Incidences of Deep Molecular Responses and Treatment-Free Remission in de Novo CP-CML Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Definitions
2.3. Statistical Analyses
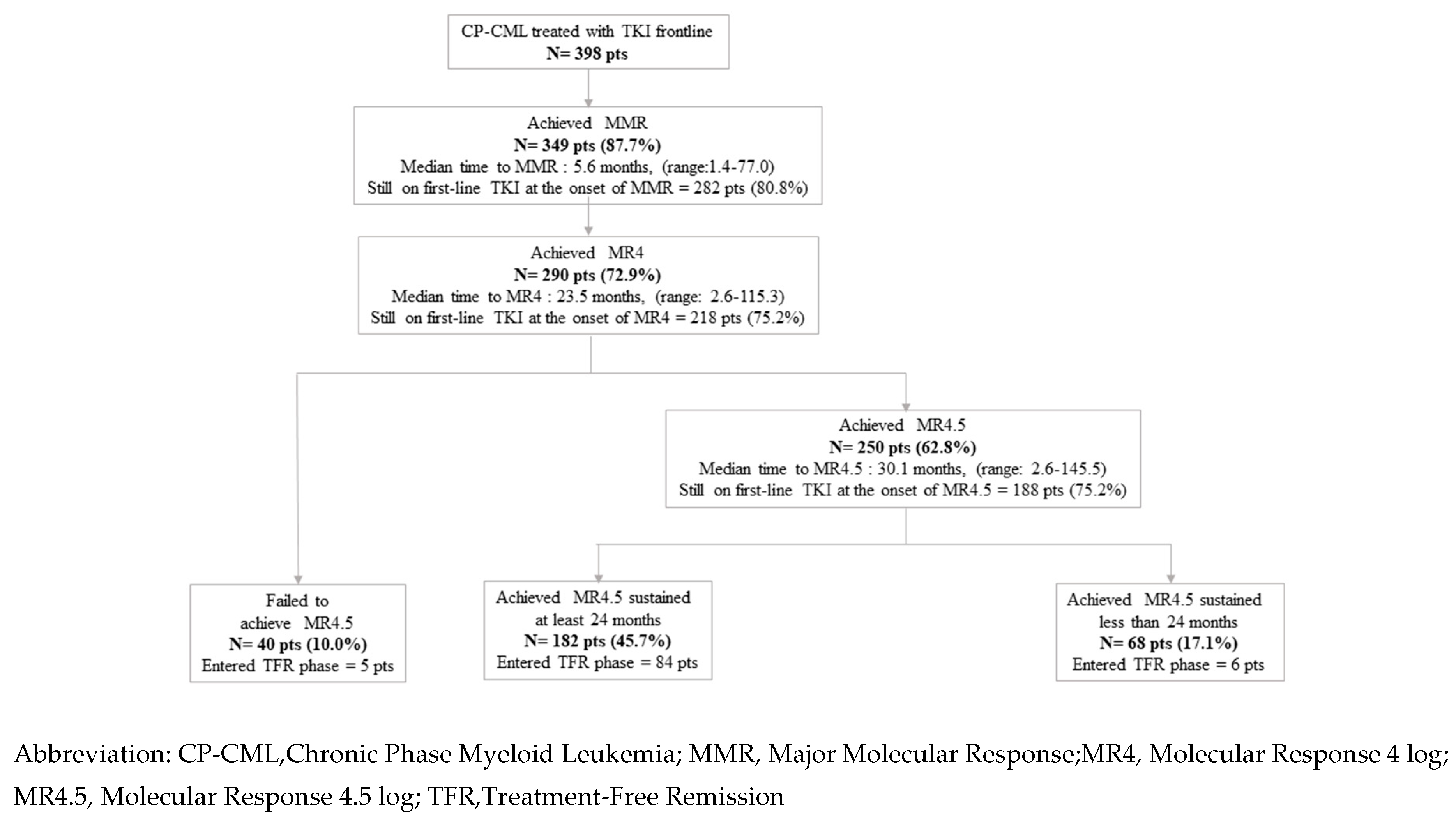
3. Results
3.1. Patients
3.2. Outcome
3.2.1. Cumulative Incidences of Deep Molecular Responses (MR4.5 and Sustained MR4.5 at Least 24 Months)
3.2.2. Predictive Factors of Deep Molecular Responses (MR4.5 and MR4.5 Sustained for at Least 24 Months)
3.2.3. Proportion of Patients Who Stopped TKI and Molecular Relapse-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gambacorti-Passerini, C.; Antolini, L.; Mahon, F.X.; Guilhot, F.; Deininger, M.; Fava, C.; Nagler, A.; Della Casa, C.M.; Morra, E.; Abruzzese, E.; et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J. Natl. Cancer Inst. 2011, 103, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Bower, H.; Bjorkholm, M.; Dickman, P.W.; Hoglund, M.; Lambert, P.C.; Andersson, T.M. Life Expectancy of Patients with Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J. Clin. Oncol. 2016, 34, 2851–2857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Hehlmann, R.; Lauseker, M.; Saussele, S.; Pfirrmann, M.; Krause, S.; Kolb, H.J.; Neubauer, A.; Hossfeld, D.K.; Nerl, C.; Gratwohl, A.; et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia 2017, 31, 2398–2406. [Google Scholar] [CrossRef]
- Dulucq, S.; Astrugue, C.; Etienne, G.; Mahon, F.X.; Benard, A. Risk of molecular recurrence after tyrosine kinase inhibitor discontinuation in chronic myeloid leukaemia patients: A systematic review of literature with a meta-analysis of studies over the last ten years. Br. J. Haematol. 2020, 189, 452–468. [Google Scholar] [CrossRef]
- Cortes, J.; Rea, D.; Lipton, J.H. Treatment-free remission with first- and second-generation tyrosine kinase inhibitors. Am. J. Hematol. 2019, 94, 346–357. [Google Scholar] [CrossRef]
- Rousselot, P.; Charbonnier, A.; Cony-Makhoul, P.; Agape, P.; Nicolini, F.E.; Varet, B.; Gardembas, M.; Etienne, G.; Rea, D.; Roy, L.; et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J. Clin. Oncol. 2014, 32, 424–430. [Google Scholar] [CrossRef]
- Lee, S.E.; Choi, S.Y.; Song, H.Y.; Kim, S.H.; Choi, M.Y.; Park, J.S.; Kim, H.J.; Kim, S.H.; Zang, D.Y.; Oh, S.; et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: The KID study. Haematologica 2016, 101, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Etienne, G.; Guilhot, J.; Rea, D.; Rigal-Huguet, F.; Nicolini, F.; Charbonnier, A.; Guerci-Bresler, A.; Legros, L.; Varet, B.; Gardembas, M.; et al. Long-Term Follow-up of the French Stop Imatinib (STIM1) Study in Patients with Chronic Myeloid Leukemia. J. Clin. Oncol. 2017, 35, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Rea, D.; Nicolini, F.E.; Tulliez, M.; Guilhot, F.; Guilhot, J.; Guerci-Bresler, A.; Gardembas, M.; Coiteux, V.; Guillerm, G.; Legros, L.; et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: Interim analysis of the STOP 2G-TKI study. Blood 2017, 129, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Nagafuji, K.; Matsumura, I.; Shimose, T.; Kawaguchi, T.; Kuroda, J.; Nakamae, H.; Miyamoto, T.; Kadowaki, N.; Ishikawa, J.; Imamura, Y.; et al. Cessation of nilotinib in patients with chronic myelogenous leukemia who have maintained deep molecular responses for 2 years: A multicenter phase 2 trial, stop nilotinib (NILSt). Int. J. Hematol. 2019, 110, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Ross, D.M. Moving treatment-free remission into mainstream clinical practice in CML. Blood 2016, 128, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, D.; Ame, S.; Berger, M.; Cayuela, J.M.; Charbonnier, A.; Coiteux, V.; Cony-Makhoul, P.; Dubruille, V.; Dulucq, S.; Etienne, G.; et al. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: Recommendations for clinical practice from the French Chronic Myeloid Leukemia Study Group. Cancer 2018, 124, 2956–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.E.; Polydoros, F.; Apperley, J.F.; Milojkovic, D.; Pocock, C.; Smith, G.; Byrne, J.L.; de Lavallade, H.; O’Brien, S.G.; Coffrey, T.; et al. De-escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): An interim analysis of a non-randomised, phase 2 trial. Lancet Haematol. 2017, 4, e310–e316. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.E.; Polydoros, F.; Apperley, J.F.; Milojkovic, D.; Rothwell, K.; Pocock, C.; Byrne, J.; de Lavallade, H.; Osborne, W.; Robinson, L.; et al. De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): A non-randomised, phase 2 trial. Lancet Haematol. 2019, 6, e375–e383. [Google Scholar] [CrossRef] [Green Version]
- Branford, S.; Yeung, D.T.; Ross, D.M.; Prime, J.A.; Field, C.R.; Altamura, H.K.; Yeoman, A.L.; Georgievski, J.; Jamison, B.A.; Phillis, S.; et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood 2013, 121, 3818–3824. [Google Scholar] [CrossRef]
- Ferrero, D.; Cerrano, M.; Crisa, E.; Aguzzi, C.; Giai, V.; Boccadoro, M. How many patients can proceed from chronic myeloid leukaemia diagnosis to deep molecular response and long-lasting imatinib discontinuation? A real life experience. Br. J. Haematol. 2017, 176, 669–671. [Google Scholar] [CrossRef] [Green Version]
- Kantarjian, H.; Shah, N.P.; Hochhaus, A.; Cortes, J.; Shah, S.; Ayala, M.; Moiraghi, B.; Shen, Z.; Mayer, J.; Pasquini, R.; et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 2010, 362, 2260–2270. [Google Scholar] [CrossRef]
- Saglio, G.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.; Etienne, G.; Lobo, C.; Pasquini, R.; Clark, R.E.; Hochhaus, A.; Hughes, T.P.; et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 2010, 362, 2251–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes, J.E.; Gambacorti-Passerini, C.; Deininger, M.W.; Mauro, M.J.; Chuah, C.; Kim, D.W.; Dyagil, I.; Glushko, N.; Milojkovic, D.; le Coutre, P.; et al. Bosutinib versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results from the Randomized BFORE Trial. J. Clin. Oncol. 2018, 36, 231–237. [Google Scholar] [CrossRef]
- Cortes, J.E.; Saglio, G.; Kantarjian, H.M.; Baccarani, M.; Mayer, J.; Boqué, C.; Shah, N.P.; Chuah, C.; Casanova, L.; Bradley-Garelik, B.; et al. Final 5-Year Study Results of DASISION: The Dasatinib versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients Trial. J. Clin. Oncol. 2016, 34, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.D.; Etienne, G.; Dorlhiac-Llacer, P.E.; Clatk, R.E.; et al. Long-term benefits and risks of frontline nilotinib vs. imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016, 30, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Guilhot, J.; Baccarani, M.; Clark, R.E.; Cervantes, F.; Guilhot, F.; Hochhaus, A.; Kulikov, S.; Mayer, J.; Petzer, A.L.; Rosti, G.; et al. Definitions, methodological and statistical issues for phase 3 clinical trials in chronic myeloid leukemia: A proposal by the European LeukemiaNet. Blood 2012, 119, 5963–5971. [Google Scholar] [CrossRef]
- Baccarani, M.; Deininger, M.W.; Rosti, G.; Hochhaus, A.; Soverini, S.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Guilhot, F.; et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013, 122, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Sokal, J.E.; Cox, E.B.; Baccarani, M.; Tura, S.; Gomez, G.A.; Robertson, J.E.; Tso, C.Y.; Braun, T.J.; Clarkson, B.D.; Cervantes, F.; et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood 1984, 63, 789–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfirrmann, M.; Baccarani, M.; Saussele, S.; Guilhot, J.; Cervantes, F.; Ossenkoppele, G.; Hoffmann, V.S.; Castagnetti, F.; Hasford, J.; Hehlmann, R.; et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia 2016, 30, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.C.; White, H.E.; Muller, M.C.; Saglio, G.; Hochhaus, A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia 2012, 26, 2172–2175. [Google Scholar] [CrossRef] [Green Version]
- Cross, N.C.; White, H.E.; Colomer, D.; Ehrencrona, H.; Foroni, L.; Gottardi, E.; Lange, T.; Lion, T.; Machova Polakova, K.; Dulucq, S.; et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia 2015, 29, 999–1003. [Google Scholar] [CrossRef] [Green Version]
- Hehlmann, R.; Muller, M.C.; Lauseker, M.; Hanfstein, B.; Fabarius, A.; Schreiber, A.; Proetel, U.; Pletsch, N.; Pfirrmann, M.; Haferlach, C.; et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: Results from the randomized CML-study IV. J. Clin. Oncol. 2014, 32, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Castagnetti, F.; Di, R.F.; De, V.A.; Spilateri, A.; Gugliotta, G.; Fabbiano, F.; Capodanno, I.; Mannina, D.; Salvucci, M.; Antolino, A.; et al. A population-based study of chronic myeloid leukemia patients treated with imatinib in first line. Am. J. Hematol. 2017, 92, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hanfstein, B.; Lauseker, M.; Hehlmann, R.; Saussele, S.; Erben, P.; Dietz, C.; Fabarius, A.; Proetel, U.; Schnittger, S.; Haferlach, C.; et al. Distinct characteristics of e13a2 versus e14a2 BCR-ABL1 driven chronic myeloid leukemia under first-line therapy with imatinib. Haematologica 2014, 99, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Kantarjian, H.; Patel, K.P.; Gonzalez, G.N.; Luthra, R.; Kanagal Shamanna, R.; Sasaki, K.; Jabbour, E.; Romo, C.G.; Kadia, T.M.; et al. Impact of BCR-ABL transcript type on outcome in patients with chronic-phase CML treated with tyrosine kinase inhibitors. Blood 2016, 127, 1269–1275. [Google Scholar] [CrossRef]
- Hughes, T.P.; Lipton, J.H.; Spector, N.; Cervantes, F.; Pasquini, R.; Clementino, N.C.D.; Dorlhiac LLacer, P.E.; Schwarer, A.P.; Mahon, F.X.; Rea, D.; et al. Deep molecular responses achieved in patients with CML-CP who are switched to nilotinib after long-term imatinib. Blood 2014, 124, 729–736. [Google Scholar] [CrossRef]
- Hughes, T.P.; Leber, B.; Cervantes, F.; Spector, N.; Pasquini, R.; Clementino, N.C.D.; Schwarer, A.P.; Dorlhiac-Llacer, P.E.; Mahon, F.X.; Rea, D.; et al. Sustained deep molecular responses in patients switched to nilotinib due to persistent BCR-ABL1 on imatinib: Final ENESTcmr randomized trial results. Leukemia 2017, 31, 2529–2531. [Google Scholar] [CrossRef]
- Castagnetti, F.; Gugliotta, G.; Baccarani, M.; Breccia, M.; Specchia, G.; Levato, L.; Abruzzese, E.; Rossi, G.; Iurlo, A.; Martino, B.; et al. Differences among young adults, adults and elderly chronic myeloid leukemia patients. Ann. Oncol. 2015, 26, 185–192. [Google Scholar] [CrossRef]
- Hijiya, N.; Schultz, K.R.; Metzler, M.; Millot, F.; Suttorp, M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood 2016, 127, 392–399. [Google Scholar] [CrossRef]
- Baccarani, M.; Castagnetti, F.; Gugliotta, G.; Rosti, G.; Soverini, S.; Albeer, A.; Pfirrmann, M.; International BCR-ABL Study Group. The proportion of different BCR-ABL1 transcript types in chronic myeloid leukemia. An international overview. Leukemia 2019, 33, 1173–1183. [Google Scholar] [CrossRef]
- Castagnetti, F.; Gugliotta, G.; Breccia, M.; Iurlo, A.; Levato, L.; Albano, F.; Vigneri, P.; Abruzzese, E.; Rossi, G.; Rupoli, S.; et al. The BCR-ABL1 transcript type influences response and outcome in Philadelphia chromosome-positive chronic myeloid leukemia patients treated frontline with imatinib. Am. J. Hematol. 2017, 92, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Ernst, T.; Busch, M.; Rinke, J.; Ernst, J.; Haferlach, C.; Beck, J.F.; Hochhaus, A.; Gruhn, B. Frequent ASXL1 mutations in children and young adults with chronic myeloid leukemia. Leukemia 2018, 32, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Kantarjian, H.; Shan, J.; Jabbour, E.; Quintas-Cardama, A.; Verstovsek, S.; Ravandi, F.; Wierda, W.; O’Brien, S.; Cortes, J. Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica 2012, 97, 1029–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, F.J.; Rea, D.; Rosti, G.; Cross, N.C.P.; Steegmann, J.L.; Griskevicius, L.; le Coutre, P.; Coriu, D.; Petrov, L.; Ossenkoppele, G.J.; et al. Impact of age on efficacy and toxicity of nilotinib in patients with chronic myeloid leukemia in chronic phase: ENEST1st subanalysis. J. Cancer Res. Clin. Oncol. 2017, 143, 1585–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nteliopoulos, G.; Bazeos, A.; Claudiani, S.; Gerrard, G.; Curry, E.; Szydlo, R.; Alikian, M.; Foong, H.E.; Nikolakopoulou, Z.; Loaiza, S.; et al. Somatic variants in epigenetic modifiers can predict failure of response to imatinib but not to second-generation tyrosine kinase inhibitors. Haematologica 2019, 104, 2400–2409. [Google Scholar] [CrossRef] [Green Version]
- Fava, C.; Rege-Cambrin, G.; Dogliotti, I.; Cerrano, M.; Berchialla, P.; Dragani, M.; Rosti, G.; Castagnetti, F.; Gugliotta, G.; Martino, B.; et al. Observational study of chronic myeloid leukemia Italian patients who discontinued tyrosine kinase inhibitors in clinical practice. Haematologica 2019, 104, 1589–1596. [Google Scholar] [CrossRef]
- Lipton, J.H.; Chuah, C.; Guerci-Bresler, A.; Rosti, G.; Simpson, D.; Assouline, S.; Etienne, G.; Nicolini, F.E.; le Coutre, P.; Clark, R.E.; et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 612–621. [Google Scholar] [CrossRef]
- Hochhaus, A.; Masszi, T.; Giles, F.J.; Radich, J.P.; Ross, D.M.; Gomez Casares, M.T.; Hellmann, A.; Stentoft, J.; Conneally, E.; Garcia-Gutiérrez, V.; et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: Results from the ENESTfreedom study. Leukemia 2017, 31, 1525–1531. [Google Scholar] [CrossRef]
- Mahon, F.X.; Boquimpani, C.; Kim, D.W.; Benyamini, N.; Clementino, N.C.D.; Shuvaev, V.; Ailawadhi, S.; Lipton, J.H.; Turkina, A.G.; De Paz, R.; et al. Treatment-Free Remission after Second-Line Nilotinib Treatment in Patients with Chronic Myeloid Leukemia in Chronic Phase: Results from a Single-Group, Phase 2, Open-Label Study. Ann. Intern. Med. 2018, 168, 461–470. [Google Scholar] [CrossRef]
- Kumagai, T.; Nakaseko, C.; Nishiwaki, K.; Yoshida, C.; Ohashi, K.; Takezako, N.; Takaho, H.; Kouzai, Y.; Murase, T.; Matsue, K.; et al. Dasatinib cessation after deep molecular response exceeding 2 years and natural killer cell transition during dasatinib consolidation. Cancer Sci. 2018, 109, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Kimura, S.; Imagawa, J.; Murai, K.; Hino, M.; Kitawaki, T.; Okada, M.; Tanaka, H.; Shindo, M.; Kumagai, T.; Ikezoe, T.; et al. Treatment-free remission after first-line dasatinib discontinuation in patients with chronic myeloid leukaemia (first-line DADI trial): A single-arm, multicentre, phase 2 trial. Lancet Haematol. 2020, 7, e218–e225. [Google Scholar] [CrossRef]
- Shah, N.P.; Garcia-Gutierrez, V.; Jimenez-Velasco, A.; Larson, S.; Saussele, S.; Rea, D.; Mahon, F.X.; Levy, M.Y.; Gomez-Casares, M.T.; Pane, F.; et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: The DASFREE study. Leuk. Lymphoma 2020, 61, 650–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristics | All Patients (N = 398) | Frontline IMA Patients (N = 291) | Frontline 2–3G TKI Patients (N = 107) | p-Value |
|---|---|---|---|---|
| Median age at diagnosis, years (range) | 61.6 (18.6–90.2) | 63.9 (18.6–90.2) | 54.0 (22.7–82.9) | <0.001 |
| ≥70, n pts (%) <45, n pts (%) | 106 (26.6) 77 (19.3) | 94 (32.3) 40 (13.7) | 12 (11.2) 37 (34.5) | <0.001 <0.001 |
| Gender, female, n pts (%) | 168 (42.2) | 123 (42.2) | 45 (42.0) | 0.532 |
| Sokal risk score, n pts (%) | 0.022 | |||
| Low | 112 (28.1) | 81 (27.8) | 31 (28.9) | |
| Intermediate | 193 (49.7) | 149 (51.2) | 44 (41.2) | |
| High | 76 (19.0) | 46 (15.8) | 30 (28.0) | |
| Unknown | 17 (4.2) | 15 (5.1) | 2 (1.8) | |
| ELTS risk score, n pts (%) | 0.054 | |||
| Low | 199 (50.0) | 138 (47.7) | 61 (56.0) | |
| Intermediate | 122 (30.6) | 98 (33.3) | 24 (23.3) | |
| High | 53 (13.3) | 35 (12.0) | 18 (16.8) | |
| Unknown | 24 (6.0) | 20 (6.8) | 4 (3.7) | |
| Non-M BCR-ABL1 transcript, n pts | 3 | 3 | 0 | 0.373 |
| ACA at diagnosis, n pts (%) | 30 (7.5) | 22 (7.5) | 8 (7.4) | 0.584 |
| ACA major route at diagnosis, n pts (%) | 12 (3.0) | 8 (2.7) | 4 (3.7) | 0.410 |
| First-line TKI type and initial daily dose, n pts (%) | ||||
| Imatinib 400 | 276 (69.3) | 276 (94.8) | NA | |
| Imatinib > 400 | 7 (1.7) | 7 (2.4) | NA | |
| Imatinib < 400 | 8 (2.0) | 8 (2.7) | NA | |
| Nilotinib 600 | 69 (17.3) | NA | 69 (64.4) | |
| Nilotinib 800 | 8 (2.0) | NA | 8 (7.4) | |
| Dasatinib 100 | 26 (6.5) | NA | 26 (24.3) | |
| Bosutinib 500 | 2 (0.5) | NA | 2 (1.8) | |
| Ponatinib 45 | 2 (0.5) | NA | 2 (1.8) |
| Cumulative Incidence of Sustained MR4.5 beyond 24 Months (n = 398) | |||||||
|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | ||||||
| Variable | No. Patients | SHR | 95%CI | p | SHR | 95%CI | p |
| Gender | |||||||
| Male | 230 | - | - | - | - | - | - |
| Female | 168 | 1.41 | 1.12–1.70 | 0.021 | 1.4 | 1.10–1.70 | 0.028 |
| Age at diagnosis (years old) | 398 | 0.99 | 0.9816–0.9984 | 0.24 | |||
| Type of transcript | |||||||
| B2A2 | 130 | - | - | - | |||
| B3A2 | 209 | 1.33 | 1.02–1.64 | 0.082 | |||
| B3A2 +B2A2 | 23 | 1.11 | 0.46–1.76 | 0.75 | |||
| E8A2 | 1 | 0 | −3.96 | <0.001 | |||
| E19A2 | 2 | 0 | −2.82 | <0.001 | |||
| ACA | |||||||
| No | 368 | - | - | - | |||
| Yes | 30 | 0.74 | 0.13–1.35 | 0.32 | |||
| Sokal | |||||||
| Low | 112 | - | - | - | |||
| Intermediate | 193 | 0.67 | 0.36–0.98 | 0.016 | |||
| High | 76 | 0.46 | 0.01–0.91 | <0.001 | |||
| ELTS | |||||||
| Low | 199 | - | - | - | - | - | - |
| Intermediate | 122 | 0.46 | 0.089–0.83 | <0.001 | 0.46 | 0.09–0.83 | <0.001 |
| High | 53 | 0.47 | −1.02 | 0.003 | 0.48 | −1.01 | 0.004 |
| TKI first-line | |||||||
| IMA | 291 | - | - | - | |||
| 2–3G TKI | 107 | 0.99 | 0.66–1.32 | 0.96 | |||
| Characteristics | All Patients (N = 83) | With MolRec (N = 42) | Without MolRec (N = 41) | p-Value |
|---|---|---|---|---|
| Median age at diagnosis, years (range) | 61.8 (19.2–85.6) | 61.31 (19.2–85.6) | 62.69 (27.6–81.7) | 0.318 |
| Gender, female, n pts (%) | 46 (55.4) | 21 (50) | 25 (61) | 0.216 |
| Sokal risk score, n pts (%) | 0.982 | |||
| Low | 20 (24.1) | 11 (26) | 9 (22) | |
| Intermediate | 40 (48.2) | 21 (50) | 19 (46) | |
| High | 19 (22.9) | 10 (24) | 9 (22) | |
| Unknown | 4 (4.8) | 0 (0) | 4 (9.8) | |
| ELTS risk score, n pts (%) | 0.774 | |||
| Low | 46 (55.4) | 26 (62) | 20 (49) | |
| Intermediate | 19 (22.9) | 9 (21) | 10 (24) | |
| High | 12 (14.5) | 6 (14) | 6 (15) | |
| Unknown | 6 (7.2) | 1 (2) | 5 (12) | |
| Type of Transcript | 0.346 | |||
| Transcript B2A2, n pts (%) | 28 (33.7) | 17 (41) | 11 (27) | |
| Transcript B3A2, n pts (%) | 45 (54.2) | 20 (48) | 25 (61) | |
| Transcripts B3A2 + B2A2, n pts (%) | 3 (3.6) | 2 (4.8) | 1 (2.4) | |
| M BCR-ABL1 Nos, n pts (%) | 7 (8.4) | 3 (7.1) | 4 (9.8) | |
| University Hospital, n pts (%) | 65 (78.3) | 30 (71.4) | 35 (85.4) | 0.101 |
| First-line TKI | 0.010 | |||
| IMA, n pts (%) | 61 (73.5) | 36 (85.7) | 25 (60.98) | |
| 2–3G TKI, n pts (%) | 22 (26.5) | 6 (14) | 16 (39) | |
| Median time from TKI start to onset of sustained MR4.5 at least 24 months, months (range) * | 21.5 (2.6–88) | 21.21 (2.6–67.4) | 21.85 (5.3–88) | 0.339 |
| Median time from TKI start to TKI discontinuation, years (range) | 5.77 (2.8–11.2) | 5.13 (2.8–10.6) | 6.46 (3–11.2) | 0.014 |
| Still on first-line TKI at the date of discontinuation, n pts (%) | 64 (77.1) | 31 (73.8) | 34 (82.9) | 0.230 |
| Sustained MR4.5 at least 24 months at the time of TKI discontinuation, n pts (%) | 74 (89.2) | 37 (88.1) | 37 (90.2) | 0.516 |
| Median MR4.5 duration at the time of TKI discontinuation, months (range) | 41.4 (13.1–113.4) | 38.4 (20–104.4) | 62.69 (27.6–81.7) | 0.038 |
| Cumulative Incidence of MolRec over Time (n = 83) | |||||||
|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | ||||||
| Variable | No Patients | SHR | 95%CI | p | SHR | 95%CI | p |
| Sex | |||||||
| Male | 37 | - | - | ||||
| Female | 46 | 0.7 | 0.38–1.28 | 0.249 | |||
| Age at diagnosis (years old) | 83 | 0.99 | 0.97–1.00 | 0.229 | |||
| Type of transcript | |||||||
| B2A2 | 28 | - | - | ||||
| B3A2 | 45 | 0.6 | 0.31–1.15 | 0.123 | |||
| B3A2 +B2A2 | 3 | 0.88 | 0.20–3.83 | 0.866 | |||
| ACA | |||||||
| No | 79 | - | - | ||||
| Yes | 4 | 0.37 | 0.05–2.69 | 0.326 | |||
| Sokal | |||||||
| Low | 20 | - | - | ||||
| Intermediate | 40 | 0.94 | 0.45–1.95 | 0.867 | |||
| High | 19 | 0.95 | 0.40–2.24 | 0.908 | |||
| ELTS | |||||||
| Low | 46 | - | - | ||||
| Intermediate | 19 | 1.07 | 0.50–2.29 | 0.867 | |||
| High | 12 | 0.81 | 0.33–1.97 | 0.64 | |||
| First-line TKI | |||||||
| IMA | 61 | - | - | - | - | ||
| 2–3G TKI | 22 | 0.41 | 0.17–0.97 | 0.043 | 0.36 | 0.15–0.87 | 0.023 |
| TKI switch | |||||||
| No | 65 | - | - | ||||
| Yes | 18 | 1.48 | 0.74–2.97 | 0.266 | 1.4 | 0.70–2.81 | 0.344 |
| TKI duration (years) | 83 | 0.87 | 0.75–1.01 | 0.066 | 0.85 | 0.73–0.98 | 0.029 |
| Time to sustained MR4.5 at least 24 months (months) | 78 | 0.99 | 0.98–1.01 | 0.467 | |||
| MR4.5 duration (months) | 78 | 0.99 | 0.97–1.00 | 0.095 | |||
| Multivariate Analysis | ||||
|---|---|---|---|---|
| Variable | No Patients | SHR | 95%CI | p |
| TKI | ||||
| IMA | 61 | - | - | |
| 2–3G TKI | 22 | 0.42 | 0.17–1.00 | 0.05 |
| Switch | ||||
| No | 65 | - | - | |
| Yes | 18 | 0.92 | 0.41–2.05 | 0.838 |
| MR4.5 duration (months) | 78 | 0.99 | 0.97–1.00 | 0.095 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etienne, G.; Dulucq, S.; Bauduer, F.; Adiko, D.; Lifermann, F.; Dagada, C.; Lenoir, C.; Schmitt, A.; Klein, E.; Madene, S.; et al. Incidences of Deep Molecular Responses and Treatment-Free Remission in de Novo CP-CML Patients. Cancers 2020, 12, 2521. https://doi.org/10.3390/cancers12092521
Etienne G, Dulucq S, Bauduer F, Adiko D, Lifermann F, Dagada C, Lenoir C, Schmitt A, Klein E, Madene S, et al. Incidences of Deep Molecular Responses and Treatment-Free Remission in de Novo CP-CML Patients. Cancers. 2020; 12(9):2521. https://doi.org/10.3390/cancers12092521
Chicago/Turabian StyleEtienne, Gabriel, Stéphanie Dulucq, Fréderic Bauduer, Didier Adiko, François Lifermann, Corinne Dagada, Caroline Lenoir, Anna Schmitt, Emilie Klein, Samia Madene, and et al. 2020. "Incidences of Deep Molecular Responses and Treatment-Free Remission in de Novo CP-CML Patients" Cancers 12, no. 9: 2521. https://doi.org/10.3390/cancers12092521
APA StyleEtienne, G., Dulucq, S., Bauduer, F., Adiko, D., Lifermann, F., Dagada, C., Lenoir, C., Schmitt, A., Klein, E., Madene, S., Fort, M.-P., Bijou, F., Moldovan, M., Turcq, B., Robbesyn, F., Durrieu, F., Versmée, L., Katsahian, S., Faberes, C., ... Mahon, F.-X. (2020). Incidences of Deep Molecular Responses and Treatment-Free Remission in de Novo CP-CML Patients. Cancers, 12(9), 2521. https://doi.org/10.3390/cancers12092521






