1. Introduction
Breast cancer is the most common diagnosed cancer and the leading cause of cancer death among women in developed countries [
1]. Despite advances in cancer prevention, diagnoses, and treatment, still approximately 5% of patients are diagnosed with metastatic disease, and 20–30% of initially primary breast cancer develops metastasis subsequently, during the course of the disease.
Extracellular DNA (ecDNA), also called cell-free DNA, is present in blood plasma in various forms [
2]. EcDNA in the circulation of cancer patients contains tumor DNA from the primary tumor, metastasis, or circulating tumor cells, as well as healthy host cells mostly of hematopoietic origin [
3,
4,
5]. Plasma ecDNA is partially free unbound DNA and, so, sensitive to rapid cleavage, but it also can be protected as ecDNA hidden in apoptotic bodies and/or bound to proteins such as histones in the form of nucleosomes [
5].
Nucleosomes are composed of DNA wound around histone proteins and represent the basic structural unit of chromatin in the nucleus [
6]. After cell death, membranes and nuclei disintegrate and cell-free nucleosomes can get into the circulation. Plasma nucleosomes might serve as a nonspecific biomarker of cell death [
7]. This might be of interest in patients not only with autoimmune diseases, but also with sepsis or cancer [
8,
9,
10]. The prognostic value of the concentration of circulating nucleosomes was shown in several types of cancer including lung, pancreatic, or colorectal cancer [
11,
12,
13,
14,
15]. For example, in pancreatic cancer, high nucleosome levels during treatment, but not pretherapeutic levels, correlate with time to progression [
16]. Similarly, in non-small cell lung cancer, high baseline nucleosome level and/or during chemotherapy was associated with poor response to treatment and these data suggested that circulating nucleosomes are a valuable tool for early prediction of chemotherapy efficacy in cancer patients [
17]. However, when it comes to primary breast cancer, data in the published literature are limited.
In this study, we aimed to analyze circulating nucleosomes in relation to patients/tumor characteristics and prognosis in primary breast cancer.
2. Methods
2.1. Study Patients
This study included 92 primary breast cancer patients (stage I–III) treated with surgery from March to November 2012, for whom plasma isolated in the morning on the day of surgery was available in the biobank. This study represents a substudy of a translational trial that aimed to evaluate prognostic value of circulating tumor cells in primary breast cancer [
18]. Study eligibility criteria and study details were described previously [
18]. The study was approved by the Institutional Review Board (IRB) of the National Cancer Institute of Slovakia (TRUSK002, 20.6.2011). Each participant provided signed informed consent before study enrollment.
2.2. Detection of Circulating Tumor Cells (CTCs) in Peripheral Blood
CTCs were detected in peripheral blood by a quantitative real-time polymerase chain reaction (qRT-PCR)-based assay of peripheral blood as described previously [
18,
19,
20].
2.3. Plasma Isolation
Venous peripheral blood samples were collected in EDTA-treated tubes in the morning on the day of surgery and centrifuged at 1000×
g for 10 min at room temperature within 2 h of venipuncture and processed as described previously [
21].
2.4. Quantification of Circulating Nucleosomes
The commercially available Cell Death Detection kit (Roche, Basel, Switzerland) was used for the measurement of nucleosomes. Briefly, 20 mL of plasma was mixed with biotin-labeled anti-histone and peroxidase-conjugated anti-DNA antibodies. After incubation and washing, the substrate for the peroxidase enzyme was added. Absorbance was measured at 405 nm in arbitrary units after stopping the reaction. Interassay and intra-assay coefficients of variation were below 10% and 5%, respectively.
2.5. Measurement of DD, TF, uPA, and PAI-1 in Plasma
Plasma tissue factor (TF), d-dimer (DD), urokinase plasminogen activator (uPA), and plasminogen activator inhibitor-1 (PAI-1) were analyzed using enzyme-linked immunosorbent assays (ELISA) as described previously [
21].
2.6. Plasma Cytokines and Angiogenic Factors Analysis
Plasma samples were analyzed for 51 plasma cytokines and angiogenic factors: TGF-β1, TGF-β2, TGF-β3, IFN-α2, IL-1α, IL-2Rα, IL-3, IL-12p40, IL-16, IL-18, CTACK, Gro-α, HGF, LIF, MCP-3, M-CSF, MIF, MIG, β-NGF, SCF, SCGF-β, SDF-1α, TNF-β, TRAIL, IL-1β, Il-1RA, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, Eotaxin, FGF basic, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF bb, RANTES, TNF-α, VEGF using predesigned panels as described previously and were available for subset of patients (Bio-Plex Pro TGF-β assay, Bio-Plex Pro Human Cytokine 21- and 27-plex immunoassays; Bio-Rad Laboratories, Hercules, CA, USA) [
22]. The large panel of cytokines was analyzed as data were available from the previous study [
22].
2.7. Complete Blood Count and Inflammation-Based Scores
Complete blood count (CBC) and CBC-derived inflammation-based scores were calculated as described previously [
23,
24]. For CBC-derived inflammation-based scores, identical cut-off values as published previously for metastatic breast cancer patients were used [
23,
24]. Data for calculation of NLR, PLR, MLR, SII were available for 54, 52, 48, and 52 patients, respectively.
2.8. Statistical Analysis
The characteristics of patients is summarized using mean (range) for continuous variables and frequency (percentage) for categorical variables. The median follow-up period was calculated as the median observation time among all patients and among those who were still alive at the time of their last follow-up. Disease-free survival (DFS) was calculated from the date of blood sampling to the date of disease recurrence (locoregional or distant), secondary cancer, death, or last follow-up. DFS was estimated using the Kaplan–Meier product limit method and compared between groups by log-rank test. For survival analysis, circulating nucleosomes were dichotomized to “low” or “high” (nucleosome level below vs. above mean, respectively). Univariate analyses with Chi squared or Fisher’s exact test were performed to find associations between prognostic factors.
A multivariate Cox proportional hazards model for DFS was used to assess differences in outcome on the basis of the nucleosomes status (above mean vs. below mean), hormone receptor status (positive for either vs. negative for both), HER-2 status (positive or negative), axillary lymph node involvement (N0 vs. N+), grade (grade 3 vs. grade 1 and 2). Stepwise regression techniques were used to build multivariate models using a significance level of 0.10 to remain in the model. All p values presented are two-sided, and associations were considered significant if the p value was less than or equal to 0.05. Statistical analyses were performed using NCSS 11 Statistical Software (2016, NCSS, LLC., Kaysville, UT, USA, ncss.com/software/ncss).
4. Discussion
In this translational study, circulating nucleosomes showed neither an association with basic patient/tumor characteristics nor a correlation to CTCs. The origin of circulating nucleosomes is unclear and likely complex [
25]. While there is no correlation between CTCs and SII and/or neutrophil/lymphocyte ratio [
23,
24], this study showed for the first time an association between plasma nucleosomes and SII. Patients with high SII had significantly higher level of nucleosomes. Similarly, there was a trend of higher nucleosomes in patients with high neutrophil/lymphocyte ratio, however, the neutrophil/lymphocyte ratio is part of the SII.
Tumor-induced systemic changes in immune cells contribute to cancer progression and metastasis. Various forms of ecDNA including extracellular nucleosomes and naked ecDNA differ in their cytotoxic and proinflammatory effects [
26]. For example, histones in the nucleosomes induce proinflammatory signaling via toll-like receptors (TLR2/4), with subsequent production of TNF-α, IL-6, IL-10, and myeloperoxidase, but they exhibit TLR-independent cytotoxicity as well [
26,
27,
28]. On the other hand, the ecDNA as part of the nucleosomes is recognized by the TLR9 [
29]. In our study, nucleosomes were associated with several proinflammatory cytokines, suggesting the association of circulating nucleosomes with systemic inflammation. Histones in the nucleosomes could induce formation of neutrophil extracellular traps (NETs), which contain nucleosomes and stimulate further NETs production in a positive feedback loop [
27]. On the other hand, nucleosomes could induce different inflammatory pathways, as they, in contrast to histones, seem not to be cytotoxic to the endothelium [
28]. The analyzed nucleosomes could be from tumor cells, but also from the released NETs. This would explain the observed association between circulating nucleosomes and systemic inflammation in primary breast cancer patients. NETs contain nuclear DNA and proteins that possess antibacterial characteristics crucial for fighting pathogens [
30,
31]. The same NETs, however, also induce intravascular coagulation [
32] and their overproduction can lead to autoimmune diseases [
33]. While circulating ecDNA correlates with activation of coagulation [
34], we for the first time describe this association for circulating nucleosomes. Further research is needed to uncover if nucleosomes directly activate PAI-1, or if high PAI-1 is a marker of coagulation activation in more aggressive disease that leads to release of more nucleosomes.
Data on the prognostic value of plasma nucleosomes in breast cancer is limited. In a small study, nucleosomes were elevated in locally confined and metastatic breast cancer in comparison to healthy individuals. During neoadjuvant chemotherapy, patients with no change of a local disease had significantly higher pretherapeutic concentrations of nucleosomes than patients in remission [
14]. In another study, plasma nucleosomes were higher in primary breast cancer patients when compared to healthy controls, and similarly to our study, there was no association between nucleosomes and patient/tumor characteristics [
15]. Circulating nucleosomes were, however, not able to discriminate between benign and malignant breast lesions [
35]. Their concentration was found to be associated with lymph node-positive breast cancer and the presence of distant metastases [
35].
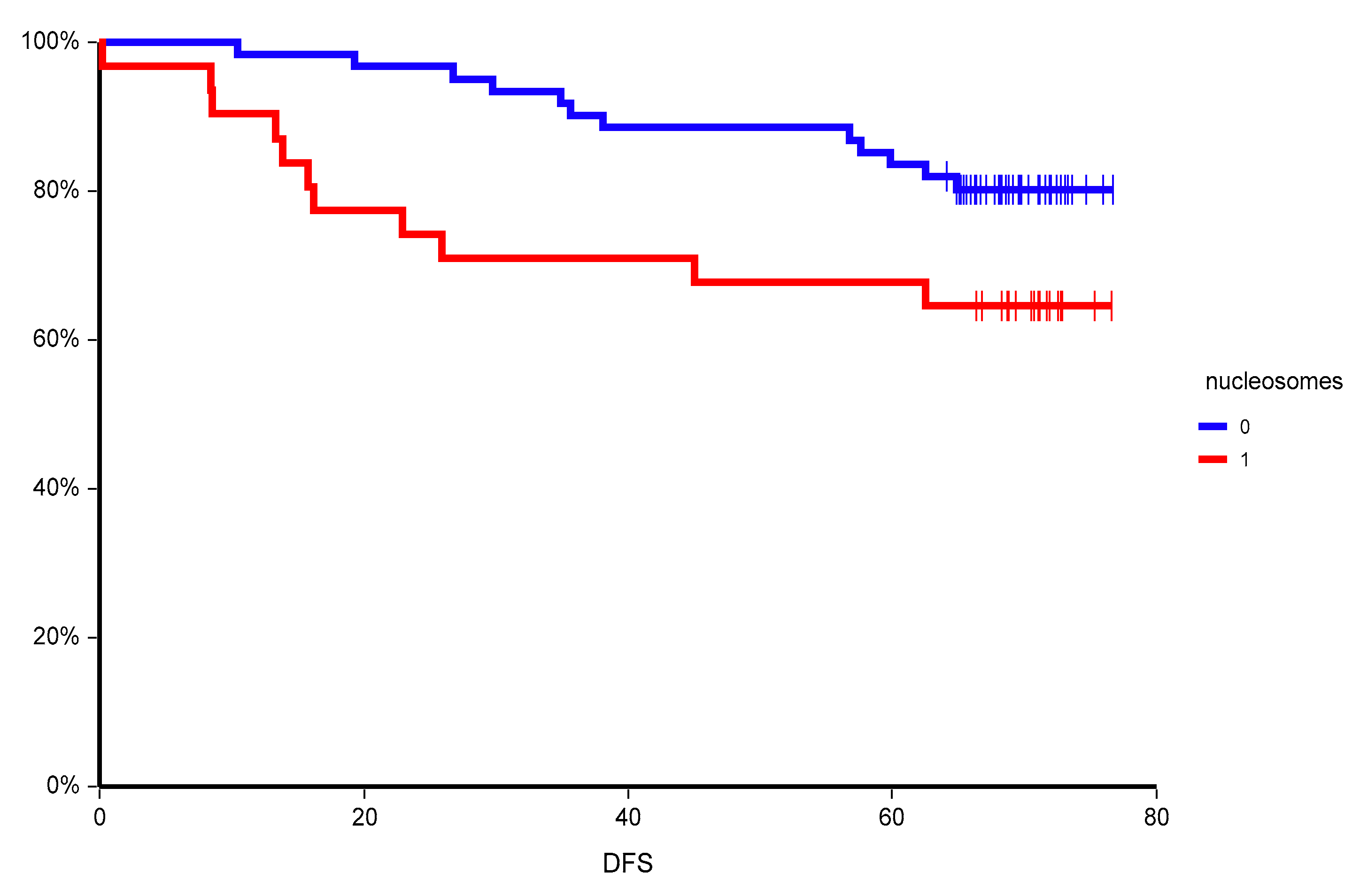
In our study, we observed an inferior outcome of primary breast cancer patients with high plasma nucleosomes. This is in contrast to a previous study, where elevated plasma nucleosomes were associated with a better prognosis in both node-negative and node-positive early breast cancer [
15]. However, the nucleosome detection method as well as the cut-off value to discriminate “low” and “high” plasma nucleosomes was different compared to our trial and therefore, these differences in results could be due to these factors. In our trial, the prognostic value of nucleosomes was consistent in various subgroups, however, it was most pronounced in poor prognostic subgroups such as lymph node-positive disease with high proliferation rate and in patients with detectable circulating tumor cells with epithelial-to-mesenchymal transition. The prognostic value of circulating nucleosomes was independent from established prognostic markers and was confirmed in a multivariate analysis. Moreover, when we combined two circulating biomarkers, circulating tumor cells, and circulating nucleosomes, we were able to uncover a subgroup of patients with extremely poor prognosis with two-year DFS of only 33.3%.
Our study has some limitations. The major one is small sample size, especially for associations between inflammatory indexes and nucleosomes. This is associated with decreased statistical power of analyses and increased confidence intervals of results. Other limitations represent the data availability for analysis of association between circulating nucleosomes and various clinic–pathological parameters, which further decreases statistical robustness and could have an impact on study results. Circulating plasma nucleosomes increase in non-neoplastic disease processes including inflammation, autoimmune diseases, sepsis, and stroke. When we analyzed association between chronic medication/comorbidities and circulating nucleosomes, no association was found, however, none of our patients received anti-inflammatory drugs and/or had inflammatory disease that could affect study results. Another limitation is lack of follow-up analysis on patient samples collected postsurgery to examine whether the presurgery baseline levels of circulating plasma nucleosomes were altered postsurgery and whether this alteration in circulating nucleosome levels is correlated with decrease in systemic inflammatory index.