The Possible Role of Cancer Stem Cells in the Resistance to Kinase Inhibitors of Advanced Thyroid Cancer
Abstract
:1. Introduction
2. Thyroid Tumor Features
2.1. Thyroid Cancer Histotypes
2.2. Genetics of Thyroid Cancer
2.2.1. Differentiated Thyroid Cancer (DTC)
2.2.2. Poorly Differentiated Thyroid Cancer (PDTC) and Anaplastic Thyroid Cancer (ATC)
2.2.3. Medullary Thyroid cancer (MTC)
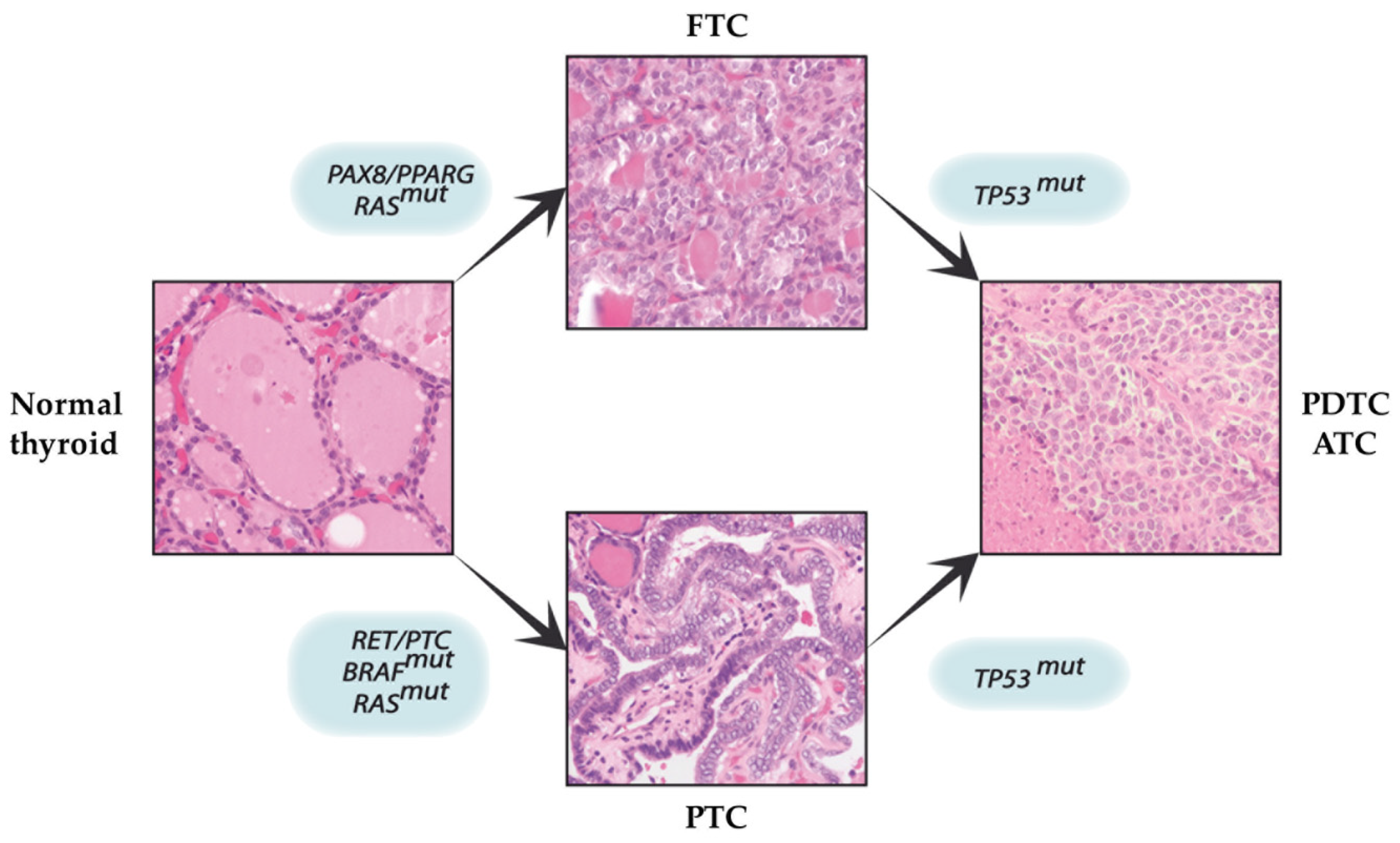
3. The Multi-Step Carcinogenesis Model
4. A New Paradigm in TC Origin and Progression: From the Multi-Step Carcinogenesis Model to the Cancer Stem Cell (CSC) Hypothesis
5. Normal and CSCs: Characteristics and Classification
6. Role of CSCs in the Development of Resistance to Thyroid Cancer Treatments
6.1. CSCs: “The Survivors” to Conventional Anti-Cancer Therapy
6.2. Over-Expression of ATP-Binding Cassette
6.3. Dysregulation of Growth Signaling Pathways of CSCs
6.4. Activation of the Bypass Pathways
6.5. Phenotypic Alterations
6.6. Non-Coding RNAs and Exosome-Based Mechanisms
7. CSC-Based Therapy
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review, 1975–2013; based on November 2015 SEER data submission, posted to the SEER web site, April 2016; Bethesda, M.D., Ed.; National Cancer Institute: Bethesda, MD, USA, 2015. Available online: http://seer.cancer.gov/csr/1975_2013/ (accessed on 20 June 2020).
- Vaccarella, S.; Franceschi, S.; Bray, F.; Wild, C.P.; Plummer, M.; Dal Maso, L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N. Engl. J. Med. 2016, 375, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide Increasing Incidence of Thyroid Cancer: Update on Epidemiology and Risk Factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef] [Green Version]
- Nixon, I.J.; Suarez, C.; Simo, R.; Sanabria, A.; Angelos, P.; Rinaldo, A.; Rodrigo, J.P.; Kowalski, L.P.; Hartl, D.M.; Hinni, M.L.; et al. The Impact of Family History on Non-Medullary Thyroid Cancer. Eur. J. Surg. Oncol. 2016, 42, 1455–1463. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-Term Outcome of 444 Patients with Distant Metastases from Papillary and Follicular Thyroid Carcinoma: Benefits and Limits of Radioiodine Therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef]
- Saini, S.; Tulla, K.; Maker, A.V.; Burman, K.D.; Prabhakar, B.S. Therapeutic Advances in Anaplastic Thyroid Cancer: A Current Perspective. Mol. Cancer 2018, 17, 154. [Google Scholar] [CrossRef] [Green Version]
- Albero, A.; Lopez, J.E.; Torres, A.; de la Cruz, L.; Martin, T. Effectiveness of Chemotherapy in Advanced Differentiated Thyroid Cancer: A Systematic Review. Endocr. Relat. Cancer 2016, 23, R71–R84. [Google Scholar] [CrossRef] [Green Version]
- Frampton, J.E. Lenvatinib: A Review in Refractory Thyroid Cancer. Target. Oncol. 2016, 11, 115–122. [Google Scholar] [CrossRef]
- Manfredi, G.I.; Dicitore, A.; Gaudenzi, G.; Caraglia, M.; Persani, L.; Vitale, G. PI3K/Akt/mTOR Signaling in Medullary Thyroid Cancer: A Promising Molecular Target for Cancer Therapy. Endocrine 2015, 48, 363–370. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. Targeting Vascular Endothelial Growth Factor (VEGF) Pathway in Iodine-Refractory Differentiated Thyroid Carcinoma (DTC): From Bench to Bedside. Crit. Rev. Oncol. Hematol. 2015, 94, 45–54. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in Radioactive Iodine-Refractory, Locally Advanced or Metastatic Differentiated Thyroid Cancer: A Randomised, Double-Blind, Phase 3 Trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib Versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asakawa, H.; Kobayashi, T.; Komoike, Y.; Maruyama, H.; Nakano, Y.; Tamaki, Y.; Matsuzawa, Y.; Monden, M. Chemosensitivity of Anaplastic Thyroid Carcinoma and Poorly Differentiated Thyroid Carcinoma. Anticancer Res. 1997, 17, 2757–2762. [Google Scholar]
- Sugawara, I.; Masunaga, A.; Itoyama, S.; Sumizawa, T.; Akiyama, S.; Yamashita, T. Expression of Multidrug Resistance-Associated Protein (MRP) in Thyroid Cancers. Cancer Lett. 1995, 95, 135–138. [Google Scholar] [CrossRef]
- Wang, S.H.; Phelps, E.; Utsugi, S.; Baker, J.R., Jr. Susceptibility of Thyroid Cancer Cells to 7-Hydroxystaurosporine-Induced Apoptosis Correlates with Bcl-2 Protein Level. Thyroid 2001, 11, 725–731. [Google Scholar] [CrossRef]
- Saylor, P.J.; Escudier, B.; Michaelson, M.D. Importance of Fibroblast Growth Factor Receptor in Neovascularization and Tumor Escape from Antiangiogenic Therapy. Clin. Genitourin. Cancer 2012, 10, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.; Fodde, R. Cancer Stemness and Metastasis: Therapeutic Consequences and Perspectives. Eur. J. Cancer 2010, 46, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cui, D.; Xu, S.; Brabant, G.; Derwahl, M. Doxorubicin Fails to Eradicate Cancer Stem Cells Derived from Anaplastic Thyroid Carcinoma Cells: Characterization of Resistant Cells. Int. J. Oncol. 2010, 37, 307–315. [Google Scholar]
- Smallridge, R.C. Approach to the Patient with Anaplastic Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2012, 97, 2566–2572. [Google Scholar] [CrossRef] [Green Version]
- Marotta, V.; Sciammarella, C.; Vitale, M.; Colao, A.; Faggiano, A. The evolving field of kinase inhibitors in thyroid cancer. Crit. Rev. Oncol. Hematol. 2015, 93, 60–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, T.J. Follicular Cell Thyroid Neoplasia: Insights from Genomics and The Cancer Genome Atlas Research Network. Curr. Opin. Oncol. 2016, 28, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Melillo, R.M. Genetics: The Genomic Landscape of Papillary Thyroid Carcinoma. Nat. Rev. Endocrinol. 2015, 11, 133–134. [Google Scholar] [CrossRef] [Green Version]
- Raman, P.; Koenig, R.J. Pax-8-PPAR-Gamma Fusion Protein in Thyroid Carcinoma. Nat. Rev. Endocrinol. 2014, 10, 616–623. [Google Scholar] [CrossRef]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT Promoter Mutations are a Major Indicator of Poor Outcome in Differentiated Thyroid Carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef] [Green Version]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and Transcriptomic Hallmarks of Poorly Differentiated and Anaplastic Thyroid Cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [Green Version]
- Ciampi, R.; Mian, C.; Fugazzola, L.; Cosci, B.; Romei, C.; Barollo, S.; Cirello, V.; Bottici, V.; Marconcini, G.; Rosa, P.M.; et al. Evidence of a Low Prevalence of RAS Mutations in a Large Medullary Thyroid Cancer Series. Thyroid 2013, 23, 50–57. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Kondo, T.; Ezzat, S.; Asa, S.L. Pathogenetic Mechanisms in Thyroid Follicular-Cell Neoplasia. Nat. Rev. Cancer 2006, 6, 292–306. [Google Scholar] [CrossRef]
- Xing, M. Molecular Pathogenesis and Mechanisms of Thyroid Cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Kim, D.S.; McCabe, C.J.; Buchanan, M.A.; Watkinson, J.C. Oncogenes in Thyroid Cancer. Clin. Otolaryngol. Allied Sci. 2003, 28, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Fagin, J.A.; Mitsiades, N. Molecular Pathology of Thyroid Cancer: Diagnostic and Clinical Implications. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Nikiforova, M.N.; Nikiforov, Y.E. Molecular Genetics of Thyroid Cancer: Implications for Diagnosis, Treatment and Prognosis. Expert Rev. Mol. Diagn. 2008, 8, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Suarez, H.G. Molecular Basis of Epithelial Thyroid Tumorigenesis. C. R. Acad. Sci. 2000, 323, 519–528. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human Acute Myeloid Leukemia is Organized as a Hierarchy that Originates from a Primitive Hematopoietic Cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Pardal, R.; Clarke, M.F.; Morrison, S.J. Applying the Principles of Stem-Cell Biology to Cancer. Nat. Rev. Cancer 2003, 3, 895–902. [Google Scholar] [CrossRef]
- Polyak, K.; Hahn, W.C. Roots and Stems: Stem Cells in Cancer. Nat. Med. 2006, 12, 296–300. [Google Scholar] [CrossRef]
- Zhang, P.; Zuo, H.; Ozaki, T.; Nakagomi, N.; Kakudo, K. Cancer Stem Cell Hypothesis in Thyroid Cancer. Pathol. Int. 2006, 56, 485–489. [Google Scholar] [CrossRef]
- Takano, T.; Amino, N. Fetal Cell Carcinogenesis: A New Hypothesis for Better Understanding of Thyroid Carcinoma. Thyroid 2005, 15, 432–438. [Google Scholar] [CrossRef]
- Zito, G.; Richiusa, P.; Bommarito, A.; Carissimi, E.; Russo, L.; Coppola, A.; Zerilli, M.; Rodolico, V.; Criscimanna, A.; Amato, M.; et al. In Vitro Identification and Characterization of CD133(pos) Cancer Stem-Like Cells in Anaplastic Thyroid Carcinoma Cell Lines. PLoS ONE 2008, 3, e3544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klonisch, T.; Hoang-Vu, C.; Hombach-Klonisch, S. Thyroid Stem Cells and Cancer. Thyroid 2009, 19, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Fierabracci, A.; Puglisi, M.A.; Giuliani, L.; Mattarocci, S.; Gallinella-Muzi, M. Identification of an Adult Stem/Progenitor Cell-Like Population in the Human Thyroid. J. Endocrinol. 2008, 198, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Dumont, J.E.; Lamy, F.; Roger, P.; Maenhaut, C. Physiological and Pathological Regulation of Thyroid Cell Proliferation and Differentiation by Thyrotropin and Other Factors. Physiol. Rev. 1992, 72, 667–697. [Google Scholar] [CrossRef]
- Thomas, T.; Nowka, K.; Lan, L.; Derwahl, M. Expression of Endoderm Stem Cell Markers: Evidence for the Presence of Adult Stem Cells in Human Thyroid Glands. Thyroid 2006, 16, 537–544. [Google Scholar] [CrossRef]
- Harach, H.R.; Vujanic, G.M.; Jasani, B. Ultimobranchial Body Nests in Human Fetal Thyroid: An Autopsy, Histological, and Immunohistochemical Study in Relation to Solid Cell Nests and Mucoepidermoid Carcinoma of the Thyroid. J. Pathol. 1993, 169, 465–469. [Google Scholar] [CrossRef]
- Cameselle-Teijeiro, J.; Febles-Perez, C.; Sobrinho-Simoes, M. Papillary and mucoepidermoid carcinoma of the thyroid with anaplastic transformation: A case report with histologic and immunohistochemical findings that support a provocative histogenetic hypothesis. Pathol. Res. Pract. 1995, 191, 1214–1221. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Preto, A.; Soares, P.; Ricardo, S.; Cameselle-Teijeiro, J.; Sobrinho-Simoes, M. p63 Expression in Solid Cell Nests of the Thyroid: Further Evidence for a Stem Cell Origin. Mod. Pathol. 2003, 16, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Campagnoli, C.; Roberts, I.; Kumar, S.; Bennett, P.R.; Fisk, N.M. Clonal Culture of Fetal Cells from Maternal Blood. Lancet 2001, 357, 962. [Google Scholar] [CrossRef]
- Sanders, J.E.; Hoffmeister, P.A.; Woolfrey, A.E.; Carpenter, P.A.; Storer, B.E.; Storb, R.F.; Appelbaum, F.R. Thyroid Function Following Hematopoietic Cell Transplantation in Children: 30 Years’ Experience. Blood 2009, 113, 306–308. [Google Scholar] [CrossRef] [Green Version]
- Hoshi, N.; Kusakabe, T.; Taylor, B.J.; Kimura, S. Side Population Cells in the Mouse Thyroid Exhibit Stem/Progenitor Cell-Like Characteristics. Endocrinology 2007, 148, 4251–4258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, L.; Cui, D.; Nowka, K.; Derwahl, M. Stem Cells Derived from Goiters in Adults Form Spheres in Response to Intense Growth Stimulation and Require Thyrotropin for Differentiation into Thyrocytes. J. Clin. Endocrinol. Metab. 2007, 92, 3681–3688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaguarnera, R.; Frasca, F.; Garozzo, A.; Giani, F.; Pandini, G.; Vella, V.; Vigneri, R.; Belfiore, A. Insulin Receptor Isoforms and Insulin-Like Growth Factor Receptor in Human Follicular Cell Precursors from Papillary Thyroid Cancer and Normal Thyroid. J. Clin. Endocrinol. Metab. 2011, 96, 766–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, S. Thyroid Regeneration: How Stem Cells Play a Role? Front. Endocrinol. 2014, 5, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, T.; Amino, N. Cancer-Specific mRNAs in Thyroid Carcinomas: Detection, Use, and Their Implication in Thyroid Carcinogenesis. Endocr. J. 2002, 49, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Takano, T. Fetal Cell Carcinogenesis of the Thyroid: A Modified Theory Based on Recent Evidence. Endocr. J. 2014, 61, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Jhiang, S.M.; Sagartz, J.E.; Tong, Q.; Parker-Thornburg, J.; Capen, C.C.; Cho, J.Y.; Xing, S.; Ledent, C. Targeted Expression of the ret/PTC1 Oncogene Induces Papillary Thyroid Carcinomas. Endocrinology 1996, 137, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Charles, R.P.; Iezza, G.; Amendola, E.; Dankort, D.; McMahon, M. Mutationally Activated BRAF(V600E) Elicits Papillary Thyroid Cancer in the Adult Mouse. Cancer Res. 2011, 71, 3863–3871. [Google Scholar] [CrossRef] [Green Version]
- Shimamura, M.; Nakahara, M.; Orim, F.; Kurashige, T.; Mitsutake, N.; Nakashima, M.; Kondo, S.; Yamada, M.; Taguchi, R.; Kimura, S.; et al. Postnatal Expression of BRAFV600E Does Not Induce Thyroid Cancer in Mouse Models of Thyroid Papillary Carcinoma. Endocrinology 2013, 154, 4423–4430. [Google Scholar] [CrossRef] [Green Version]
- Todaro, M.; Iovino, F.; Eterno, V.; Cammareri, P.; Gambara, G.; Espina, V.; Gulotta, G.; Dieli, F.; Giordano, S.; De Maria, R.; et al. Tumorigenic and Metastatic Activity of Human Thyroid Cancer Stem Cells. Cancer Res. 2010, 70, 8874–8885. [Google Scholar] [CrossRef] [Green Version]
- Gibelli, B.; El-Fattah, A.; Giugliano, G.; Proh, M.; Grosso, E. Thyroid Stem Cells—Danger or Resource? Acta Otorhinolaryngol. Ital. 2009, 29, 290–295. [Google Scholar] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem Cells, Cancer, and Cancer Stem Cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicha, M.S.; Liu, S.; Dontu, G. Cancer Stem Cells: An Old Idea—A Paradigm Shift. Cancer Res. 2006, 66, 1883–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sell, S. Stem Cell Origin of Cancer and Differentiation Therapy. Crit. Rev. Oncol. Hematol. 2004, 51, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.M.; Jordan, C.T. The Increasing Complexity of the Cancer Stem Cell Paradigm. Science 2009, 324, 1670–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer Stem Cells. N. Engl. J. Med. 2006, 355, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Yang, X.; Lassus, H.; Liang, S.; Kaur, S.; Ye, Q.; Li, C.; Wang, L.P.; Roby, K.F.; Orsulic, S.; et al. Distinct Expression Levels and Patterns of Stem Cell Marker, Aldehyde Dehydrogenase Isoform 1 (ALDH1), in Human Epithelial Cancers. PLoS ONE 2010, 5, e10277. [Google Scholar] [CrossRef]
- Kimlin, L.; Virador, V. Cellular Populations Isolated from Newborn Mouse Skin Including Mesenchymal Stem Cells. Methods Mol. Biol. 2013, 989, 217–233. [Google Scholar] [CrossRef]
- Weiswald, L.B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hardin, H.; Montemayor-Garcia, C.; Lloyd, R.V. Thyroid Cancer Stem-Like Cells and Epithelial-Mesenchymal Transition in Thyroid Cancers. Hum. Pathol. 2013, 44, 1707–1713. [Google Scholar] [CrossRef]
- Lan, L.; Luo, Y.; Cui, D.; Shi, B.Y.; Deng, W.; Huo, L.L.; Chen, H.L.; Zhang, G.Y.; Deng, L.L. Epithelial- Mesenchymal Transition Triggers Cancer Stem Cell Generation in Human Thyroid Cancer Cells. Int. J. Oncol. 2013, 43, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varjosalo, M.; Taipale, J. Hedgehog: Functions and Mechanisms. Genes Dev. 2008, 22, 2454–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Matsui, W.; Khaki, L.; Stearns, D.; Chun, J.; Li, Y.M.; Eberhart, C.G. Notch Pathway Inhibition Depletes Stem-Like Cells and Blocks Engraftment in Embryonal Brain Tumors. Cancer Res. 2006, 66, 7445–7452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.; Zhao, X.; Chen, S.; Liu, S.; Wicha, M.S.; Guan, J.L. Distinct FAK Activities Determine Progenitor and Mammary Stem Cell Characteristics. Cancer Res. 2013, 73, 5591–5602. [Google Scholar] [CrossRef] [Green Version]
- Van Amerongen, R.; Bowman, A.N.; Nusse, R. Developmental Stage and Time Dictate the Fate of Wnt/Beta-Catenin-Responsive Stem Cells in the Mammary Gland. Cell Stem Cell 2012, 11, 387–400. [Google Scholar] [CrossRef] [Green Version]
- Kamran, M.Z.; Patil, P.; Gude, R.P. Role of STAT3 in Cancer Metastasis and Translational Advances. Biomed. Res. Int. 2013, 2013, 421821. [Google Scholar] [CrossRef]
- Wang, M.L.; Chiou, S.H.; Wu, C.W. Targeting Cancer Stem Cells: Emerging Role of Nanog Transcription Factor. Onco. Targets Ther. 2013, 6, 1207–1220. [Google Scholar] [CrossRef] [Green Version]
- Giani, F.; Vella, V.; Nicolosi, M.L.; Fierabracci, A.; Lotta, S.; Malaguarnera, R.; Belfiore, A.; Vigneri, R.; Frasca, F. Thyrospheres From Normal or Malignant Thyroid Tissue Have Different Biological, Functional, and Genetic Features. J. Clin. Endocrinol. Metab. 2015, 100, E1168–E1178. [Google Scholar] [CrossRef] [Green Version]
- Han, S.A.; Jang, J.H.; Won, K.Y.; Lim, S.J.; Song, J.Y. Prognostic Value of Putative Cancer Stem Cell Markers (CD24, CD44, CD133, and ALDH1) in Human Papillary Thyroid Carcinoma. Pathol. Res. Pract. 2017, 213, 956–963. [Google Scholar] [CrossRef]
- Liotti, F.; Collina, F.; Pone, E.; La Sala, L.; Franco, R.; Prevete, N.; Melillo, R.M. Interleukin-8, but not the Related Chemokine CXCL1, Sustains an Autocrine Circuit Necessary for the Properties and Functions of Thyroid Cancer Stem Cells. Stem Cells 2017, 35, 135–146. [Google Scholar] [CrossRef]
- Tseng, L.M.; Huang, P.I.; Chen, Y.R.; Chen, Y.C.; Chou, Y.C.; Chen, Y.W.; Chang, Y.L.; Hsu, H.S.; Lan, Y.T.; Chen, K.H.; et al. Targeting Signal Transducer and Activator of Transcription 3 Pathway by Cucurbitacin I Diminishes Self-Renewing and Radiochemoresistant Abilities in Thyroid Cancer-Derived CD133+ Cells. J. Pharmacol. Exp. Ther. 2012, 341, 410–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belfiore, A.; Malaguarnera, R.; Vella, V.; Lawrence, M.C.; Sciacca, L.; Frasca, F.; Morrione, A.; Vigneri, R. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr. Rev. 2017, 38, 379–431. [Google Scholar] [CrossRef] [PubMed]
- Vella, V.; Malaguarnera, R. The Emerging Role of Insulin Receptor Isoforms in Thyroid Cancer: Clinical Implications and New Perspectives. Int. J. Mol. Sci. 2018, 19, 3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vella, V.; Milluzzo, A.; Scalisi, N.M.; Vigneri, P.; Sciacca, L. Insulin Receptor Isoforms in Cancer. Int. J. Mol. Sci. 2018, 19, 3615. [Google Scholar] [CrossRef] [Green Version]
- Ciavardelli, D.; Bellomo, M.; Consalvo, A.; Crescimanno, C.; Vella, V. Metabolic Alterations of Thyroid Cancer as Potential Therapeutic Targets. Biomed. Res. Int. 2017, 2017, 2545031. [Google Scholar] [CrossRef] [Green Version]
- Vella, V.; Nicolosi, M.L.; Giuliano, M.; Morrione, A.; Malaguarnera, R.; Belfiore, A. Insulin Receptor Isoform A Modulates Metabolic Reprogramming of Breast Cancer Cells in Response to IGF2 and Insulin Stimulation. Cells 2019, 8, 1017. [Google Scholar] [CrossRef] [Green Version]
- Vella, V.; Malaguarnera, R.; Nicolosi, M.L.; Morrione, A.; Belfiore, A. Insulin/IGF Signaling and Discoidin Domain Receptors: An Emerging Functional Connection. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118522. [Google Scholar] [CrossRef]
- Vella, V.; Malaguarnera, R.; Nicolosi, M.L.; Palladino, C.; Spoleti, C.; Massimino, M.; Vigneri, P.; Purrello, M.; Ragusa, M.; Morrione, A.; et al. Discoidin Domain Receptor 1 Modulates Insulin Receptor Signaling and Biological Responses in Breast Cancer Cells. Oncotarget 2017, 8, 43248–43270. [Google Scholar] [CrossRef] [Green Version]
- Vella, V.; Nicolosi, M.L.; Cantafio, P.; Massimino, M.; Lappano, R.; Vigneri, P.; Ciuni, R.; Gangemi, P.; Morrione, A.; Malaguarnera, R.; et al. DDR1 Regulates Thyroid Cancer Cell Differentiation via IGF-2/IR-A Autocrine Signaling Loop. Endocr. Relat. Cancer 2019, 26, 197–214. [Google Scholar] [CrossRef]
- Belfiore, A.; Malaguarnera, R.; Nicolosi, M.L.; Lappano, R.; Ragusa, M.; Morrione, A.; Vella, V. A Novel Functional Crosstalk Between DDR1 and the IGF Axis and its Relevance for Breast Cancer. Cell Adh. Migr. 2018, 12, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Piscazzi, A.; Costantino, E.; Maddalena, F.; Natalicchio, M.I.; Gerardi, A.M.; Antonetti, R.; Cignarelli, M.; Landriscina, M. Activation of the RAS/RAF/ERK Signaling Pathway Contributes to Resistance to Sunitinib in Thyroid Carcinoma Cell Lines. J. Clin. Endocrinol. Metab. 2012, 97, E898–E906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frasca, F.; Vella, V.; Nicolosi, M.L.; Messina, R.L.; Giani, F.; Lotta, S.; Vigneri, P.; Regalbuto, C.; Vigneri, R. Thyroid Cancer Cell Resistance to Gefitinib Depends on the Constitutive Oncogenic Activation of the ERK Pathway. J. Clin. Endocrinol. Metab. 2013, 98, 2502–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero-Conde, C.; Ruiz-Llorente, S.; Dominguez, J.M.; Knauf, J.A.; Viale, A.; Sherman, E.J.; Ryder, M.; Ghossein, R.A.; Rosen, N.; Fagin, J.A. Relief of Feedback Inhibition of HER3 Transcription by RAF and MEK Inhibitors Attenuates Their Antitumor Effects in BRAF-Mutant Thyroid Carcinomas. Cancer Discov. 2013, 3, 520–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byeon, H.K.; Na, H.J.; Yang, Y.J.; Kwon, H.J.; Chang, J.W.; Ban, M.J.; Kim, W.S.; Shin, D.Y.; Lee, E.J.; Koh, Y.W.; et al. c-Met-Mediated Reactivation of PI3K/AKT Signaling Contributes to Insensitivity of BRAF(V600E) Mutant Thyroid Cancer to BRAF Inhibition. Mol. Carcinog. 2016, 55, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Danysh, B.P.; Rieger, E.Y.; Sinha, D.K.; Evers, C.V.; Cote, G.J.; Cabanillas, M.E.; Hofmann, M.C. Long-Term Vemurafenib Treatment Drives Inhibitor Resistance Through a Spontaneous KRAS G12D Mutation in a BRAF V600E Papillary Thyroid Carcinoma Model. Oncotarget 2016, 7, 30907–30923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isham, C.R.; Netzel, B.C.; Bossou, A.R.; Milosevic, D.; Cradic, K.W.; Grebe, S.K.; Bible, K.C. Development and Characterization of a Differentiated Thyroid Cancer Cell Line Resistant to VEGFR-Targeted Kinase Inhibitors. J. Clin. Endocrinol. Metab. 2014, 99, E936–E943. [Google Scholar] [CrossRef] [Green Version]
- Wagle, N.; Grabiner, B.C.; Van Allen, E.M.; Amin-Mansour, A.; Taylor-Weiner, A.; Rosenberg, M.; Gray, N.; Barletta, J.A.; Guo, Y.; Swanson, S.J.; et al. Response and Acquired Resistance to Everolimus in Anaplastic Thyroid Cancer. N. Engl. J. Med. 2014, 371, 1426–1433. [Google Scholar] [CrossRef] [Green Version]
- Prete, A.; Lo, A.S.; Sadow, P.M.; Bhasin, S.S.; Antonello, Z.A.; Vodopivec, D.M.; Ullas, S.; Sims, J.N.; Clohessy, J.; Dvorak, A.M.; et al. Pericytes Elicit Resistance to Vemurafenib and Sorafenib Therapy in Thyroid Carcinoma via the TSP-1/TGFbeta1 Axis. Clin. Cancer Res. 2018, 24, 6078–6097. [Google Scholar] [CrossRef] [Green Version]
- Giuffrida, R.; Adamo, L.; Iannolo, G.; Vicari, L.; Giuffrida, D.; Eramo, A.; Gulisano, M.; Memeo, L.; Conticello, C. Resistance of Papillary Thyroid Cancer Stem Cells to Chemotherapy. Oncol. Lett. 2016, 12, 687–691. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jiang, X.; Chen, P.; Wu, X.; Duan, A.; Qin, Y. Combined Effects of Octreotide and Cisplatin on the Proliferation of Side Population Cells from Anaplastic Thyroid Cancer Cell Lines. Oncol. Lett. 2018, 16, 4033–4042. [Google Scholar] [CrossRef] [Green Version]
- Carina, V.; Zito, G.; Pizzolanti, G.; Richiusa, P.; Criscimanna, A.; Rodolico, V.; Tomasello, L.; Pitrone, M.; Arancio, W.; Giordano, C. Multiple Pluripotent Stem Cell Markers in Human Anaplastic Thyroid Cancer: The Putative Upstream Role of SOX2. Thyroid 2013, 23, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borah, A.; Raveendran, S.; Rochani, A.; Maekawa, T.; Kumar, D.S. Targeting Self-Renewal Pathways in Cancer Stem Cells: Clinical Implications for Cancer Therapy. Oncogenesis 2015, 4, e177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiden, K.B.; Williamson, A.J.; Doscas, M.E.; Ye, J.; Wang, Y.; Liu, D.; Xing, M.; Prinz, R.A.; Xu, X. The Sonic Hedgehog Signaling Pathway Maintains the Cancer Stem Cell Self-Renewal of Anaplastic Thyroid Cancer by Inducing Snail Expression. J. Clin. Endocrinol. Metab. 2014, 99, E2178–E2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.Q.; Ahmed, E.I.; Elareer, N.; Fathima, H.; Prabhu, K.S.; Siveen, K.S.; Kulinski, M.; Azizi, F.; Dermime, S.; Ahmad, A.; et al. Curcumin-Mediated Apoptotic Cell Death in Papillary Thyroid Cancer and Cancer Stem-Like Cells through Targeting of the JAK/STAT3 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Cai, L.; Zhang, F.; Shang, X.; Xiao, R.; Zhou, H. Inhibition of EZH2 Attenuates Sorafenib Resistance by Targeting NOTCH1 Activation-Dependent Liver Cancer Stem Cells via NOTCH1-Related MicroRNAs in Hepatocellular Carcinoma. Transl. Oncol. 2020, 13, 100741. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.Y.; Zhang, X.C.; Yang, S.Q.; An, S.J.; Chen, Z.H.; Su, J.; Xie, Z.; Gou, L.Y.; Wu, Y.L. Blockade of Hedgehog Signaling Synergistically Increases Sensitivity to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-small-Cell Lung Cancer Cell Lines. PLoS ONE 2016, 11, e0149370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giani, F.; Russo, G.; Pennisi, M.; Sciacca, L.; Frasca, F.; Pappalardo, F. Computational Modeling Reveals MAP3K8 as Mediator of Resistance to Vemurafenib in Thyroid Cancer Stem Cells. Bioinformatics 2019, 35, 2267–2275. [Google Scholar] [CrossRef]
- Singh, A.; Settleman, J. EMT, Cancer Stem Cells and Drug Resistance: An Emerging Axis of Evil in the War on Cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Kim, S.M.; Kim, B.W.; Chang, H.J.; Kim, S.Y.; Park, C.S.; Park, K.C.; Chang, H.S. Anti-Cancer Effects of HNHA and Lenvatinib by the Suppression of EMT-Mediated Drug Resistance in Cancer Stem Cells. Neoplasia 2018, 20, 197–206. [Google Scholar] [CrossRef]
- Dima, M.; Pecce, V.; Biffoni, M.; Di Gioia, C.R.; Tallini, G.; Biffoni, M.; Rosignolo, F.; Verrienti, A.; Sponziello, M.; Damante, G.; et al. Molecular profiles of cancer stem-like cell populations in aggressive thyroid cancers. Endocrine 2016, 53, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Heery, R.; Finn, S.P.; Cuffe, S.; Gray, S.G. Long Non-Coding RNAs: Key Regulators of Epithelial-Mesenchymal Transition, Tumour Drug Resistance and Cancer Stem Cells. Cancers 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.C.; Chang, H.Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, X.; Han, S.; Wu, G.; Latchoumanin, O.; Zhou, G.; Hebbard, L.; George, J.; Qiao, L. Dysregulated Long Noncoding RNAs (lncRNAs) in Hepatocellular Carcinoma: Implications for Tumorigenesis, Disease Progression, and Liver Cancer Stem Cells. Mol. Cancer 2017, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, K.; Ma, L.; Xu, J.; Chang, W. The Role of Long Non-Coding RNAs in Thyroid Cancer. Front. Oncol. 2020, 10, 941. [Google Scholar] [CrossRef]
- Jiang, W.; Xia, J.; Xie, S.; Zou, R.; Pan, S.; Wang, Z.W.; Assaraf, Y.G.; Zhu, X. Long Non-Coding RNAs as a Determinant of Cancer Drug Resistance: Towards the Overcoming of Chemoresistance via Modulation of lncRNAs. Drug Resist. Updates 2020, 50, 100683. [Google Scholar] [CrossRef]
- Castro-Oropeza, R.; Melendez-Zajgla, J.; Maldonado, V.; Vazquez-Santillan, K. The Emerging Role of lncRNAs in the Regulation of Cancer Stem Cells. Cell. Oncol. 2018, 41, 585–603. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Q.; Li, S.; Liang, N.; Li, F.; Li, C.; Sui, C.; Dionigi, G.; Sun, H. LncRNA FOXD2-AS1 Functions as a Competing Endogenous RNA to Regulate TERT Expression by Sponging miR-7-5p in Thyroid Cancer. Front. Endocrinol. 2019, 10, 207. [Google Scholar] [CrossRef]
- Kabir, T.D.; Ganda, C.; Brown, R.M.; Beveridge, D.J.; Richardson, K.L.; Chaturvedi, V.; Candy, P.; Epis, M.; Wintle, L.; Kalinowski, F.; et al. A MicroRNA-7/Growth Arrest Specific 6/TYRO3 Axis Regulates the Growth and Invasiveness of Sorafenib-Resistant Cells in Human Hepatocellular Carcinoma. Hepatology 2018, 67, 216–231. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lu, X.; Geng, Z.; Yang, G.; Shi, Y. LncRNA PTCSC3/miR-574-5p Governs Cell Proliferation and Migration of Papillary Thyroid Carcinoma via Wnt/β-Catenin Signaling. J. Cell. Biochem. 2017, 118, 4745–4752. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Fan, Y.; Liu, Z.; Yuan, Q.; Jia, M.; Geng, Z.; Gu, L.; Lu, X. LncRNA PTCSC3 Affects Drug Resistance of Anaplastic Thyroid Cancer Through STAT3/INO80 Pathway. Cancer Biol. Ther. 2018, 19, 590–597. [Google Scholar] [CrossRef] [Green Version]
- Sheng, W.; Chen, Y.; Gong, Y.; Dong, T.; Zhang, B.; Gao, W. Mir-148a Inhibits Self-Renewal of Thyroid Cancer Stem Cells via Repressing ino80 Expression. Oncol. Rep. 2016, 36, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Lin, X.D.; Fu, X.H.; Yan, W.; Lin, F.S.; Kuang, P.H.; Luo, Y.; Lin, E.; Hong, X.; Wu, G. Long Non-Coding RNA BANCR Regulates Cancer Stem Cell Markers in Papillary Thyroid Cancer via the RAF/MEK/ERK Signaling Pathway. Oncol. Rep. 2018, 40, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Su, Z.; Zhang, Z.; Gao, T. LncRNA NEAT1 Enhances the Resistance of Anaplastic Thyroid Carcinoma Cells to Cisplatin by Sponging miR-9-5p and Regulating SPAG9 Expression. Int. J. Oncol. 2019, 55, 988–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.; Tang, G.; Wu, X.; Wu, C. LncRNA NEAT1 Modulates Sorafenib Resistance in Hepatocellular Carcinoma Through Regulating the miR-149-5p/AKT1 Axis. Saudi J. Gastroenterol. 2020. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-Mediated Transfer of mRNAs and microRNAs is a Novel Mechanism of Genetic Exchange Between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Hardin, H.; Helein, H.; Meyer, K.; Robertson, S.; Zhang, R.; Zhong, W.; Lloyd, R.V. Thyroid Cancer Stem-Like Cell Exosomes: Regulation of EMT via Transfer of lncRNAs. Lab. Investig. 2018, 98, 1133–1142. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Wang, L.; Dong, L.; Wang, X. Emerging Role of Exosome Signalling in Maintaining Cancer Stem Cell Dynamic Equilibrium. J. Cell Mol. Med. 2018, 22, 3719–3728. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Caires, H.R.; Barbosa, M.A.G.; Bergantim, R.; Guimaraes, J.E.; Vasconcelos, M.H. The Role of Extracellular Vesicles in the Hallmarks of Cancer and Drug Resistance. Cells 2020, 9, 1141. [Google Scholar] [CrossRef]
- Lim, S.M.; Chang, H.; Yoon, M.J.; Hong, Y.K.; Kim, H.; Chung, W.Y.; Park, C.S.; Nam, K.H.; Kang, S.W.; Kim, M.K.; et al. A Multicenter, Phase II Trial of Everolimus in Locally Advanced or Metastatic Thyroid Cancer of All Histologic Subtypes. Ann. Oncol. 2013, 24, 3089–3094. [Google Scholar] [CrossRef]
- Tirrò, E.; Martorana, F.; Romano, C.; Vitale, S.R.; Motta, G.; Di Gregorio, S.; Massimino, M.; Pennisi, M.S.; Stella, S.; Puma, A.; et al. Molecular Alterations in Thyroid Cancer: From Bench to Clinical Practice. Genes 2019, 10, 709. [Google Scholar] [CrossRef] [Green Version]
- Shiraiwa, K.; Matsuse, M.; Nakazawa, Y.; Ogi, T.; Suzuki, K.; Saenko, V.A.; Xu, S.; Umezawa, K.; Yamashita, S.; Tsukamoto, K.; et al. JAK/STAT3 and NF-κB Signaling Pathways Regulate Cancer Stem-Cell Properties in Anaplastic Thyroid Cancer Cells. Thyroid 2019, 29, 674–682. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianì, F.; Vella, V.; Tumino, D.; Malandrino, P.; Frasca, F. The Possible Role of Cancer Stem Cells in the Resistance to Kinase Inhibitors of Advanced Thyroid Cancer. Cancers 2020, 12, 2249. https://doi.org/10.3390/cancers12082249
Gianì F, Vella V, Tumino D, Malandrino P, Frasca F. The Possible Role of Cancer Stem Cells in the Resistance to Kinase Inhibitors of Advanced Thyroid Cancer. Cancers. 2020; 12(8):2249. https://doi.org/10.3390/cancers12082249
Chicago/Turabian StyleGianì, Fiorenza, Veronica Vella, Dario Tumino, Pasqualino Malandrino, and Francesco Frasca. 2020. "The Possible Role of Cancer Stem Cells in the Resistance to Kinase Inhibitors of Advanced Thyroid Cancer" Cancers 12, no. 8: 2249. https://doi.org/10.3390/cancers12082249





