [CrossRef]
- Sha, C.; Barrans, S.; Cucco, F.; Bentley, M.A.; Care, M.A.; Cummin, T.; Kennedy, H.; Thompson, J.S.; Uddin, R.; Worrillow, L.; et al. Molecular High-Grade B-Cell Lymphoma: Defining a Poor-Risk Group That Requires Different Approaches to Therapy. J. Clin. Oncol. 2019, 37, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.J.; Slack, G.W.; Mottok, A.; Sehn, L.H.; Villa, D.; Kansara, R.; Kridel, R.; Steidl, C.; Ennishi, D.; Tan, K.L.; et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood 2016, 127, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Aviles, A.; Neri, N.; Nambo, M.J. The role of genotype in 104 cases of diffuse large B-cell lymphoma primary of breast. Am. J. Clin. Oncol. 2012, 35, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Nakamura, N.; Sasaki, Y.; Yoshida, S.; Yasuda, M.; Sagara, H.; Ohtake, T.; Takenoshita, S.; Abe, M. Primary breast diffuse large B-cell lymphoma shows a non-germinal center B-cell phenotype. Mod. Pathol. 2005, 18, 398–405. [Google Scholar] [CrossRef]
- Yoon, D.H.; Sohn, B.S.; Oh, S.Y.; Lee, W.S.; Lee, S.M.; Yang, D.H.; Huh, J.; Suh, C. Feasibility of abbreviated cycles of immunochemotherapy for completely resected limited-stage CD20+ diffuse large B-cell lymphoma (CISL 12-09). Oncotarget 2017, 8, 13367–13374. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Lunning, M.A.; Zhang, Z.; Migliacci, J.C.; Moskowitz, C.H.; Zelenetz, A.D. Excellent outcomes and lack of prognostic impact of cell of origin for localized diffuse large B-cell lymphoma in the rituximab era. Br. J. Haematol. 2015, 171, 776–783. [Google Scholar] [CrossRef]
- Grommes, C.; Pastore, A.; Palaskas, N.; Tang, S.S.; Campos, C.; Schartz, D.; Codega, P.; Nichol, D.; Clark, O.; Hsieh, W.Y.; et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov. 2017, 7, 1018–1029. [Google Scholar] [CrossRef]
- Houillier, C.; Choquet, S.; Touitou, V.; Martin-Duverneuil, N.; Navarro, S.; Mokhtari, K.; Soussain, C.; Hoang-Xuan, K. Lenalidomide monotherapy as salvage treatment for recurrent primary CNS lymphoma. Neurology 2015, 84, 325–326. [Google Scholar] [CrossRef]
- Younes, A.; Sehn, L.H.; Johnson, P.; Zinzani, P.L.; Hong, X.; Zhu, J.; Patti, C.; Belada, D.; Samoilova, O.; Suh, C.; et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 1285–1295. [Google Scholar] [CrossRef]
- Ayed, A.O.; Chiappella, A.; Pederson, L.; Laplant, B.R.; Congiu, A.G.; Gaidano, G.; Spina, M.; Re, A.; Cavallo, F.; Musuraca, G.; et al. CNS relapse in patients with DLBCL treated with lenalidomide plus R-CHOP (R2CHOP): Analysis from two phase 2 studies. Blood Cancer J. 2018, 8, e63. [Google Scholar] [CrossRef]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Zeynalova, S.; Nickelsen, M.; Kansara, R.; Villa, D.; Sehn, L.H.; Glass, B.; Scott, D.W.; Gascoyne, R.D.; Connors, J.M.; et al. CNS International Prognostic Index: A Risk Model for CNS Relapse in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP. J. Clin. Oncol. 2016, 34, 3150–3156. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Patients (n = 33) | |
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 50 | |
| Range | 29–75 | |
| Primary site involved | ||
| Right | 18 | 54.5 |
| Left | 14 | 42.4 |
| Bilateral | 1 | 3.0 |
| Lymph node involvement | ||
| No | 17 | 51.5 |
| Regional node | 14 | 42.4 |
| Distant node | 2 | 6.1 |
| Ann Arbor stage | ||
| IE | 17 | 51.5 |
| IIE | 13 | 39.4 |
| IIIE | 2 | 6.1 |
| IVE | 1 | 3.0 |
| ECOG performance status | ||
| 0 or 1 | 32 | 97.0 |
| 2 | 1 | 3.0 |
| B symptoms | ||
| Absence | 31 | 93.9 |
| Presence | 2 | 6.1 |
| Serum lactate dehydrogenase level | ||
| Normal | 24 | 72.7 |
| Elevated | 9 | 27.3 |
| Bulky disease | ||
| No | 32 | 97.0 |
| Yes | 1 | 3.0 |
| IPI | ||
| Low | 28 | 84.8 |
| Low-intermediate | 2 | 6.1 |
| High-intermediate | 3 | 9.1 |
| High | 0 | 0 |
| CNS-IPI | ||
| Low | 28 | 84.8 |
| Intermediate | 5 | 15.2 |
| High | 0 | 0 |
| Toxicities | Treatment Emergent Adverse Events (Total = 196 Cycles, %) | Grade 1–2 | Grade 3–4 | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Hematological | |||||
| Neutropenia | 70 (35.7) | 2 | 1.0 | 68 | 34.7 |
| Anemia | 49 (25.0) | 43 | 21.9 | 6 | 3.1 |
| Thrombocytopenia | 22 (11.2) | 14 | 7.1 | 8 | 4.1 |
| Infectious | |||||
| Febrile neutropenia | 8 (4.1) | 0 | 0 | 8 | 4.1 |
| Other infection | 3 (1.5) | 2 | 1.0 | 1 | 0.5 |
| Gastrointestinal | |||||
| Mucositis | 28 (14.3) | 26 | 13.3 | 2 | 1.0 |
| Nausea/vomiting | 15 (7.7) | 14 | 7.1 | 1 | 0.5 |
| Constipation | 7 (3.6) | 6 | 3.1 | 1 | 0.5 |
| Neurological | |||||
| Sensory | 66 (33.7) | 64 | 32.7 | 2 | 1.0 |
| Motor | 10 (5.1) | 8 | 4.1 | 2 | 1.0 |
| Central nervous system | 1 (0.5) | 0 | 0 | 1 | 0.5 |
| Cutaneous | 9 (4.6) | 9 | 4.6 | 0 | 0 |
| Musculoskeletal | 14 (7.1) | 14 | 7.1 | 0 | 0 |
| Case | Age | Lymphoma Involved | Time From Last Treatment to Breast Cancer Diagnosis (Months) | Radiation Therapy During Lymphoma Treatment | Breast Cancer Developed | Breast Cancer Stage |
|---|---|---|---|---|---|---|
| #5 | 55 | Left | 27.0 | No | Contralateral | 1 |
| #18 | 47 | Left | 14.9 | No | Ipsilateral | 2 |
| #20 | 46 | Right | 80.3 | No | Ipsilateral | 2 |
| #31 | 50 | Right | 14.0 | No | Ipsilateral | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yhim, H.-Y.; Yoon, D.H.; Kim, S.J.; Yang, D.-H.; Eom, H.-S.; Kim, K.H.; Park, Y.; Kim, J.S.; Kim, H.J.; Suh, C.; et al. First-Line Treatment for Primary Breast Diffuse Large B-Cell Lymphoma Using Immunochemotherapy and Central Nervous System Prophylaxis: A Multicenter Phase 2 Trial. Cancers 2020, 12, 2192. https://doi.org/10.3390/cancers12082192
Yhim H-Y, Yoon DH, Kim SJ, Yang D-H, Eom H-S, Kim KH, Park Y, Kim JS, Kim HJ, Suh C, et al. First-Line Treatment for Primary Breast Diffuse Large B-Cell Lymphoma Using Immunochemotherapy and Central Nervous System Prophylaxis: A Multicenter Phase 2 Trial. Cancers. 2020; 12(8):2192. https://doi.org/10.3390/cancers12082192
Chicago/Turabian StyleYhim, Ho-Young, Dok Hyun Yoon, Seok Jin Kim, Deok-Hwan Yang, Hyeon-Seok Eom, Kyoung Ha Kim, Yong Park, Jin Seok Kim, Hyo Jung Kim, Cheolwon Suh, and et al. 2020. "First-Line Treatment for Primary Breast Diffuse Large B-Cell Lymphoma Using Immunochemotherapy and Central Nervous System Prophylaxis: A Multicenter Phase 2 Trial" Cancers 12, no. 8: 2192. https://doi.org/10.3390/cancers12082192
APA StyleYhim, H.-Y., Yoon, D. H., Kim, S. J., Yang, D.-H., Eom, H.-S., Kim, K. H., Park, Y., Kim, J. S., Kim, H. J., Suh, C., Kim, W. S., & Kwak, J.-Y. (2020). First-Line Treatment for Primary Breast Diffuse Large B-Cell Lymphoma Using Immunochemotherapy and Central Nervous System Prophylaxis: A Multicenter Phase 2 Trial. Cancers, 12(8), 2192. https://doi.org/10.3390/cancers12082192





