Health-Related Quality of Life and Experiences of Sarcoma Patients during the COVID-19 Pandemic
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Loneliness
2.3. Resilient Coping
2.4. Treatment Characteristics
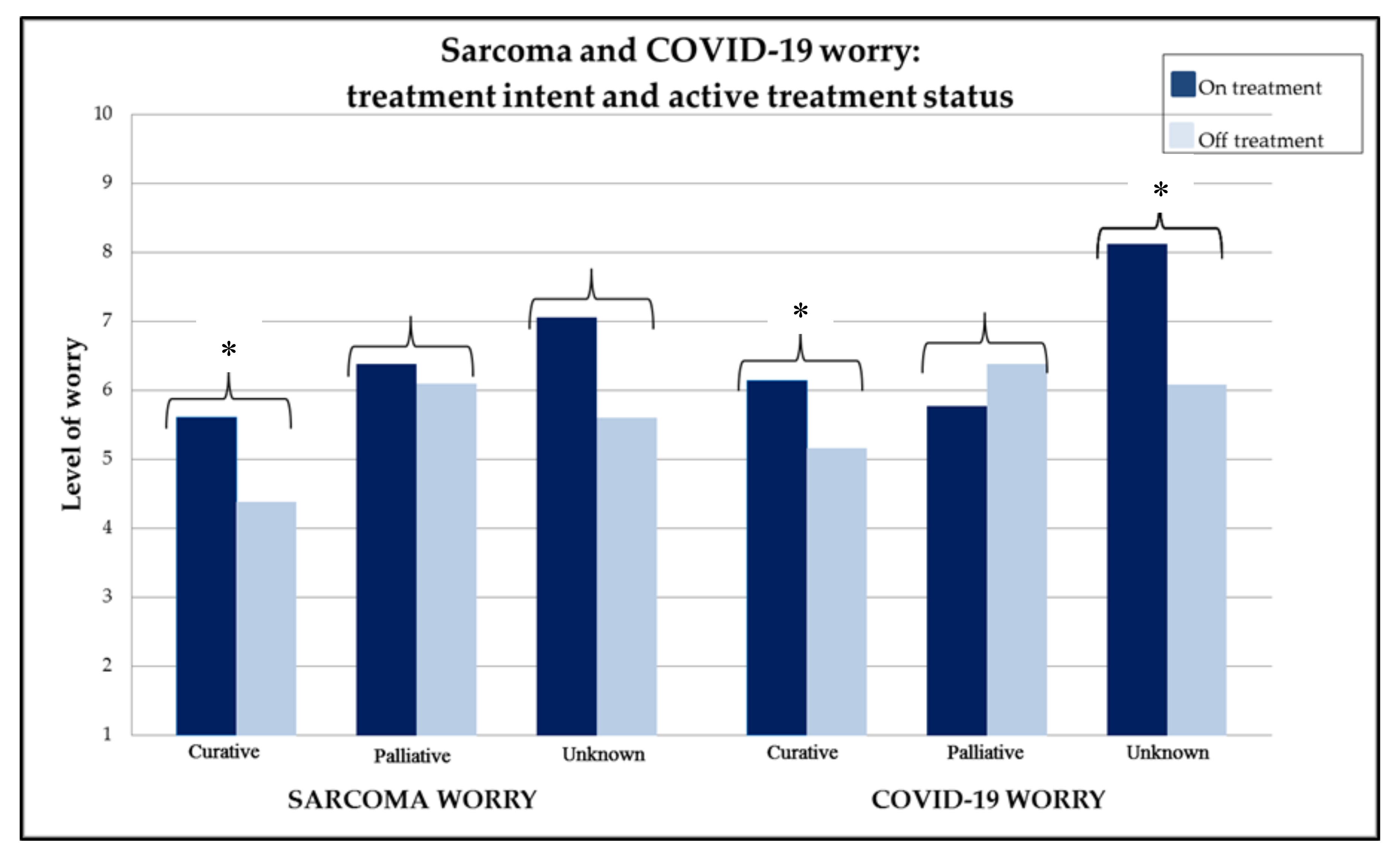
2.5. COVID-19: Worry and Psychosocial Impact
2.6. Experiences of Care during COVID-19 Pandemic
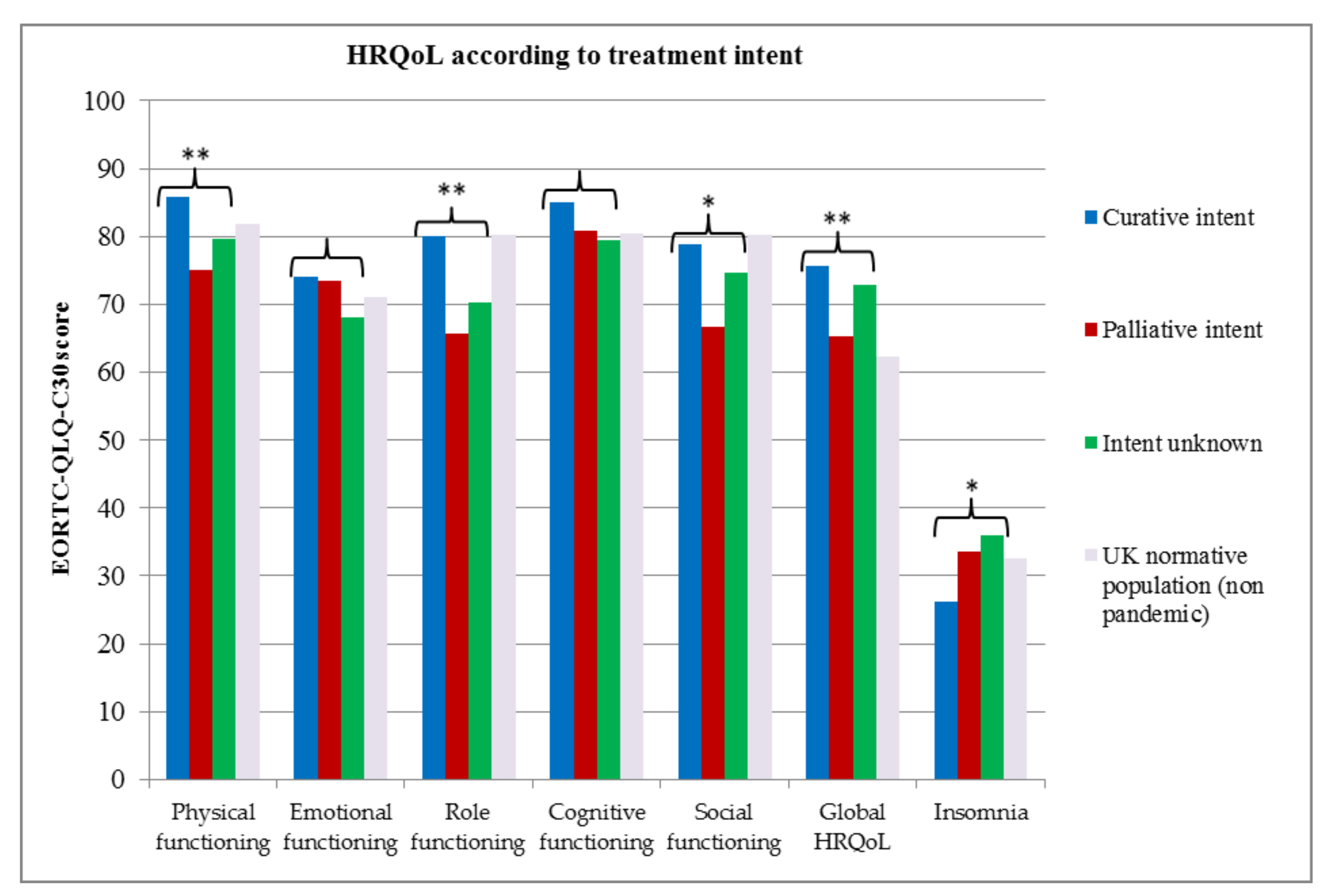
2.7. Health-Related Quality of Life (HRQoL)
2.8. Multivariate Analysis: Cancer Worry and COVID-19 Worry
3. Discussion
3.1. Implications for Care
3.2. Limitations
4. Materials and Methods
4.1. Participants
4.2. Measures
4.3. Statistical Analysis
4.4. Ethical approval
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Patient Recruitment

Appendix B. Questionnaire
- How old are you?……. years
- What is your gender?MaleFemaleOther, please specify__________________________
- What is your ethnicity?European/CaucasianAsianBlackMixedOther, please specify____________________________
- What is your current living situation? (Please check all that apply)I live aloneI live with my spouse/partnerI live with children in the homeI live with friend(s) or roommate(s) who are not related to me (e.g., students or lodgers)I live with my parent(s)Other, please specify____________________________
- What is your marital status?Married/in a relationshipDivorcedWidowedSingle
- What is your highest level of education?None/Primary schoolSecondary schoolCollege/diploma/vocational qualificationUniversity/postgraduate degree
- Do you have any of the following medical conditions? (please tick all that apply)Chronic Obstructive Pulmonary Disease (COPD)Hypertension (high blood pressure)DiabetesCoronary artery disease/heart disease (e.g., previous heart attack)ObesityNone of the above
- How long is your usual journey time from your home to the Royal Marsden Hospital?Less than 1 h journey timeMore than 1 h journey time
- How do you usually travel to the Royal Marsden Hospital? (please tick all that apply)I walkBusTrainUndergroundCarOther, please specify____________________________
- On a scale of 1 to 10, please rate your current level of worry about the impact of COVID-19 on your health. (1—not worried at all, 10—extremely worried).

- 2.
- Do you think you are at higher risk of contracting COVID-19 than others in the population:Yes, higher riskEqual, same riskNo, lower riskDon’t know
- 3.
- Do you think you have had COVID-19?Yes, confirmed with swab (test)Yes, I think I had symptoms of the COVID-19, but have not had a test to confirmYes, I think I had symptoms of COVID-19, but I had a negative testNo, I have not had symptoms of COVID-19I don’t know
- 4.
- Has the COVID-19 pandemic had an impact on any of the following? (please tick all that apply)EmploymentFinancial situationFamily lifeEmotional wellbeingSocial life/activitiesOther, please specify____________________________
- 5.
- In the next 12 months, if you developed a life threatening respiratory disease (due to COVID-19 or other cause), do you think you would accept treatment with a ventilator (a machine to breathe for you)?YesNoI don’t know.
- On a scale of 1 to 10, please rate your current level of worry about your sarcoma?(1—not worried at all, 10—extremely worried)

- 2.
- Are you currently having treatment for sarcoma?Yes, intravenous (through a vein) anticancer treatment given at the hospitalYes, oral (tablets) anticancer treatmentsYes, radiotherapyYes, other treatment for sarcoma (please specify)No, I am not receiving treatment.I don’t know.
- 3.
- Are you currently taking part in a clinical trial (i.e., research study)?Yes, I am currently receiving treatment as part of a clinical trialNo, I was taking part in a trial but my participation was paused due to COVID-19 pandemicNo, I was taking part in a clinical trial but my participation was stopped due to COVID-19 pandemicNo, I was being considered for a clinical trial but this was put on hold due to COVID-19 pandemicNo, I am not taking part in a clinical trial.
- 4.
- What is the aim of your current treatment or management plan (including surveillance or monitoring with scans)?Curative (cure of disease) or I have no evidence of sarcomaPalliative (control of disease)I do not know.
- 5.
- Over the last year, what was the usual frequency of your follow-up appointments with the sarcoma team?Intervals of less than three months (e.g., every few weeks)Three or four month intervalSix month intervalsAnnualOther, please specify____________________________
- Have you had an appointment with the sarcoma team at the Royal Marsden Hospital since 23 March 2020?Yes, I have had a face to face appointment at the Royal Marsden Hospital.Yes, I have had a telephone appointment with a doctor from the Royal Marsden Hospital.Yes, other e.g., video consultationNo, I have not had an appointmentI don’t know
- If you have had a face to face appointment with a doctor in the hospital since 23 March 2020, how satisfied were you with the consultation? (Please select a number on the scale from 1–10: 1 = not satisfied at all and 10 = extremely satisfied). If you have not had a face to face appointment during this period, please tick not applicable.Not applicable

- 3.
- If you have had a telephone appointment with a doctor since 23 March 2020, how satisfied where you with the consultation? (Please select a number on the scale from 1–10: 1 = not satisfied at all and 10 = extremely satisfied). If you have not had a telephone appointment during this period, please tick not applicable.Not applicable

- 4.
- If you had a video appointment (e.g., Zoom/Skype) with a doctor since 23 March 2020, how satisfied where you with the consultation? (Please select a number on the scale from 1–10: 1 = not satisfied at all and 10 = extremely satisfied). If you have not had a video appointment during this period, please tick not applicable.Not applicable

- 5.
- I had previously met the doctor who performed my telemedicine appointment.YesNoI don’t knowNot applicable
- 6.
- If you had a choice for your future routine appointments:
- (a)
- Which type of consultation would you prefer? (please choose one option)Face to face appointments onlyMostly face to face appointments, occasional telemedicineMostly telemedicine appointments, occasional face to face appointmentsOnly telemedicine appointmentsI don’t know.
- (b)
- What are the main reasons that you chose this option? (please select all that apply)Reduced time travelling to the hospitalReduced cost of travelling to the hospitalReduced waiting in the hospitalI would find it more reassuringI think my treatment plan would be clearerI think it is more convenientOther, please specify____________________________
- 7.
- If telemedicine (phone or video) was an option for patient care at the Royal Marsden Hospital moving forward, I would prefer using the following method: (please select one option)TelephoneVideo call (such as Zoom, or Skype)
- 8.
- I would not wish to hear the following information by a telemedicine (phone or video) appointment (please select all that apply)Any results of imaging tests (CT scan or MRI results)Bad news from imaging tests (i.e., growth of cancer on CT or MRI test, new area of cancer seen on imaging test)Blood test resultsResults from a team discussion regarding my care (for example, whether the team recommended to continue with my current treatment or suggested that I need to see a surgeon to manage my cancer)
- 9.
- Do you think having a specialist nurse in sarcoma participate in the telemedicine (phone or video) appointment would be useful?AlwaysSometimes—for example when the doctor is telling me bad news (like CT scan showing cancer is growing or new areas of cancer)Sometimes—I would like the option of asking for a nurse to be present during my telemedicine appointmentNeverI don’t know
- Have your follow-up appointments been postponed due to the COVID-19 pandemic?No, my follow-up has not changedYes, postponed for up to three months.Yes, postponed for more than three monthsI don’t know
- If your follow-up appointments have been postponed, what are your views on that decision?I think it was a good decisionI think it was a bad decisionI am not sure whether it was a good or bad decision.Not applicable, my appointments have not been changed.
- Have your scans been postponed due to due to the COVID-19 pandemic?No, my routine scans have not changedYes, postponed for up to three months.Yes, postponed for more than three monthsNot applicable, I do not have scansI don’t know
- If your scanshave been postponed, what are your views on that decision?I think it was a good decisionI think it was a bad decisionI am not sure whether it was a good or bad decision.Not applicable (my routine scans have not been changed, or I do not have scans)
- Has your treatment been postponed or discontinued due to the COVID-19 pandemic?No, my treatment has continued as normalYes, postponed for up to three monthsYes, postponed for more than three monthsYes, my current treatment has been discontinuedNot applicable, I am not on treatmentI don’t know
- If your treatment has been postponed or discontinued, what are your views on that decision?I think it was a good decisionI think it was a bad decisionI am not sure whether it was a good or bad decision.Not applicable, my treatment has not been postponedNot applicable, I am not on treatment.
- Are you concerned about the quality of your care during the COVID-19 pandemicMy care has not been affectedThe pandemic has had a negative impact on my careThe pandemic has had a positive impact on my careI don’t know
- Do you feel informed about the plan for your follow-up (and treatment if applicable)?Yes, very well informedYes, informedI received very little informationNot informed at all
- We are interested in some things about you and your health. Please answer all of the questions yourself by choosing the option that best applies to you. There are no “right” or “wrong” answers.
| Not at All | A Little | Quite a Bit | Very Much | |
| Do you have any trouble doing strenuous activities like carrying a heavy shopping bag or a suitcase? | ||||
| Do you have any trouble taking a long walk? | ||||
| Do you have any trouble taking a short walk outside of the house? | ||||
| Do you need to stay in bed or a chair during the day? | ||||
| Do you need help with eating, dressing, washing yourself or using the toilet? | ||||
| During the past week: | ||||
| Were you limited in doing either your work or other daily activities? | ||||
| Were you limited in pursuing your hobbies or other leisure time activities? | ||||
| Have you had trouble sleeping? | ||||
| Have you had difficulty in concentrating on things, like reading a newspaper or watching television? | ||||
| Did you feel tense? | ||||
| Did you worry? | ||||
| Did you feel irritable? | ||||
| Did you feel depressed? | ||||
| Have you had difficulty remembering things? | ||||
| Has your physical condition or medical treatment interfered with your family life? | ||||
| Has your physical condition or medical treatment interfered with your social activities? |
- 2.
- How would you rate your overall health during the past week?

- 3.
- How would you rate your overall quality of life during the past week?

- How often do you feel that you lack companionship?Hardly everSome of the timeOften
- How often do you feel left out?Hardly everSome of the timeOften
- How often do you feel isolated from others?Hardly everSome of the timeOften
- Who would you choose to contact for any problems related to your sarcoma, or questions about your cancer care during the COVID-19 pandemic? (please tick all that apply)Sarcoma clinical nurse specialistRoyal Marsden Hospital helplineGeneral practitioner (GP)Sarcoma charity (e.g., Sarcoma UK, BCRT)Other cancer charity (e.g., Macmillan, Cancer Research UK)Online peer support groupsNHS helpline (111)Other, please specify____________________________
- Do you feel comfortable contacting your healthcare providers (GP or sarcoma team) during the COVID-19 pandemic?I feel able to contact my healthcare providers as normalI am worried about availability of healthcare providersI would try not to contact healthcare providers unless absolutely essentialI don’t know
- Consider how well the following statements describe your usual behaviour and actions:
| (1) Does not describe me at all | (2) Does not describe me | (3) Neutral | (4) Describes me | (5) Describes me very well. | |
| I look for creative ways to alter difficult situations | |||||
| Regardless of what happens to me, I believe I can control my reaction to it. | |||||
| I believe I can grow in positive ways by dealing with difficult situations | |||||
| I actively look for ways to replace the losses I encounter in life. |

References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- World Health Organisation: Coronavirus Disease (COVID19) Pandemic Statistics. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 1 June 2020).
- Prem, K.; Liu, Y.; Russell, T.W.; Kucharski, A.J.; Eggo, R.M.; Davies, N.; Jit, M.; Klepac, P.; Flasche, S.; Clifford, S.; et al. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: A modelling study. Lancet Public Health 2020, 5, e261–e270. [Google Scholar] [CrossRef] [Green Version]
- Wilder-Smith, A.; Freedman, D.O. Isolation, quarantine, social distancing and community containment: Pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J. Travel Med. 2020, 27, taaa020. [Google Scholar] [CrossRef] [PubMed]
- The Rt Hon Boris Johnson MP. Statement on Coronavirus 23rd March 2020. Available online: https://www.gov.uk/government/speeches/pm-address-to-the-nation-on-coronavirus-23-march-2020 (accessed on 8 June 2020).
- World Health Organisation. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available online: https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) (accessed on 8 June 2020).
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 2020, 20, 669–677. [Google Scholar] [CrossRef]
- The OpenSAFELY Collaborative; Williamson, E.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.; Mehrkar, A.; Evans, D.; et al. OpenSAFELY: Factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. Available online: https://doi.org/10.1101/2020.05.06.20092999 (accessed on 12 August 2020).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A.; et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020, 10, 935–941. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef]
- Rogado, J.; Obispo, B.; Pangua, C.; Serrano-Montero, G.; Marino, A.M.; Pérez-Pérez, M.; López-Alfonso, A.; Gullón, P.; Lara, M.Á. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin. Transl. Oncol. 2020, 1–5. [Google Scholar] [CrossRef]
- National Institute for Clinical Excellence. COVID-19 Rapid Guideline: Delivery of Systemic Anticancer Treatments (NG161). Available online: https://www.nice.org.uk/guidance/ng161 (accessed on 8 June 2020).
- Büntzel, J.; Klein, M.; Keinki, C.; Walter, S.; Büntzel, J.; Hübner, J. Oncology services in corona times: A flash interview among German cancer patients and their physicians. J. Cancer Res. Clin. Oncol. 2020, 1–3. [Google Scholar] [CrossRef]
- Qiu, J.; Shen, B.; Zhao, M.; Wang, Z.; Xie, B.; Xu, Y.-F. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. Gen. Psychiatry 2020, 33, e100213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazza, C.; Ricci, E.; Biondi, S.; Colasanti, M.; Ferracuti, S.; Napoli, C.; Roma, P. A Nationwide Survey of Psychological Distress among Italian People during the COVID-19 Pandemic: Immediate Psychological Responses and Associated Factors. Int. J. Environ. Res. Public Health 2020, 17, 3165. [Google Scholar] [CrossRef] [PubMed]
- Shuja, K.H.; Aqeel, M.; Jaffar, A.; Ahmed, A. COVID-19 Pandemic and Impending Global Mental Health Implications. Psychiatr. Danub. 2020, 32, 32–35. [Google Scholar] [CrossRef]
- Nolte, S.; Liegl, G.; Petersen, M.; Aaronson, N.K.; Costantini, A.; Fayers, P.; Groenvold, M.; Holzner, B.; Johnson, C.; Kemmler, G.; et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur. J. Cancer 2019, 107, 153–163. [Google Scholar] [CrossRef]
- Torales, J.; O’Higgins, M.; Castaldelli-Maia, J.M.; Ventriglio, A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int. J. Soc. Psychiatry 2020, 66, 317–320. [Google Scholar] [CrossRef] [Green Version]
- Thaler, M.; Khosravi, I.; Leithner, A.; Papagelopoulos, P.J.; Ruggieri, P. Impact of the COVID-19 pandemic on patients suffering from musculoskeletal tumours. Int. Orthop. 2020, 44, 1503–1509. [Google Scholar] [CrossRef]
- Casali, P.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; Broto, J.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv51–iv67. [Google Scholar] [CrossRef]
- Casali, P.G.; Bielack, S.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brennan, B.; et al. Bone sarcomas: ESMO–PaedCan–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv79–iv95. [Google Scholar] [CrossRef] [PubMed]
- Smrke, A.; Younger, E.; Wilson, R.; Husson, O.; Farag, S.; Merry, E.; Macklin-Doherty, A.; Cojocaru, E.; Arthur, A.; Benson, C.; et al. Telemedicine During the COVID-19 Pandemic: Impact on Care for Rare Cancers. JCO Glob. Oncol. 2020, 6, 1046–1051. [Google Scholar] [CrossRef]
- Ernst, J.; Mehnert-Theuerkauf, A.; Dietz, A.; Hornemann, B.; Esser, P. Perceived stigmatization and its impact on quality of life—results from a large register-based study including breast, colon, prostate and lung cancer patients. BMC Cancer 2017, 17, 741. [Google Scholar] [CrossRef]
- A Holmes, E.; O’Connor, R.C.; Perry, V.H.; Tracey, I.; Wessely, S.; Arseneault, L.; Ballard, C.; Christensen, H.; Silver, R.C.; Everall, I.; et al. Multidisciplinary research priorities for the COVID-19 pandemic: A call for action for mental health science. Lancet Psychiatry 2020, 7, 547–560. [Google Scholar] [CrossRef]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.S.H.; Ho, R.C.M. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ma, Z.F. Impact of the COVID-19 Pandemic on Mental Health and Quality of Life among Local Residents in Liaoning Province, China: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 2381. [Google Scholar] [CrossRef] [Green Version]
- Armitage, R.; Nellums, L.B. COVID-19 and the consequences of isolating the elderly. Lancet Public Health 2020, 5, e256. [Google Scholar] [CrossRef] [Green Version]
- Kroenke, C.; Kubzansky, L.D.; Schernhammer, E.; Holmes, M.D.; Kawachi, I. Social Networks, Social Support, and Survival After Breast Cancer Diagnosis. J. Clin. Oncol. 2006, 24, 1105–1111. [Google Scholar] [CrossRef]
- Nausheen, B.; Carr, N.J.; Peveler, R.C.; Moss-Morris, R.; Verrill, C.; Robbins, E.; Nugent, K.; Baker, A.M.; Judd, M.; Gidron, Y. Relationship between Loneliness and Proangiogenic Cytokines in Newly Diagnosed Tumors of Colon and Rectum. Psychosom. Med. 2010, 72, 912–916. [Google Scholar] [CrossRef]
- Nipp, R.D.; El-Jawahri, A.; Fishbein, J.N.; Eusebio, J.; Stagl, J.M.; Gallagher, E.R.; Park, E.R.; Jackson, V.A.; Pirl, W.F.; Greer, J.A.; et al. The relationship between coping strategies, quality of life, and mood in patients with incurable cancer. Cancer 2016, 122, 2110–2116. [Google Scholar] [CrossRef]
- Vindegaard, N.; Benros, M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav. Immun. 2020, 0889. [Google Scholar] [CrossRef] [PubMed]
- Van de Poll-Franse, L.V.; de Rooij, B.H.; Horevoorts, N.J.E.; May, A.M.; Vink, G.R.; Koopman, M.; van Laarhoven, H.W.M.; Besselink, M.G.; Oerlemans, S.; Husson, O.; et al. The impact of the COVID-19 crisis on perceived changes in care and wellbeing of cancer patients and norm participants: Results of the PROFILES registry. In Proceedings of the European Society Medical Oncology Conference, 19–21 July 2020. Abstract 3203. [Google Scholar]
- Juanjuan, L.; Santa-Maria, C.A.; Hongfang, F.; Lingcheng, W.; Pengcheng, Z.; Yuangbing, X.; Yuyan, T.; Zhongchun, L.; Bo, D.; Meng, L.; et al. Patient-reported Outcomes of Patients With Breast Cancer During the COVID-19 Outbreak in the Epicenter of China: A Cross-sectional Survey Study. Clin. Breast Cancer 2020, S1526-8209(20)30147-6. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.K.; Ellis, A.E.; Zeligs, K.; Chapman-Davis, E.; Thomas, C.; Christos, P.J.; Kolev, V.; Prasad-Hayes, M.; Cohen, S.; Holcomb, K.; et al. Impact of the COVID-19 Pandemic on Quality of Life for Women with Ovarian Cancer. Am. J. Obstet. Gynecol. 2020, S0002-9378(20)30674-8. [Google Scholar] [CrossRef]
- Romito, F.; Dellino, M.; Loseto, G.; Opinto, G.; Silvestris, E.; Cormio, C.; Guarini, A.; Minoia, C. Psychological Distress in Outpatients with Lymphoma During the COVID-19 Pandemic. Front. Oncol. 2020, 10, 1270. [Google Scholar] [CrossRef] [PubMed]
- Falcone, R.; Grani, G.; Ramundo, V.; Melcarne, R.; Giacomelli, L.; Filetti, S.; Durante, C. Cancer Care During COVID-19 Era: The Quality of Life of Patients with Thyroid Malignancies. Front. Oncol. 2020, 10, 1128. [Google Scholar] [CrossRef]
- Fernando, A. Mental Health and Cancer: Why It Is Time to Innovate and Integrate—A Call to Action. Eur. Urol. Focus 2020, S2405-4569(20)30191-7. [Google Scholar] [CrossRef]
- Donovan, K.A.; Grassi, L.; McGinty, H.L.; Jacobsen, P.B. Validation of the Distress Thermometer worldwide: State of the science. Psycho-Oncology 2013, 23, 241–250. [Google Scholar] [CrossRef]
- Grassi, L.; Spiegel, D.; Riba, M. Advancing psychosocial care in cancer patients. F1000Research 2017, 6, 2083. [Google Scholar] [CrossRef] [Green Version]
- Hinz, A.; Friedrich, M.; Kuhnt, S.; Zenger, M.; Schulte, T. The influence of self-efficacy and resilient coping on cancer patients’ quality of life. Eur. J. Cancer Care 2018, 28, e12952. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, V.G.; Wallston, K.A. The Development and Psychometric Evaluation of the Brief Resilient Coping Scale. Assessment 2004, 11, 94–101. [Google Scholar] [CrossRef]
- Ludolph, P.; Kunzler, A.M.; Stoffers-Winterling, J.; Helmreich, I.; Lieb, K. Interventions to Promote Resilience in Cancer Patients. Dtsch. Aerzteblatt Online 2019, 51–52, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.R.; Bradford, M.C.; McCauley, E.; Curtis, J.R.; Wolfe, J.; Baker, K.S.; Yi-Frazier, J.P. Promoting resilience in adolescents and young adults with cancer: Results from the PRISM randomized controlled trial. Cancer 2018, 124, 3909–3917. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.W.; Smith, T.J. Reasons Why Physicians Do Not Have Discussions About Poor Prognosis, Why It Matters, and What Can Be Improved. J. Clin. Oncol. 2012, 30, 2715–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagerty, R.; Butow, P.; Ellis, P.M.; Lobb, E.A.; Pendlebury, S.C.; Leighl, N.; Mac Leod, C.; Tattersall, M.H.N. Communicating with Realism and Hope: Incurable Cancer Patients’ Views on the Disclosure of Prognosis. J. Clin. Oncol. 2005, 23, 1278–1288. [Google Scholar] [CrossRef] [Green Version]
- Husson, O.; Zebrack, B.J.; Aguilar, C.; Hayes-Lattin, B.; Cole, S. Cancer in adolescents and young adults: Who remains at risk of poor social functioning over time? Cancer 2017, 123, 2743–2751. [Google Scholar] [CrossRef] [Green Version]
- Masi, C.M.; Chen, H.-Y.; Hawkley, L.C.; Cacioppo, J.T. A Meta-Analysis of Interventions to Reduce Loneliness. Pers. Soc. Psychol. Rev. 2010, 15, 219–266. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.; Cheevers, K.; Armes, J. The emerging role of digital health in monitoring and supporting people living with cancer and the consequences of its treatments. Curr. Opin. Support. Palliat. Care 2018, 12, 268–275. [Google Scholar] [CrossRef]
- Gebbia, V.; Piazza, D.; Valerio, M.R.; Borsellino, N.; Firenze, A. Patients with Cancer and COVID-19: A WhatsApp Messenger-Based Survey of Patients’ Queries, Needs, Fears, and Actions Taken. JCO Glob. Oncol. 2020, 6, 722–729. [Google Scholar] [CrossRef]
- Weeks, J.C.; Cook, E.F.; O’Day, S.J.; Peterson, L.M.; Wenger, N.; Reding, U.; Harrell, F.E.; Kussin, P.; Dawson, N.V.; Connors, A.F.; et al. Relationship Between Cancer Patients’ Predictions of Prognosis and Their Treatment Preferences. JAMA 1998, 279, 1709–1714. [Google Scholar] [CrossRef]
- Survey Monkey Security and Compliance. Available online: https://www.surveymonkey.com/mp/data-security-and-compliance/ (accessed on 1 June 2020).
- Fayers, P.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- Russell, D.W. UCLA Loneliness Scale (Version 3): Reliability, Validity, and Factor Structure. J. Pers. Assess. 1996, 66, 20–40. [Google Scholar] [CrossRef]
| Patient Characteristics | All Participants (n = 350) n (%) | Curative Intent (n = 150) n (%) | Palliative Intent (n = 117) n (%) | Intent Unknown (n = 77) n (%) | p-Value |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean | 56.1 | 52.0 | 61.4 | 56.3 | 0.060 |
| (SD) | (17.3) | (17.9) | (14.6) | (17.8) | |
| Gender | |||||
| Male | 158 (45) | 65 (43) | 58 (50) | 31 (30) | 0.394 |
| Female | 192 (55) | 85 (57) | 59 (50) | 46 (60) | |
| Ethnicity | |||||
| Caucasian/European | 286 (82) | 121 (81) | 100 (87) | 59 (77) | 0.168 |
| Other | 62 (18) | 29 (19) | 15 (13) | 18 (23) | |
| Living situation | |||||
| Alone | 47 (13) | 16 (11) | 18 (15) | 12 (16) | 0.439 |
| Cohabiting | 303 (87) | 134 (89) | 99 (85) | 65 (84) | |
| Marital status | |||||
| Partner | 245 (70) | 115 (77) | 81 (69) | 45 (58) | 0.017 |
| No partner | 105 (30) | 35 (23) | 36 (31) | 32 (42) | |
| Education level | |||||
| Low | 94 (27) | 35 (23) | 28 (24) | 28 (36) | 0.081 |
| Medium | 114 (33) | 48 (32) | 37 (32) | 28 (36) | |
| High | 141 (40) | 67 (45) | 51 (44) | 21 (27) | |
| Loneliness | |||||
| Not lonely | 85 (24) | 120 (80) | 89 (76) | 52 (68) | 0.115 |
| Lonely | 265 (76) | 30 (20) | 28 (24) | 25 (32) | |
| Resilient coping | |||||
| Low | 115 (33) | 51 (34) | 30 (26) | 32 (42) | 0.066 |
| Medium | 161 (46) | 73 (49) | 60 (51) | 26 (34) | |
| High | 74 (21) | 26 (17) | 27 (23) | 19 (25) | |
| Comorbidities | |||||
| COPD | 9 (3) | 2 (1) | 5 (4) | 2 (3) | 0.380 |
| Hypertension | 76 (22) | 28 (19) | 30 (26) | 17 (22) | 0.388 |
| Diabetes | 29 (8) | 7 (5) | 12 (10) | 10 (13) | 0.061 |
| Coronary artery disease | 17 (5) | 5 (3) | 10 (9) | 1 (1) | 0.055 |
| Obesity | 31 (9) | 10 (7) | 9 (8) | 12 (16) | 0.085 |
| None of above | 232 (66) | 110 (73) | 68 (58) | 49 (64) | 0.030 |
| Journey to hospital > 1 h | 243 (70) | 93 (62) | 90 (77) | 57 (77) | 0.014 |
| Transport to hospital ≥ 2 modes | 165 (47) | 69 (46) | 62 (53) | 30 (39) | 0.152 |
| Cancer items | |||||
| Current treatment | |||||
| IV chemotherapy | 38 (11) | 11 (7) | 24 (21) | 3 (4) | 0.0001 |
| Oral chemotherapy/TKI | 66 (19) | 14 (9) | 42 (36) | 10 (13) | |
| Radiotherapy | 13 (4) | 6 (4) | 6 (5) | 1 (1) | |
| Other treatment | 10 (3) | 3 (2) | 3 (3) | 1 (1) | |
| No treatment | 218 (63) | 113 (76) | 41 (35) | 59 (77) | |
| Clinical trial | |||||
| Yes | 29 (8) | 6 (4) | 19 (16) | 4 (5) | 0.001 |
| Follow-up frequency | |||||
| Less than 3 monthly | 93 (27) | 25 (17) | 48 (41) | 19 (25) | 0.0001 |
| 3–4 monthly | 148 (43) | 61 (41) | 48 (41) | 34 (44) | |
| 6 monthly | 47 (14) | 29 (20) | 9 (8) | 9 (12) | |
| Annual | 23 (7) | 19 (13) | 2 (2) | 2 (3) | |
| Other | 37 (11) | 14 (10) | 10 (9) | 13 (17) |
| COVID-19 Items | All n (%) | Curative Intent n (%) | Palliative Intent n (%) | Intent Unknown n (%) | p-Value |
|---|---|---|---|---|---|
| Perceived risk vs. Population | |||||
| Higher | 167 (48) | 52 (35) | 70 (60) | 44 (57) | 0.0001 |
| Equal | 135 (39) | 77 (51) | 30 (26) | 26 (34) | |
| Lower | 30 (9) | 14 (9) | 13 (11) | 1 (1) | |
| Do not know | 18 (5) | 7 (5) | 4 (3) | 6 (8) | |
| COVID-19 symptoms/test | |||||
| Symptoms, negative test | 6 (2) | 5 (3) | 1 (1) | 0 (0) | 0.150 |
| Symptoms, no test | 34 (10) | 15 (10) | 13 (11) | 6 (8) | |
| No symptoms | 265 (76) | 113 (75) | 91 (78) | 55 (71) | |
| Do not know | 45 (13) | 17 (11) | 12 (10) | 16 (21) | |
| COVID-19 pandemic impact | |||||
| Employment | 86 (25) | 37 (25) | 32 (27) | 15 (20) | 0.459 |
| Financial situation | 84 (24) | 33 (22) | 29 (25) | 21 (27) | 0.661 |
| Family life | 210 (60) | 85 (57) | 79 (68) | 42 (55) | 0.112 |
| Emotional wellbeing | 145 (41) | 62 (41) | 48 (41) | 33 (43) | 0.979 |
| Social life/activities | 306 (87) | 130 (87) | 103 (88) | 67 (87) | 0.978 |
| Would accept ventilator | |||||
| Yes | 255 (73) | 119 (79) | 82 (70) | 50 (65) | 0.175 |
| No | 19 (5) | 6 (4) | 8 (7) | 5 (7) | |
| Do not know | 75 (22) | 25 (17) | 27 (23) | 22 (29) |
| Experiences of Care | All Participants | Curative Intent | Palliative Intent | Intent Unknown | p-Value |
|---|---|---|---|---|---|
| Appointment type | |||||
| Face to face appointment | 75 (26) | 32 (27) | 35 (33) | 8 (14) | 0.003 |
| Telemedicine appointment | 211 (74) | 86 (73) | 70 (67) | 51 (86) | |
| Preferred appointment type | |||||
| Face to face (F2F) only * | 78 (22) | 29 (19) | 25 (22) | 23 (30) | 0.047 |
| Mostly F2F, occasional telemedicine | 129 (37) | 68 (45) | 36 (31) | 23 (30) | |
| Mostly telemedicine, occasional F2F | 118 (34) | 44 (29) | 48 (41) | 23 (30) | |
| Only telemedicine | 12 (4) | 6 (4) | 4 (3) | 2 (3) | |
| Unsure | 12 (4) | 3 (2) | 3 (3) | 6 (8) | |
| Appointment modification | |||||
| Postponed | 117 (34) | 48 (33) | 40 (35) | 28 (36) | 0.853 |
| Opinion on postponement | |||||
| Positive | 82 (70) | 39 (81) | 24 (60) | 18 (64) | 0.220 |
| Negative | 10 (9) | 2 (4) | 4 (10) | 4 (14) | |
| Neutral | 24 (21) | 7 (15) | 11 (28) | 6 (21) | |
| Imaging modification | |||||
| Postponed | 106 (31) | 42 (29) | 33 (30) | 28 (37) | 0.422 |
| Opinion on postponement | |||||
| Positive | 63 (59) | 28 (67) | 18 (55) | 14 (50) | 0.212 |
| Negative | 11 (10) | 2 (5) | 3 (9) | 6 (21) | |
| Neutral | 32 (30) | 12 (29) | 12 (36) | 8 (29) | |
| Treatment modification | |||||
| Postponed | 29 (9) | 5 (3) | 20 (18) | 4 (5) | 0.0001 |
| Discontinued | 5 (2) | 2 (1) | 3 (3) | 0 (0) | |
| Opinion on postponed/stopped | |||||
| Positive | 14 (48) | 3 (75) | 8 (40) | 3 (75) | 0.093 |
| Negative | 2 (7) | 1 (25) | 1 (5) | 0 (0) | |
| Neutral | 12 (41) | 0 (0) | 11 (55) | 1 (25) | |
| Impact of pandemic on care quality | |||||
| Positive | 4 (1) | 3 (2) | 0 (0) | 1 (1) | 0.155 |
| Negative | 50 (15) | 18 (12) | 23 (20) | 8 (10) | |
| Not affected | 250 (72) | 113 (76) | 78 (68) | 55 (71) | |
| Unsure | 42 (12) | 14 (10) | 14 (12) | 13 (17) | |
| Informed about care plan | |||||
| Very well informed | 190 (55) | 94 (63) | 57 (49) | 35 (47) | 0.017 |
| Informed | 126 (36) | 44 (30) | 53 (45) | 27 (36) | |
| Little information | 23 (7) | 8 (5) | 5 (4) | 10 (13) | |
| Not informed | 8 (2) | 3 (2) | 2 (2) | 3 (4) | |
| Contacting HCP during pandemic ** | |||||
| Contact as normal | 223 (64) | 98 (66) | 79 (68) | 44 (57) | 0.019 |
| Worried about availability | 19 (5) | 6 (4) | 10 (9) | 2 (3) | |
| Only if essential | 95 (27) | 39 (26) | 28 (24) | 25 (33) | |
| Do not know | 12 (3) | 6 (4) | 0 (0) | 6 (8) | |
| Contact for cancer support | |||||
| Clinical nurse specialist | 272 (78) | 114 (76) | 96 (82) | 60 (78) | 0.482 |
| Sarcoma helpline (RMH/UCLH) | 179 (51) | 80 (53) | 61 (52) | 33 (43) | 0.300 |
| General practitioner (GP) | 72 (21) | 29 (19) | 22 (19) | 29 (26) | 0.420 |
| NHS helpline | 15 (4) | 5 (3) | 4 (3) | 5 (7) | 0.474 |
| Sarcoma charity | 19 (5) | 10 (7) | 5 (4) | 4 (5) | 0.690 |
| Cancer charity (any) | 44 (13) | 14 (9) | 22 (19) | 8 (10) | 0.055 |
| Online peer support | 16 (5) | 6 (4) | 6 (5) | 4 (5) | 0.880 |
| Worry about COVID-19 on Health | Worry about Sarcoma | |||||
|---|---|---|---|---|---|---|
| Patient Factors | Curative Intent | Palliative Intent | Unknown Intent | Curative Intent | Palliative Intent | Unknown Intent |
| Age (continuous) | - | - | - | - | β = −0.026 p = 0.782 | - |
| Ethnicity (Caucasian vs. other) | - | - | β = 0.102 p = 0.233 | - | - | - |
| Loneliness (continuous) | β = 0.077 p = 0.393 | - | - | β = −0.110 p = 0.242 | - | - |
| Resilient coping (continuous) | β = −0.206 p = 0.012 | - | - | β = −0.060 p = 0.475 | - | - |
| Comorbidities (Yes/No) | - | - | - | - | β = −0.127 p = 0.160 | - |
| Care Factors | ||||||
| On treatment (Yes/No) | β = 0.056 p = 0.475 | - | β = 0.175 p = 0.062 | β = 0.116 p = 0.183 | - | β = 0.221 p = 0.038 |
| Telemedicine (Yes/No) | - | - | - | β = −0.101 p = 0.232 | β = −0.282 p = 0.001 | β = −0.069 p = 0.525 |
| Appointment postponed (Yes/No) | - | - | - | β = −0.057 p = 0.494 | - | - |
| Treatment postponed (Yes/No) | - | - | - | - | β = 0.094 p = 0.260 | - |
| Impact of Pandemic | ||||||
| Financial impact (Yes/No) | - | - | β = 0.037 p = 0.690 | - | β = 0.064 p = 0.438 | - |
| Family impact (Yes/No) | - | β = 0.090 p = 0.300 | β = 0.036 p = 0.696 | - | β = 0.041 p = 0.716 | |
| Emotional impact (Yes/No) | β = 0.173 p = 0.056 | β = 0.175 p = 0.071 | β = 0.048 p = 0.620 | - | β = −0.060 p = 0.541 | β = 0.180 p = 0.117 |
| Social life impact (Yes/No) | - | β = 0.119 p = 0.173 | - | β = 0.171 p = 0.039 | - | |
| HRQoL | ||||||
| Sarcoma worry (continuous) | β = 0.340 p = 0.0001 | β = 0.464 p = 0.0001 | β = 0.582 p = 0.0001 | - | - | - |
| Physical functioning (continuous) | β = −0.187 p = 0.122 | β = −0.011 p = 0.928 | β = −0.009 p = 0.941 | β = −0.051 p = 0.686 | β = −0.037 p = 0.758 | β = 0.114 p = 0.424 |
| Emotional functioning (continuous) | β = 0.054 p = 0.661 | β = 0.011 p = 0.923 | β = 0.105 p = 0.427 | β = −0.409 p = 0.0001 | β = −0.410 p = 0.0001 | β = −0.572 p = 0.0001 |
| Role functioning (continuous) | β = 0.065 p = 0.547 | β = −0.058 p = 0.644 | β = 0.013 p = 0.915 | β = −0.103 p = 0.370 | β = 0.101 p = 0.423 | β = −0.101 p = 0.510 |
| Cognitive functioning (continuous) | β = 0.030 p = 0.778 | β = −0.109 p = 0.283 | β = 0.136 p = 0.218 | β = 0.215 p = 0.061 | - | β = 0.046 p = 0.735 |
| Social functioning (continuous) | β = −0.129 p = 0.218 | β = 0.012 p = 0.925 | β = 0.067 p = 0.571 | β = −0.098 p = 0.364 | β = −0.201 p = 0.127 | β = −0.187 p = 0.192 |
| Global HRQoL (continuous) | β = 0.064 p = 0.540 | β = 0.108 p = 0.347 | β = −0.285 p = 0.012 | β = 0.032 p = 0.775 | β = −0.051 p = 0.662 | β = 0.117 p = 0.404 |
| Insomnia (continuous) | β = −0.042 p = 0.639 | - | β = 0.238 p = 0.053 | β = 0.088 p = 0.353 | - | β = −0.166 p = 0.259 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younger, E.; Smrke, A.; Lidington, E.; Farag, S.; Ingley, K.; Chopra, N.; Maleddu, A.; Augustin, Y.; Merry, E.; Wilson, R.; et al. Health-Related Quality of Life and Experiences of Sarcoma Patients during the COVID-19 Pandemic. Cancers 2020, 12, 2288. https://doi.org/10.3390/cancers12082288
Younger E, Smrke A, Lidington E, Farag S, Ingley K, Chopra N, Maleddu A, Augustin Y, Merry E, Wilson R, et al. Health-Related Quality of Life and Experiences of Sarcoma Patients during the COVID-19 Pandemic. Cancers. 2020; 12(8):2288. https://doi.org/10.3390/cancers12082288
Chicago/Turabian StyleYounger, Eugenie, Alannah Smrke, Emma Lidington, Sheima Farag, Katrina Ingley, Neha Chopra, Alessandra Maleddu, Yolanda Augustin, Eve Merry, Roger Wilson, and et al. 2020. "Health-Related Quality of Life and Experiences of Sarcoma Patients during the COVID-19 Pandemic" Cancers 12, no. 8: 2288. https://doi.org/10.3390/cancers12082288
APA StyleYounger, E., Smrke, A., Lidington, E., Farag, S., Ingley, K., Chopra, N., Maleddu, A., Augustin, Y., Merry, E., Wilson, R., Benson, C., Miah, A., Zaidi, S., McTiernan, A., Strauss, S. J., Dileo, P., Gennatas, S., Husson, O., & Jones, R. L. (2020). Health-Related Quality of Life and Experiences of Sarcoma Patients during the COVID-19 Pandemic. Cancers, 12(8), 2288. https://doi.org/10.3390/cancers12082288








