Sigma 1 Receptor is Overexpressed in Hepatocellular Adenoma: Involvement of ERα and HNF1α
Abstract
:1. Introduction
2. Results
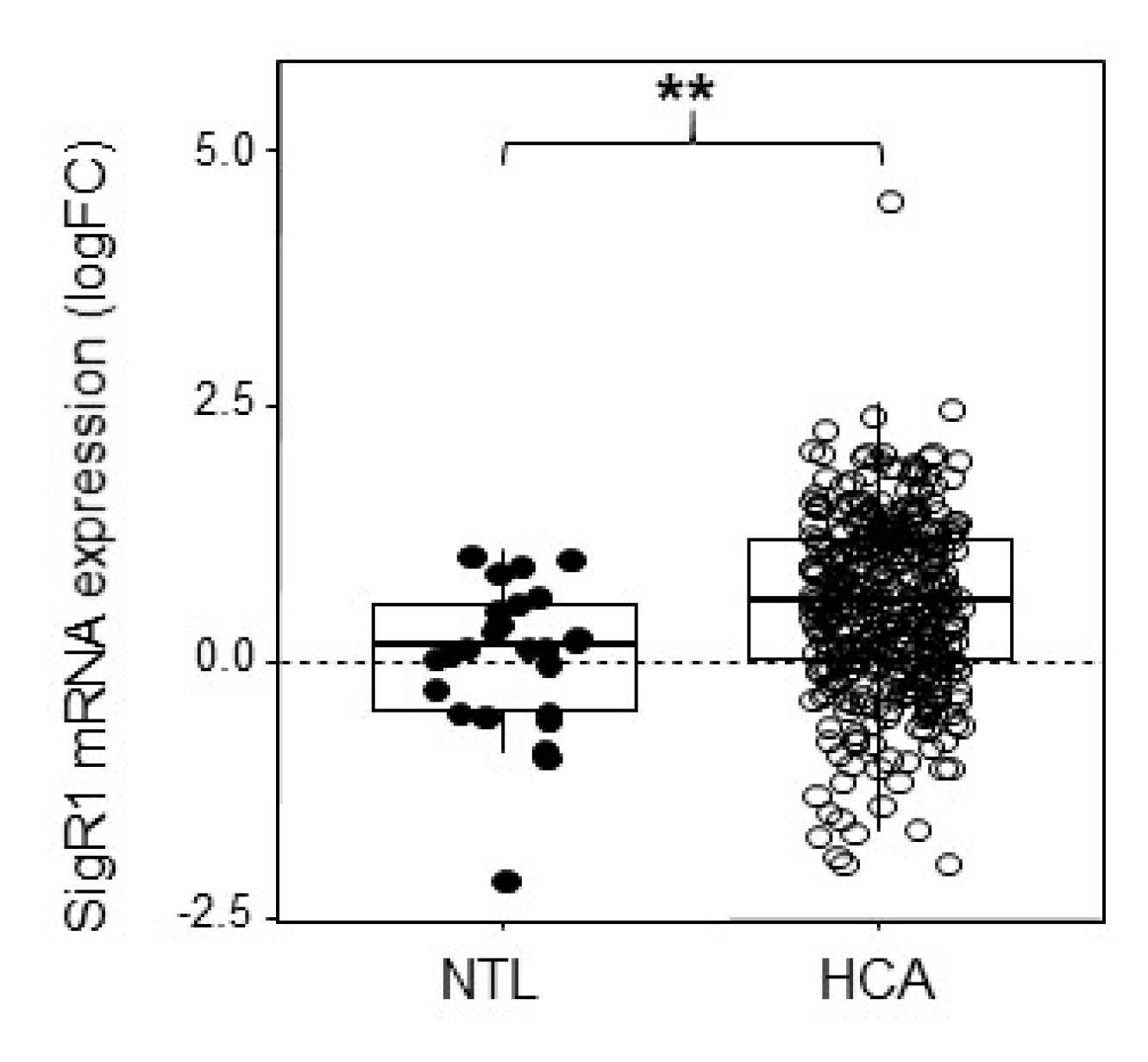
2.1. HCA Overexpress SigR1
2.2. Estrogens Induce Expression of SigR1
2.3. Estrogens Use ERα to Regulate the Expression of SigR1
2.4. H-HCA Overexpress SigR1 Compared to Other Subgroups of HCA
2.5. Loss of HNF1α Leads to Increased SigR1 Expression
2.6. SigR1 Overexpression Affects Cell Proliferation
2.7. SigR1 Overexpression Affects Lipid Storage
3. Discussion
3.1. SigR1 Expression is Upregulated by Estrogens in Hepatocytes
3.2. SigR1 Expression is Repressed by HNF1
3.3. SigR1 Overexpression Controls Hepatocyte Proliferation and Fat Accumulation
4. Materials and Methods
4.1. Human Tissue Samples and Animals
4.2. Cell Culture and Proliferation Assay
4.3. Transfection with Luciferase Reporter and ERα Overexpression
4.4. 17βestradiol Treatment
4.5. Generating Myc-SigR1 Stably Expressing HepG2 Cell Line
4.6. Protein Extraction and Western Blotting
4.7. RT-qPCR
4.8. Oil Red O Staining
4.9. Chromatin Immunoprecipitation (ChIP)
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ChIP | Chromatin immunoprecipitation |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| E2 | 17βestradiol |
| HCA | hepatocellular adenomas |
| H-HCA | human hepatocyte nuclear factor 1α-mutated hepatocellular adenoma |
| HNF1α | hepatocyte nuclear factor 1α |
| RT-PCR | reverse transcription-polymerase chain reaction |
| SigR1 | Sigma receptor 1 |
| siRNA | small interfering RNA |
References
- Aydar, E.; Palmer, C.P.; Djamgoz, M.B. Sigma receptors and cancer: Possible involvement of ion channels. Cancer Res. 2004, 64, 5029–5035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganapathy, M.E.; Prasad, P.D.; Huang, W.; Seth, P.; Leibach, F.H.; Ganapathy, V. Molecular and Ligand-Binding Characterization of the ς-Receptor in the Jurkat Human T Lymphocyte Cell Line. J. Pharmacol. Exp. Ther. 1999, 289, 251–260. [Google Scholar] [PubMed]
- Hayashi, T.; Su, T.P. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T. The Sigma-1 Receptor in Cellular Stress Signaling. Front. Neurosci. 2019, 13, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Rizzuto, R.; Hajnoczky, G.; Su, T.P. MAM: More than just a housekeeper. Trends Cell Biol. 2009, 19, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef] [Green Version]
- Su, T.P.; Su, T.C.; Nakamura, Y.; Tsai, S.Y. The sigma-1 receptor as a pluripotent modulator in living systems. Trends Pharmacol. Sci. 2016, 37, 262–278. [Google Scholar] [CrossRef] [Green Version]
- Maurice, T.; Goguadze, N. Role of σ1 Receptors in Learning and Memory and Alzheimer’s Disease-Type Dementia. Adv. Exp. Med. Biol. 2017, 964, 213–233. [Google Scholar]
- Benarroch, E.E. Sigma-1 receptor and amyotrophic lateral sclerosis. Neurology 2018, 91, 743–747. [Google Scholar] [CrossRef]
- Vilner, B.J.; John, C.S.; Bowen, W.D. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995, 55, 408–413. [Google Scholar]
- Aydar, E.; Onganer, P.; Perrett, R.; Djamgoz, M.B.; Palmer, C.P. The expression and functional characterization of sigma (σ) 1 receptors in breast cancer cell lines. Cancer Lett. 2006, 242, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Simony-Lafontaine, J.; Esslimani, M.; Bribes, E.; Gourgou, S.; Lequeux, N.; Lavail, R.; Grenier, J.; Kramar, A.; Casellas, P. Immunocytochemical assessment of sigma-1 receptor and human sterol isomerase in breast cancer and their relationship with a series of prognostic factors. Br. J. Cancer 2000, 82, 1958. [Google Scholar] [PubMed] [Green Version]
- Maurice, T.; Su, T.P. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 2009, 124, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Gueguinou, M.; Crottès, D.; Chantôme, A.; Rapetti-Mauss, R.; Potier-Cartereau, M.; Clarysse, L.; Girault, A.; Fourbon, Y.; Jézéquel, P.; Guérin-Charbonnel, C.; et al. The SigmaR1 chaperone drives breast and colorectal cancer cell migration by tuning SK3-dependent Ca2+ homeostasis. Oncogene 2017, 36, 3640–3647. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, L.; Han, C.; Wei, L.; Kong, L.; Lin, F. Sigma-1 receptor (σ1R) is downregulated in hepatic malignant tumors and regulates HepG2 cell proliferation, migration and apoptosis. Oncol. Rep. 2018, 39, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Brent, P.J.; Pang, G.T. σ binding site ligands inhibit cell proliferation in mammary and colon carcinoma cell lines and melanoma cells in culture. Eur. J. Pharmacol. 1995, 278, 151–160. [Google Scholar] [CrossRef]
- Tesei, A.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Zanoni, M.; Paolillo, M.; Curti, D.; Rossi, D.; Collina, S. Sigma Receptors as Endoplasmic Reticulum Stress “Gatekeepers” and their Modulators as Emerging New Weapons in the Fight Against Cancer. Front. Pharmacol. 2018, 9, 711. [Google Scholar] [CrossRef] [Green Version]
- John, C.S.; Gulden, M.E.; Li, J.; Bowen, W.D.; McAfee, J.G.; Thakur, M.L. Synthesis, in vitro binding, and tissue distribution of radioiodinated 2-[125I] N-(N-benzylpiperidin-4-yl)-2-iodo benzamide, 2-[125I] BP: A potential σ receptor marker for human prostate tumors. Nucl. Med. Biol. 1998, 25, 189–194. [Google Scholar] [CrossRef]
- Nault, J.C.; Paradis, V.; Cherqui, D.; Vilgrain, V.; Zucman-Rossi, J. Molecular classification of hepatocellular adenoma in clinical practice. J. Hepatol. 2017, 67, 1074–1083. [Google Scholar] [CrossRef]
- Pelletier, L.; Rebouissou, S.; Paris, A.; Rathahao-Paris, E.; Perdu, E.; Bioulac-Sage, P.; Imbeaud, S.; Zucman-Rossi, J. Loss of hepatocyte nuclear factor 1α function in human hepatocellular adenomas leads to aberrant activation of signaling pathways involved in tumorigenesis. Hepatology 2010, 51, 557–566. [Google Scholar] [CrossRef]
- Rebouissou, S.; Imbeaud, S.; Balabaud, C.; Boulanger, V.; Bertrand-Michel, J.; Tercé, F.; Auffray, C.; Bioulac-Sage, P.; Zucman-Rossi, J. HNF1α inactivation promotes lipogenesis in human hepatocellular adenoma independently of SREBP-1 and carbohydrate-response element-binding protein (ChREBP) activation. J. Biol. Chem. 2007, 282, 14437–14446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannitrapani, L.; Soresi, M.; La Spada, E.; Cervello, M.; D’Alessandro, N.; Montalto, G. Sex hormones and risk of liver tumor. Ann. N.Y. Acad. Sci. 2006, 1089, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Ciana, P.; Raviscioni, M.; Mussi, P.; Vegeto, E.; Que, I.; Parker, M.G.; Lowik, C.; Maggi, A. In vivo imaging of transcriptionally active estrogen receptors. Nat. Med. 2003, 9, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Uebi, T.; Umeda, M.; Imai, T. Estrogen induces estrogen receptor alpha expression and hepatocyte proliferation in the livers of male mice. Genes Cells 2015, 20, 217–223. [Google Scholar] [CrossRef]
- Yamagata, K.; Oda, N.; Kaisaki, P.J.; Menzel, S.; Furuta, H.; Vaxillaire, M.; Southam, L.; Cox, R.D.; Lathrop, G.M.; Boriraj, V.V.; et al. Mutations in the hepatocyte nuclear factor-1α gene in maturity-onset diabetes of the young (MODY3). Nature 1996, 384, 455. [Google Scholar] [CrossRef]
- Pontoglio, M.; Barra, J.; Hadchouel, M.; Doyen, A.; Kress, C.; Bach, J.P.; Babinet, C.; Yaniv, M. Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell 1996, 84, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Patitucci, C.; Couchy, G.; Bagattin, A.; Cañeque, T.; De Reyniès, A.; Scoazec, J.Y.; Rodriguez, R.; Pontoglio, M.; Zucman-Rossi, J.; Pende, M.; et al. Hepatocyte nuclear factor 1α suppresses steatosis-associated liver cancer by inhibiting PPARγ transcription. J. Clin. Investig. 2017, 127, 1873–1888. [Google Scholar] [CrossRef] [Green Version]
- Shih, D.Q.; Bussen, M.; Sehayek, E.; Ananthanarayanan, M.; Shneider, B.L.; Suchy, F.J.; Shefer, S.; Bollileni, J.S.; Gonzalez, F.J.; Breslow, J.L.; et al. Hepatocyte nuclear factor-1α is an essential regulator of bile acid and plasma cholesterol metabolism. Nat. Genet. 2001, 27, 375. [Google Scholar] [CrossRef]
- Ryffel, G.U. Mutations in the human genes encoding the transcription factors of the hepatocyte nuclear factor HNF1 and HNF4 families: Functional and pathological consequences. J. Mol. Endocrinol. 2001, 27, 11–29. [Google Scholar] [CrossRef] [Green Version]
- Lau, H.H.; Ng, N.H.J.; Loo, L.S.W.; Jasmen, J.B.; Teo, A.K.K. The molecular functions of hepatocyte nuclear factors—In and beyond the liver. J. Hepatol. 2018, 68, 1033–1048. [Google Scholar] [CrossRef] [Green Version]
- Ben-David, U.; Siranosian, B.; Ha, G.; Tang, H.; Oren, Y.; Hinohara, K.; Strathdee, C.A.; Dempster, J.; Lyons, N.J.; Burns, R.; et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature 2018, 560, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tajima, S.; Izawa-Ishizawa, Y.; Kihira, Y.; Ishizawa, K.; Tomita, S.; Tsuchiya, K.; Tamaki, T. Estrogen regulates hepcidin expression via GPR30-BMP6-dependent signaling in hepatocytes. PLoS ONE 2012, 7, e40465. [Google Scholar] [CrossRef] [PubMed]
- Han, E.H.; Gorman, A.A.; Singh, P.; Chi, Y.I. Repression of HNF1α-mediated transcription by amino-terminal enhancer of split (AES). Biochem. Biophys. Res. Commun. 2015, 468, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Odom, D.T.; Zizlsperger, N.; Gordon, D.B.; Bell, G.W.; Rinaldi, N.J.; Murray, H.L.; Volkert, T.L.; Schreiber, J.; Rolfe, P.A.; Gifford, D.; et al. Control of pancreas and liver gene expression by HNF transcription factors. Science 2004, 303, 1378–1381. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Bai, X.; Li, X.; Zhu, C.; Wu, Z.P. Overexpression of sigma-1 receptor in MCF-7 cells enhances proliferation via the classic protein kinase C subtype signaling pathway. Oncol. Lett. 2018, 16, 6763–6769. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef]
- Mori, T.; Hayashi, T.; Hayashi, E.; Su, T.P. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS ONE 2013, 8, e76941. [Google Scholar] [CrossRef] [Green Version]
- Madden, E.; Logue, S.E.; Healy, S.J.; Manie, S.; Samali, A. The role of the unfolded protein response in cancer progression: From oncogenesis to chemoresistance. Biol. Cell 2019, 111, 1–17. [Google Scholar] [CrossRef]
- Vance, J.E. MAM (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2014, 1841, 595–609. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujimoto, M. Detergent-resistant microdomains determine the localization of σ-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol. Pharmacol. 2010, 77, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Weng, T.Y.; Tsai, S.Y.A.; Su, T.P. Roles of sigma-1 receptors on mitochondrial functions relevant to neurodegenerative diseases. J. Biomed. Sci. 2017, 24, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marriott, K.S.C.; Prasad, M.; Thapliyal, V.; Bose, H.S. σ-1 receptor at the mitochondrial-associated endoplasmic reticulum membrane is responsible for mitochondrial metabolic regulation. J. Pharmacol. Exp. Ther. 2012, 343, 578–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baiceanu, A.; Mesdom, P.; Lagouge, M.; Foufelle, F. Endoplasmic reticulum proteostasis in hepatic steatosis. Nat. Rev. Endocrinol. 2016, 12, 710. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.N.; Ye, J. Proteolytic activation of sterol regulatory element-binding protein induced by cellular stress through depletion of Insig-1. J. Biol. Chem. 2004, 279, 45257–45265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Hayashi, E.; Fujimoto, M.; Sprong, H.; Su, T.P. The lifetime of UDP-galactose: Ceramide galactosyltransferase is controlled by a distinct endoplasmic reticulum-associated degradation (ERAD) regulated by sigma-1 receptor chaperones. J. Biol. Chem. 2012, 287, 43156–43169. [Google Scholar] [CrossRef] [Green Version]
- Pillon, A.; Servant, N.; Vignon, F.; Balaguer, P.; Nicolas, J.C. In vivo bioluminescence imaging to evaluate estrogenic activities of endocrine disrupters. Anal. Biochem. 2005, 340, 295–302. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villemain, L.; Prigent, S.; Abou-Lovergne, A.; Pelletier, L.; Chiral, M.; Pontoglio, M.; Foufelle, F.; Caruso, S.; Pineau, R.; Rebouissou, S.; et al. Sigma 1 Receptor is Overexpressed in Hepatocellular Adenoma: Involvement of ERα and HNF1α. Cancers 2020, 12, 2213. https://doi.org/10.3390/cancers12082213
Villemain L, Prigent S, Abou-Lovergne A, Pelletier L, Chiral M, Pontoglio M, Foufelle F, Caruso S, Pineau R, Rebouissou S, et al. Sigma 1 Receptor is Overexpressed in Hepatocellular Adenoma: Involvement of ERα and HNF1α. Cancers. 2020; 12(8):2213. https://doi.org/10.3390/cancers12082213
Chicago/Turabian StyleVillemain, Laure, Sylvie Prigent, Aurélie Abou-Lovergne, Laura Pelletier, Magali Chiral, Marco Pontoglio, Fabienne Foufelle, Stefano Caruso, Raphael Pineau, Sandra Rebouissou, and et al. 2020. "Sigma 1 Receptor is Overexpressed in Hepatocellular Adenoma: Involvement of ERα and HNF1α" Cancers 12, no. 8: 2213. https://doi.org/10.3390/cancers12082213
APA StyleVillemain, L., Prigent, S., Abou-Lovergne, A., Pelletier, L., Chiral, M., Pontoglio, M., Foufelle, F., Caruso, S., Pineau, R., Rebouissou, S., Chevet, E., Zucman-Rossi, J., & Combettes, L. (2020). Sigma 1 Receptor is Overexpressed in Hepatocellular Adenoma: Involvement of ERα and HNF1α. Cancers, 12(8), 2213. https://doi.org/10.3390/cancers12082213






