Incidence of Hepatocellular Carcinoma after Treatment with Sofosbuvir-Based or Sofosbuvir-Free Regimens in Patients with Chronic Hepatitis C
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
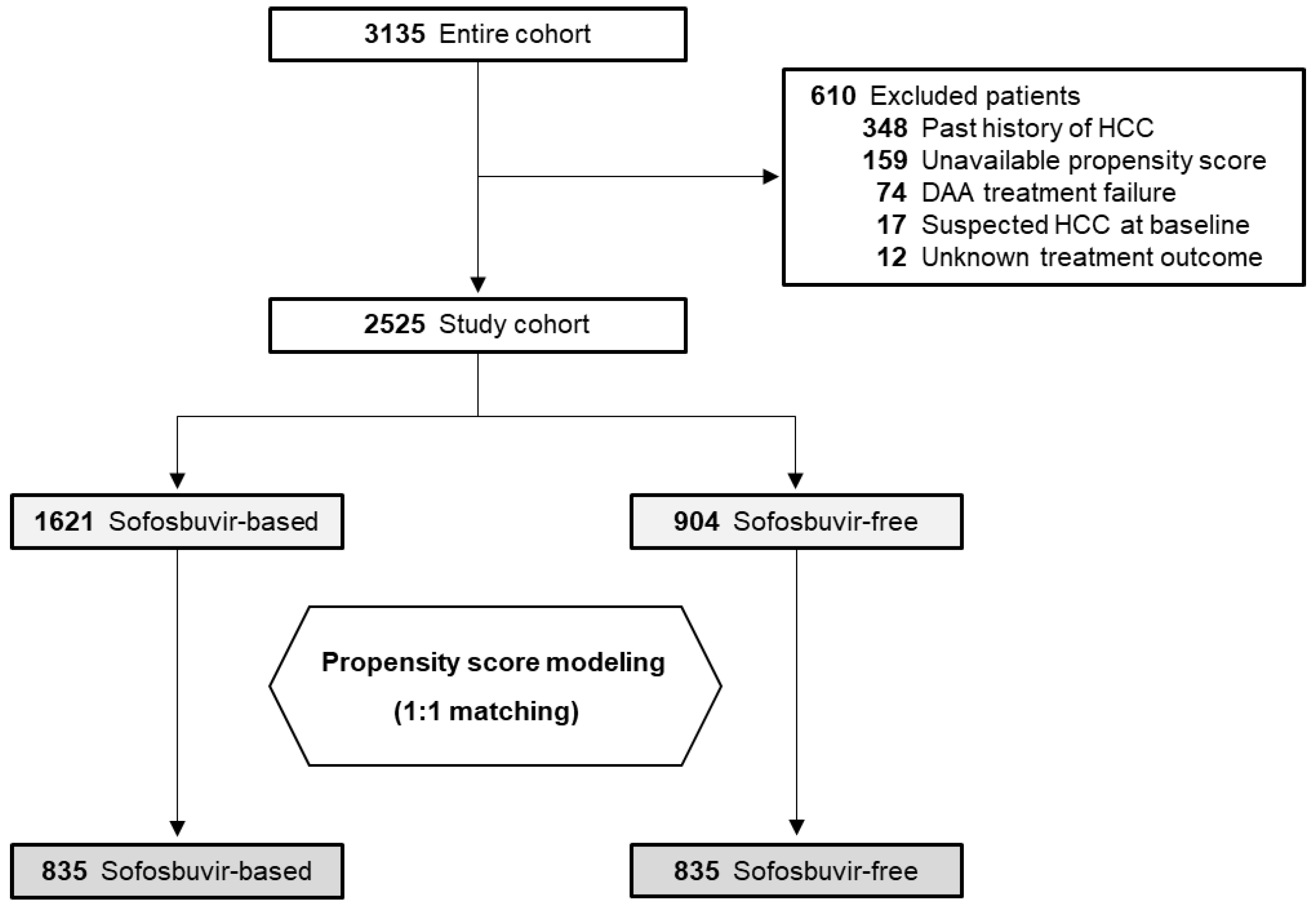
2.1. Baseline Demographics
2.2. Cumulative Rates of the Development of HCC
2.3. Incidence Rates for the Development of HCC within the First Two Years
2.4. Incidence Rates for the Development of HCC over the Entire Follow-Up Period
2.5. Predictors of HCC Development for the Overall Cohort
3. Discussion
4. Patients and Methods
4.1. Study Cohort
4.2. Study Assessments
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HCV | hepatitis C virus |
| HCC | hepatocellular carcinoma |
| DAA | direct-acting antiviral |
| SVR | sustained viral response |
| AFP | α-fetoprotein |
| SOF | sofosbuvir |
| EGFR | epidermal growth factor receptor |
| PSM | propensity score matching |
| pw12 | 12 weeks after the end of DAA treatment |
| eGFR | estimated glomerular filtration rate |
| sCr | serum creatinine |
| CI | confidence interval |
| PY | person-years |
| ALT | alanine aminotransferase |
| HR | hazard ratio |
| CXCL | C-X-C motif chemokine |
References
- Spengler, U. Direct antiviral agents (DAAs)-A new age in the treatment of hepatitis C virus infection. Pharmacol Ther. 2018, 183, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Yeo, Y.H.; Wei, M.T.; Enomoto, M.; Lee, D.H.; Iio, E.; Lubel, J.; Wang, W.; Wei, B.; Ide, T.; et al. Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 473–485. [Google Scholar] [CrossRef]
- Ogawa, E.; Toyoda, H.; Iio, E.; Jun, D.W.; Huang, C.F.; Enomoto, M.; Hsu, Y.C.; Haga, H.; Iwane, S.; Wong, G.; et al. HCV Cure Rates are Reduced in Patients with Active but not Inactive Hepatocellular Carcinoma—A Practice Implication. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated with Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Waziry, R.; Hajarizadeh, B.; Grebely, J.; Amin, J.; Law, M.; Danta, M.; George, J.; Dore, G.J. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J. Hepatol. 2017, 67, 1204–1212. [Google Scholar] [CrossRef]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; Tinè, F.; Distefano, M.; Licata, A.; Giannitrapani, L.; et al. Incidence of Hepatocellular Carcinoma in Patients with HCV-Associated Cirrhosis Treated with Direct-Acting Antiviral Agents. Gastroenterology 2018, 155, 411–421. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Feld, J.J. What Are the Benefits of a Sustained Virologic Response to Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection? Gastroenterology 2019, 156, 446–460. [Google Scholar] [CrossRef]
- Rinaldi, L.; Nevola, R.; Franci, G.; Perrella, A.; Corvino, G.; Marrone, A.; Berretta, M.; Morone, M.V.; Galdiero, M.; Giordano, M.; et al. Risk of Hepatocellular Carcinoma after HCV Clearance by Direct-Acting Antivirals Treatment Predictive Factors and Role of Epigenetics. Cancers (Basel) 2020, 12, 1351. [Google Scholar] [CrossRef]
- Flemming, J.A.; Kim, W.R.; Brosgart, C.L.; Terrault, N.A. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology 2017, 65, 804–812. [Google Scholar] [CrossRef]
- Belli, L.S.; Perricone, G.; Adam, R.; Cortesi, P.A.; Strazzabosco, M.; Facchetti, R.; Karam, V.; Salizzoni, M.; Andujar, R.L.; Fondevila, C.; et al. Impact of DAAs on liver transplantation: Major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J. Hepatol. 2018, 69, 810–817. [Google Scholar] [CrossRef]
- Ogawa, E.; Nomura, H.; Nakamuta, M.; Furusyo, N.; Kajiwara, E.; Dohmen, K.; Kawano, A.; Ooho, A.; Azuma, K.; Takahashi, K.; et al. Development of Hepatocellular Carcinoma by Patients Aged 75-84 with Chronic Hepatitis C Treated with Direct-acting Antivirals. J. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pons, M.; Rodríguez-Tajes, S.; Esteban, J.I.; Mariño, Z.; Vargas, V.; Lens, S.; Buti, M.; Augustin, S.; Forns, X.; Mínguez, B.; et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J. Hepatol. 2020, 72, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Roche, B.; Coilly, A.; Duclos-Vallee, J.C.; Samuel, D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018, 38, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Debes, J.D.; van Tilborg, M.; Groothuismink, Z.M.A.; Hansen, B.E.; Schulze Zur Wiesch, J.; von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate with Development of Hepatocellular Carcinoma in Patients with HCV Infection Treated with Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517. [Google Scholar] [CrossRef]
- Faillaci, F.; Marzi, L.; Critelli, R.; Milosa, F.; Schepis, F.; Turola, E.; Andreani, S.; Vandelli, G.; Bernabucci, V.; Lei, B.; et al. Liver Angiopoietin-2 Is a Key Predictor of De Novo or Recurrent Hepatocellular Cancer after Hepatitis C Virus Direct-Acting Antivirals. Hepatology 2018, 68, 1010–1024. [Google Scholar] [CrossRef]
- Nahon, P.; Layese, R.; Bourcier, V.; Cagnot, C.; Marcellin, P.; Guyader, D.; Pol, S.; Larrey, D.; De Lédinghen, V.; Ouzan, D.; et al. Incidence of Hepatocellular Carcinoma after Direct Antiviral Therapy for HCV in Patients with Cirrhosis Included in Surveillance Programs. Gastroenterology 2018, 155, 1436–1450. [Google Scholar] [CrossRef]
- Nagata, H.; Nakagawa, M.; Asahina, Y.; Sato, A.; Asano, Y.; Tsunoda, T.; Miyoshi, M.; Kaneko, S.; Otani, S.; Kawai-Kitahata, F.; et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J. Hepatol. 2017, 67, 933–939. [Google Scholar] [CrossRef]
- Douglas, M.W.; Tay, E.S.E.; Wang, D.S.; Ong, A.; Wilson, C.; Phu, A.; Kok, J.; Dwyer, D.E.; Bull, R.A.; Lloyd, A.R.; et al. Impact of an Open Access Nationwide Treatment Model on Hepatitis C Virus Antiviral Drug Resistance. Hepatol. Commun. 2020, 4, 904–915. [Google Scholar] [CrossRef]
- Bojkova, D.; Westhaus, S.; Costa, R.; Timmer, L.; Funkenberg, N.; Korencak, M.; Streeck, H.; Vondran, F.; Broering, R.; Heinrichs, S.; et al. Sofosbuvir Activates EGFR-Dependent Pathways in Hepatoma Cells with Implications for Liver-Related Pathological Processes. Cells 2020, 9, 1003. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Charlton, M.; Curry, M.P.; O’Leary, J.G.; Brown, R.S.; Hunt, S. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: An exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol. Hepatol. 2016, 1, 122–132. [Google Scholar] [CrossRef]
- Ogawa, E.; Furusyo, N.; Nomura, H.; Dohmen, K.; Higashi, N.; Takahashi, K.; Kawano, A.; Azuma, K.; Satoh, T.; Nakamuta, M.; et al. NS5A resistance-associated variants undermine the effectiveness of ledipasvir and sofosbuvir for cirrhotic patients infected with HCV genotype 1b. J. Gastroenterol. 2017, 52, 845–854. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Lee, M.H.; Lin, J.W.; Liu, C.J.; Su, T.H.; Tseng, T.C.; Chen, P.J.; Chen, D.S.; Kao, J.H. Evolution of eGFR in chronic HCV patients receiving sofosbuvir-based or sofosbuvir-free direct-acting antivirals. J. Hepatol. 2020, 72, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Furusyo, N.; Nomura, H.; Dohmen, K.; Higashi, N.; Takahashi, K.; Kawano, A.; Azuma, K.; Satoh, T.; Nakamuta, M.; et al. Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment. Pharmacol. Ther. 2018, 47, 104–113. [Google Scholar] [CrossRef]
- Abu Dayyeh, B.K.; Yang, M.; Fuchs, B.C.; Karl, D.L.; Yamada, S.; Sninsky, J.J.; O’Brien, T.R.; Dienstag, J.L.; Tanabe, K.K.; Chung, R.T.; et al. A functional polymorphism in the epidermal growth factor gene is associated with risk for hepatocellular carcinoma. Gastroenterology 2011, 141, 141–149. [Google Scholar] [CrossRef]
- Fuchs, B.C.; Hoshida, Y.; Fujii, T.; Wei, L.; Yamada, S.; Lauwers, G.Y.; McGinn, C.M.; DePeralta, D.K.; Chen, X.; Kuroda, T.; et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology 2014, 59, 1577–1590. [Google Scholar] [CrossRef]
- Alanazi, I.; Ebrahimie, E.; Hoffmann, P.; Adelson, D.L. Combined gene expression and proteomic analysis of EGF induced apoptosis in A431 cells suggests multiple pathways trigger apoptosis. Apoptosis 2013, 18, 1291–1305. [Google Scholar] [CrossRef]
- Breuhahn, K.; Longerich, T.; Schirmacher, P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene 2006, 25, 3787–3800. [Google Scholar] [CrossRef]
- Xu, X.; Xia, J.; Wang, X. Potential anticancer therapies via CXCL5 and its receptors. Expert Rev. Clin. Pharmacol. 2012, 5, 347–350. [Google Scholar] [CrossRef]
- Cassim, S.; Raymond, V.A.; Dehbidi-Assadzadeh, L.; Lapierre, P.; Bilodeau, M. Metabolic reprogramming enables hepatocarcinoma cells to efficiently adapt and survive to a nutrient-restricted microenvironment. Cell Cycle 2018, 17, 903–916. [Google Scholar] [CrossRef]
- Cassim, S.; Raymond, V.A.; Lacoste, B.; Lapierre, P.; Bilodeau, M. Metabolite profiling identifies a signature of tumorigenicity in hepatocellular carcinoma. Oncotarget 2018, 9, 26868–26883. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Perrella, A.; Guarino, M.; De Luca, M.; Piai, G.; Coppola, N.; Pafundi, P.C.; Ciardiello, F.; Fasano, M.; Martinelli, E.; et al. Incidence and risk factors of early HCC occurrence in HCV patients treated with direct acting antivirals: A prospective multicentre study. J. Trans. Med. 2019, 17, 292. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M.; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.R.; Rubin, D.B. Constructing a Control-Group Using Multivariate Matched Sampling Methods That Incorporate the Propensity Score. Am. Stat. 1985, 39, 33–38. [Google Scholar]


| Characteristics | Sofosbuvir-Based (n = 835) | Sofosbuvir-Free (n = 835) | Standardized Mean Difference * |
|---|---|---|---|
| Age | 67 (56–75) | 67 (57–74) | 0.04 |
| Male | 365 (43.7) | 360 (43.1) | 0.02 |
| Body Mass Index (kg/m2) | 22.9 (20.6–25.3) | 22.7 (20.6–25.0) | 0.11 |
| Cirrhosis | 225 (26.9) | 218 (26.1) | 0.02 |
| Diabetes | 151 (18.2) | 129 (15.4) | 0.10 |
| Treatment-Naïve | 613 (73.4) | 621 (74.4) | 0.03 |
| Total Bilirubin (mg/dL) | 0.7 (0.6–0.9) | 0.7 (0.5–0.9) | 0.09 |
| Albumin (g/dL) | 4.1 (3.8–4.3) | 4.1 (3.8–4.3) | 0.03 |
| AST (U/L) | 42 (29–62) | 42 (29–63) | 0.08 |
| ALT (U/L) | 40 (26–63) | 39 (25–66) | 0.07 |
| γGTP (U/L) | 32 (20–58) | 32 (20–57) | 0.02 |
| eGFR (mL/min/1.73 m2) | 72 (62–84) | 74 (62–86) | 0.01 |
| Platelet count (103/μL) | 159 (117–205) | 161 (118–205) | 0.05 |
| α-fetoprotein (ng/mL) | 4.5 (2.9–8.0) | 4.4 (2.8–8.3) | 0.03 |
| HCV RNA (logIU/mL) | 6.1 (5.5–6.5) | 6.1 (5.6–6.5) | 0.14 |
| HCV Genotype | |||
| Genotype 1 | 497 (59.5) | 705 (84.4) | |
| Genotype 2 | 338 (40.5) | 130 (15.6) | 0.82 |
| Other Genotypes | 0 | 0 | |
| DAA regimen | |||
| LDV/SOF | 500 (59.9) | - | |
| SOF + RBV | 335 (40.1) | - | |
| ASV + DCV | - | 314 (37.6) | |
| EBR + GZR | - | 227 (27.2) | |
| GLE/PIB | - | 216 (25.9) | |
| 2D | - | 78 (9.3) |
| Treatment | Patients, n | HCC, n | PY of Follow-Up * | Incidence of HCC per 100 PY (95% CI) | Crude HR (95% CI) | p Value | Adjusted HR ** (95% CI) | p Value |
|---|---|---|---|---|---|---|---|---|
| Within the First Two Years | ||||||||
| Sofosbuvir | ||||||||
| - Free | 835 | 15 | 1539.4 | 0.97 (0.58–1.62) | 1 | 0.19 | 1 | 0.06 |
| - Based | 835 | 23 | 1534.8 | 1.50 (0.99–2.25) | 1.55 (0.81–2.96) | 2.05 (0.98–4.25) | ||
| Ribavirin *** | ||||||||
| - Free | 500 | 16 | 909.8 | 1.76 (1.06–2.86) | 1 | 0.33 | 1 | 0.43 |
| - Based | 335 | 7 | 625.1 | 1.12 (0.49–2.34) | 0.64 (0.26–1.56) | 0.67 (0.25–1.81) | ||
| Entire Follow-Up Period | ||||||||
| Sofosbuvir | ||||||||
| - Free | 835 | 32 | 2565.1 | 1.25 (0.88–1.76) | 1 | 0.90 | 1 | 0.39 |
| - Based | 835 | 37 | 2918.8 | 1.27 (0.92–1.75) | 0.97 (0.60–1.56) | 1.26 (0.75–2.12) | ||
| Ribavirin *** | ||||||||
| - Free | 500 | 28 | 1710.3 | 1.64 (1.13–2.37) | 1 | 0.046 | 1 | 0.17 |
| - Based | 335 | 9 | 1208.4 | 0.74 (0.37–1.43) | 0.47 (0.22–0.99) | 0.56 (0.24–1.29) | ||
| Characteristics | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | |
| Age | ||||
| <70 | 1 (Referent) | <0.001 | 1 (Referent) | 0.001 |
| ≥70 | 2.30 (1.42–3.73) | 2.39 (1.41–4.06) | ||
| Sex | ||||
| Female | 1 (Referent) | 0.034 | 1 (Referent) | 0.006 |
| Male | 1.67 (1.04–2.68) | 2.07 (1.23–3.48) | ||
| Body Mass Index (kg/m2) | ||||
| <25 | 1 (Referent) | 0.85 | ||
| ≥25 | 0.95 (0.55–1.64) | |||
| Fibrosis Status | 1 (Referent) | 1 (Referent) | ||
| Non-Cirrhosis | 3.56 (2.21–5.79) | <0.001 | 2.97 (1.78–4.92) | <0.001 |
| Cirrhosis | ||||
| Diabetes | 1.08 (0.57–2.06) | 0.82 | ||
| History of HCV Treatment | ||||
| Treatment-Naïve | 1 (Referent) | 0.016 | 1 (Referent) | 0.057 |
| Treatment-Experienced | 1.82 (1.13–2.94) | 1.65 (0.97–2.80) | ||
| HCV RNA (log10 IU/mL) | ||||
| <6.0 | 1 (Referent) | 0.43 | ||
| ≥6.0 | 1.22 (0.75–1.99) | |||
| Serum Albumin (g/dL) at pw12 | ||||
| >3.5 | 1 (Referent) | 0.007 | 1 (Referent) | 0.003 |
| ≤3.5 | 2.86 (1.46–5.58) | 2.72 (1.39–5.33) | ||
| ALT (U/L) at pw12 | ||||
| <30 | 1 (Referent) | 0.078 | 1 (Referent) | 0.57 |
| ≥30 | 1.70 (0.94–3.05) | 1.20 (0.64–2.27) | ||
| α-Fetoprotein (ng/mL) at pw12 | ||||
| ≤7 | 1 (Referent) | < 0.001 | 1 (Referent) | <0.001 |
| >7 | 6.17 (3.72–10.2) | 4.92 (2.72–8.88) | ||
| DAA Regimen | ||||
| Sofosbuvir-Based | 1 (Referent) | 0.90 | ||
| Sofosbuvir-Free | 0.97 (0.60–1.56) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, E.; Nomura, H.; Nakamuta, M.; Furusyo, N.; Kajiwara, E.; Dohmen, K.; Kawano, A.; Ooho, A.; Azuma, K.; Takahashi, K.; et al. Incidence of Hepatocellular Carcinoma after Treatment with Sofosbuvir-Based or Sofosbuvir-Free Regimens in Patients with Chronic Hepatitis C. Cancers 2020, 12, 2602. https://doi.org/10.3390/cancers12092602
Ogawa E, Nomura H, Nakamuta M, Furusyo N, Kajiwara E, Dohmen K, Kawano A, Ooho A, Azuma K, Takahashi K, et al. Incidence of Hepatocellular Carcinoma after Treatment with Sofosbuvir-Based or Sofosbuvir-Free Regimens in Patients with Chronic Hepatitis C. Cancers. 2020; 12(9):2602. https://doi.org/10.3390/cancers12092602
Chicago/Turabian StyleOgawa, Eiichi, Hideyuki Nomura, Makoto Nakamuta, Norihiro Furusyo, Eiji Kajiwara, Kazufumi Dohmen, Akira Kawano, Aritsune Ooho, Koichi Azuma, Kazuhiro Takahashi, and et al. 2020. "Incidence of Hepatocellular Carcinoma after Treatment with Sofosbuvir-Based or Sofosbuvir-Free Regimens in Patients with Chronic Hepatitis C" Cancers 12, no. 9: 2602. https://doi.org/10.3390/cancers12092602
APA StyleOgawa, E., Nomura, H., Nakamuta, M., Furusyo, N., Kajiwara, E., Dohmen, K., Kawano, A., Ooho, A., Azuma, K., Takahashi, K., Satoh, T., Koyanagi, T., Ichiki, Y., Kuniyoshi, M., Yanagita, K., Amagase, H., Morita, C., Sugimoto, R., Kato, M., ... Hayashi, J. (2020). Incidence of Hepatocellular Carcinoma after Treatment with Sofosbuvir-Based or Sofosbuvir-Free Regimens in Patients with Chronic Hepatitis C. Cancers, 12(9), 2602. https://doi.org/10.3390/cancers12092602





