Survival in Cytologically Proven Node-Positive Breast Cancer Patients with Nodal Pathological Complete Response after Neoadjuvant Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Clinicopathological Characteristics and Survival Outcomes According to Pathological LN Response to NAC
2.2. Clinicopathological Factors Associated with Axillary pCR
2.3. Survival Outcomes between Patients with Axillary pCR and Matched Pairs with Axillary pN- without NAC Using Propensity Score Matching
3. Discussion
4. Materials and Methods
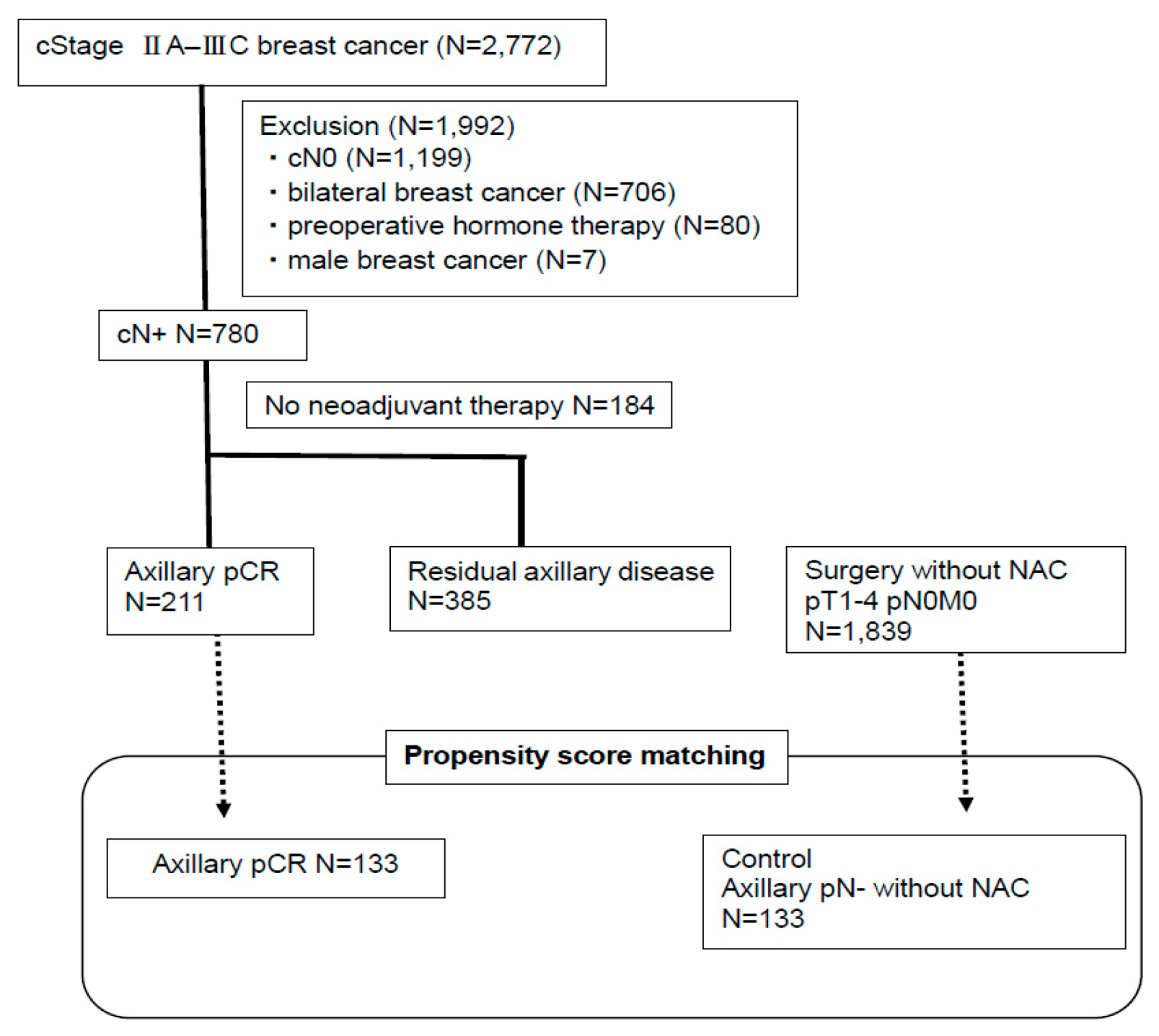
4.1. Patients
4.2. Definition of Clinical LN Status at Diagnosis
4.3. NAC
4.4. Adjuvant Therapy
4.5. Nodal Surgery and LN Pathology
4.6. Definition of Breast-pCR
4.7. Immunohistochemical (IHC) Analysis
4.8. Follow-Up Data
4.9. Propensity Score Matching
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiechmann, L.; Sampson, M.; Stempel, M.; Jacks, L.M.; Patil, S.M.; King, T.; Morrow, M. Presenting features of breast cancer differ by molecular subtype. Ann. Surg Oncol. 2009, 16, 2705–2710. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Dale, P.S.; Turner, R.R.; Morton, D.L.; Evans, S.W.; Krasne, D.L. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann. Surg. 1995, 222, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E.; Smeets, A.; Laenen, A.; Reynders, A.; Soens, J.; Van Ongeval, C.; Moerman, P.; Paridaens, R.; Wildiers, H.; Neven, P.; et al. Predictors of axillary lymph node metastases in early breast cancer and their applicability in clinical practice. Breast 2013, 22, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Viale, G.; Zurrida, S.; Maiorano, E.; Mazzarol, G.; Pruneri, G.; Paganelli, G.; Maisonneuve, P.; Veronesi, U. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer 2005, 103, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Crabb, S.J.; Cheang, M.C.; Leung, S.; Immonen, T.; Nielsen, T.O.; Huntsman, D.D.; Bajdik, C.D.; Chia, S.K. Basal breast cancer molecular subtype predicts for lower incidence of axillary lymph node metastases in primary breast cancer. Clin. Breast Cancer 2008, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yang, Z.; Liu, X.; Niu, Y. Lymph node status in different molecular subtype of breast cancer: Triple negative tumours are more likely lymph node negative. Oncotarget 2017, 8, 55534–55543. [Google Scholar] [CrossRef][Green Version]
- Yin, L.; Shuang, H.; Sheng, C.; Liang, H.; Sun, X.J.; Yang, W.T.; Shao, Z.M. The Prognostic Value of Nodal Staging in Triple-Negative Breast Cancer-A Cohort from China. Sci. Rep. 2018, 8, 9007. [Google Scholar] [CrossRef]
- Hernandez-Aya, L.F.; Chavez-Macgregor, M.; Lei, X.; Meric-Bernstam, F.; Buchholz, T.A.; Hsu, L.; Sahin, A.A.; Do, K.A.; Valero, V.; Hortobagyi, G.N.; et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J. Clin. Oncol. 2011, 29, 2628–2634. [Google Scholar] [CrossRef]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Fisher, B.; Bryant, J.; Wolmark, N.; Mamounas, E.; Brown, A.; Fisher, E.R.; Wickerham, D.L.; Begovic, M.; DeCillis, A.; Robidoux, A.; et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 1998, 16, 2672–2685. [Google Scholar] [CrossRef]
- Van Nes, J.G.; Putter, H.; Julien, J.P.; Tubiana-Hulin, M.; van de Vijver, M.; Bogaerts, J.; de Vos, M.; van de Velde, C.J.; Cooperating Investigators of the EORTC. Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up; clinical and translational results from the EORTC trial 10902. Breast Cancer Res. Treat. 2009, 115, 101–113. [Google Scholar] [CrossRef]
- Wolmark, N.; Wang, J.; Mamounas, E.; Bryant, J.; Fisher, B. Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J. Natl. Cancer Inst. Monogr. 2001, 2001, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Anderson, S.J.; Bear, H.D.; Geyer, C.E.; Kahlenberg, M.S.; Robidoux, A.; Margolese, R.G.; Hoehn, J.L.; Vogel, V.G.; Dakhil, S.R.; et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 2008, 26, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Fukada, I.; Araki, K.; Kobayashi, K.; Shibayama, T.; Takahashi, S.; Horii, R.; Akiyama, F.; Iwase, T.; Ohno, S.; Hatake, K.; et al. Predictive Factors and Value of ypN+ after Neoadjuvant Chemotherapy in Clinically Lymph Node-Negative Breast Cancer. PLoS ONE 2016, 11, e0162616. [Google Scholar] [CrossRef][Green Version]
- Fukuda, T.; Horii, R.; Gomi, N.; Miyagi, Y.; Takahashi, S.; Ito, Y.; Akiyama, F.; Ohno, S.; Iwase, T. Accuracy of magnetic resonance imaging for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy: Association with breast cancer subtype. Springerplus 2016, 5, 152. [Google Scholar] [CrossRef]
- Fukada, I.; Araki, K.; Kobayashi, K.; Shibayama, T.; Takahashi, S.; Gomi, N.; Kokubu, Y.; Oikado, K.; Horii, R.; Akiyama, F.; et al. Pattern of Tumor Shrinkage during Neoadjuvant Chemotherapy Is Associated with Prognosis in Low-Grade Luminal Early Breast Cancer. Radiology 2018, 286, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Fayanju, O.M.; Ren, Y.; Thomas, S.M.; Greenup, R.A.; Plichta, J.K.; Rosenberger, L.H.; Tamirisa, N.; Force, J.; Boughey, J.C.; Hyslop, T.; et al. The Clinical Significance of Breast-only and Node-only Pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT): A Review of 20,000 Breast Cancer Patients in the National Cancer Data Base (NCDB). Ann. Surg. 2018, 268, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.C.; Zhang, Y.F.; Xu, F.P.; Qian, X.K.; Guo, Z.B.; Ren, C.Y.; Yao, M. Axillary lymph node status, adjusted for pathologic complete response in breast and axilla after neoadjuvant chemotherapy, predicts differential disease-free survival in breast cancer. Curr. Oncol. 2013, 20, e180–e192. [Google Scholar] [CrossRef] [PubMed]
- Mougalian, S.S.; Hernandez, M.; Lei, X.; Lynch, S.; Kuerer, H.M.; Symmans, W.F.; Theriault, R.L.; Fornage, B.D.; Hsu, L.; Buchholz, T.A.; et al. Ten-Year Outcomes of Patients with Breast Cancer with Cytologically Confirmed Axillary Lymph Node Metastases and Pathologic Complete Response After Primary Systemic Chemotherapy. JAMA Oncol. 2016, 2, 508–516. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Hortobagyi, G.N.; Rouzier, R.; Kuerer, H.; Sneige, N.; Buzdar, A.U.; Kau, S.W.; Fornage, B.; Sahin, A.; Broglio, K.; et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J. Clin. Oncol. 2005, 23, 9304–9311. [Google Scholar] [CrossRef] [PubMed]
- Meric, F.; Mirza, N.Q.; Buzdar, A.U.; Hunt, K.K.; Ames, F.C.; Ross, M.I.; Pollock, R.E.; Newman, L.A.; Feig, B.W.; Strom, E.A.; et al. Prognostic implications of pathological lymph node status after preoperative chemotherapy for operable T3N0M0 breast cancer. Ann. Surg. Oncol. 2000, 7, 435–440. [Google Scholar] [CrossRef] [PubMed]
- van Nijnatten, T.J.; Simons, J.M.; Moossdorff, M.; de Munck, L.; Lobbes, M.B.; van der Pol, C.C.; Koppert, L.B.; Luiten, E.J.; Smidt, M.L. Prognosis of residual axillary disease after neoadjuvant chemotherapy in clinically node-positive breast cancer patients: Isolated tumor cells and micrometastases carry a better prognosis than macrometastases. Breast Cancer Res. Treat. 2017, 163, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Samiei, S.; van Nijnatten, T.J.A.; de Munck, L.; Keymeulen, K.B.M.I.; Simons, J.M.; Kooreman, L.F.S.; Siesling, S.; Lobbes, M.B.I.; Smidt, M.L. Correlation Between Pathologic Complete Response in the Breast and Absence of Axillary Lymph Node Metastases After Neoadjuvant Systemic Therapy. Ann. Surg. 2020, 271, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Neuman, H.; Mamounas, E.P.; Bedrosian, I.; Moulder, S.; Montero, A.J.; Jagsi, R. Debating the Optimal Approach to Nodal Management After Pathologic Complete Response to Neoadjuvant Chemotherapy in Patients with Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 42–48. [Google Scholar] [CrossRef]
- Barron, A.U.; Hoskin, T.L.; Day, C.N.; Hwang, E.S.; Kuerer, H.M.; Boughey, J.C. Association of Low Nodal Positivity Rate Among Patients with ERBB2-Positive or Triple-Negative Breast Cancer and Breast Pathologic Complete Response to Neoadjuvant Chemotherapy. JAMA Surg. 2018, 153, 1120–1126. [Google Scholar] [CrossRef]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef]
- Classe, J.M.; Loaec, C.; Gimbergues, P.; Alran, S.; de Lara, C.T.; Dupre, P.F.; Rouzier, R.; Faure, C.; Paillocher, N.; Chauvet, M.P.; et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: The GANEA 2 study. Breast Cancer Res. Treat 2019, 173, 343–352. [Google Scholar] [CrossRef]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Boileau, J.F.; Poirier, B.; Basik, M.; Holloway, C.M.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J. Clin. Oncol. 2015, 33, 258–264. [Google Scholar] [CrossRef]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; DeSnyder, S.M.; Hwang, R.F.; et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection. J. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef]
- Mamtani, A.; Barrio, A.V.; King, T.A.; Van Zee, K.J.; Plitas, G.; Pilewskie, M.; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.S.; Sclafani, L.M.; et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients with Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann. Surg. Oncol. 2016, 23, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Mo, M.; Yu, K.D.; Chen, C.M.; Hu, Z.; Hou, Y.F.; Di, G.H.; Wu, J.; Shen, Z.Z.; Shao, Z.M.; et al. ER-poor and HER2-positive: A potential subtype of breast cancer to avoid axillary dissection in node positive patients after neoadjuvant chemo-trastuzumab therapy. PLoS ONE. 2014, 9, e114646. [Google Scholar] [CrossRef]
- Tadros, A.B.; Yang, W.T.; Krishnamurthy, S.; Rauch, G.M.; Smith, B.D.; Valero, V.; Black, D.M.; Lucci, A., Jr.; Caudle, A.S.; DeSnyder, S.M.; et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 2017, 152, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Ogiya, A.; Kimura, K.; Nakashima, E.; Sakai, T.; Miyagi, Y.; Iijima, K.; Morizono, H.; Makita, M.; Horii, R.; Akiyama, F.; et al. Long-term prognoses and outcomes of axillary lymph node recurrence in 2578 sentinel lymph node-negative patients for whom axillary lymph node dissection was omitted: Results from one Japanese hospital. Breast Cancer 2016, 23, 318–322. [Google Scholar] [CrossRef]
- Stachs, A.; Thi, A.T.; Dieterich, M.; Stubert, J.; Hartmann, S.; Glass, Ä.; Reimer, T.; Gerber, B. Assessment of Ultrasound Features Predicting Axillary Nodal Metastasis in Breast Cancer: The Impact of Cortical Thickness. Ultrasound Int. Open. 2015, 1, E19–E24. [Google Scholar] [CrossRef]
- The Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer 2018. Available online: http://jbcs.gr.jp/guidline/2018/index/ (accessed on 4 January 2019).
- Japanese Breast Cancer Society. General Rules for Clinical and Pathological Recording of Breast Cancer, 18th ed.; Japanese Breast Cancer Society: Kanahara, Japan, 2018; p. 94. [Google Scholar]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley: New York, NY, USA, 2017; pp. 151–159. [Google Scholar]
- Hao, S.; Zhao, Y.Y.; Peng, J.J.; Ren, F.; Yang, W.T.; Yu, K.D.; Shao, Z.M. Invasive micropapillary carcinoma of the breast had no difference in prognosis compared with invasive ductal carcinoma: A propensity-matched analysis. Sci. Rep. 2019, 9, 286. [Google Scholar] [CrossRef]
- Li, F.; Zaslavsky, A.M.; Landrum, M.B. Propensity score weighting with multilevel data. Stat. Med. 2013, 32, 3373–3387. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]



| Characteristics | Patients with Axillary pCR (n = 211) | Patients with Residual Axillary Disease (n = 385) | p-Value |
|---|---|---|---|
| Age, years (mean ± SD) | 50.7 ± 10.9 | 51.1 ± 10.8 | 0.65 |
| Menopausal status | |||
| Pre- | 106 (50%) | 195 (51%) | 0.93 |
| Post- | 105 (50%) | 190 (49%) | |
| Clinical T stage a | |||
| T1 | 31 (14.5%) | 48 (13%) | 0.02 |
| T2 | 139 (66%) | 221 (57%) | |
| T3 | 24(11%) | 67 (17%) | |
| T4 | 16 (8%) | 49 (13%) | |
| TX | 1 (0.5%) | 0 | |
| Clinical N stage a | |||
| N1 | 162 (77%) | 306 (80%) | 0.65 |
| N2 | 9 (4%) | 18 (4%) | |
| N3 | 40 (19%) | 61 (16%) | |
| Clinical stage a | |||
| II | 137 (65%) | 224 (58%) | 0.12 |
| III | 74 (35%) | 161 (42%) | |
| ER status | |||
| Negative | 115 (55%) | 70 (18%) | <0.01 |
| Positive | 83 (39%) | 295 (77%) | |
| Unknown | 13 (6%) | 20 (5%) | |
| HER2 status | |||
| Negative | 90 (43%) | 288 (75%) | <0.01 |
| Positive | 100 (47%) | 52 (13%) | |
| Unknown | 21 (10%) | 45 (12%) | |
| NG | |||
| 1 | 47 (22%) | 170 (44%) | <0.01 |
| 2 | 58 (28%) | 111 (29%) | |
| 3 | 82 (38%) | 56 (15%) | |
| Unknown | 24 (12%) | 48 (12%) | |
| Type of surgery | |||
| Partial mastectomy | 86 (40%) | 70 (18%) | <0.01 |
| Mastectomy | 125 (60%) | 315 (82%) | |
| Breast pCR | |||
| No | 120 (57%) | 372 (97%) | <0.01 |
| Yes | 91 (43%) | 13 (3%) | |
| LI status of the surgical specimens | |||
| Negative | 179 (85%) | 211 (55%) | <0.01 |
| Positive | 32 (15%) | 174 (45%) | |
| Pre and Postoperative Chemotherapy | |||
| Anthracycline | 6 (3%) | 11 (3%) | 0.29 |
| Taxane | 3 (2%) | 1 (0/3%) | |
| Anthracycline followed by taxane | 202 (95%) | 373 (96.7%) | |
| Pre and Postoperative Trastuzumab for HER2-positive disease | |||
| Yes | 95 (95%) | 48 (92%) | 0.90 |
| No | 5 (5%) | 4 (8%) | |
| Hormone therapy after surgery for ER-positive disease | |||
| Yes | 81 (98%) | 289 (98%) | 0.90 |
| No | 2 (2%) | 6 (2%) | |
| Radiotherapy after surgery | |||
| Yes | 141 (67%) | 281 (73%) | 0.13 |
| No | 70 (33%) | 104 (27%) |
| Characteristics | Hazard Ratio | 95%CI | p-Value |
|---|---|---|---|
| Pathological lymph node status after NAC | |||
| Axillary pCR | 1 | ||
| Residual axillary disease | 4.55 | 2.48–8.35 | <0.01 |
| Clinical stage a | |||
| II | 1 | ||
| III | 2.92 | 1.96–4.35 | <0.01 |
| ER status | |||
| Negative | 1 | ||
| Positive | 0.60 | 0.37–0.96 | 0.03 |
| HER2 status | |||
| Negative | 1 | ||
| Positive | 0.92 | 0.77–1.09 | 0.35 |
| NG | |||
| 1,2 | 1 | ||
| 3 | 1.23 | 0.96–1.57 | 0.10 |
| Characteristics | Odds Ratio | 95%CI | p-Value |
|---|---|---|---|
| Menopausal status | |||
| Pre- | 1 | ||
| Post- | 0.63 | 0.37–1.04 | 0.07 |
| Clinical T a | |||
| 1 | 1 | ||
| 2–4 | 0.91 | 0.40–1.29 | 0.79 |
| Clinical N a | |||
| 1 | 1 | ||
| 2,3 | 0.72 | 0.40–1.29 | 0.27 |
| ER status | |||
| Negative | 1 | ||
| Positive | 0.40 | 0.23–0.70 | <0.01 |
| HER2 status | |||
| Negative | 1 | ||
| Positive | 1.49 | 1.24–1.78 | <0.01 |
| NG | |||
| 1,2 | 1 | ||
| 3 | 1.51 | 1.15–2.00 | <0.01 |
| Breast-pCR | |||
| No | 1 | ||
| Yes | 3.81 | 2.52–5.76 | <0.01 |
| Characteristics | Axillary pCR (n = 133) | Axillary pN- without NAC (n = 133) | p-Value |
|---|---|---|---|
| Menopausal status | |||
| Pre- | 68 | 69 | 1.00 |
| Post- | 65 | 64 | |
| Clinical T a | |||
| 1 | 23 | 27 | 0.78 |
| 2 | 102 | 99 | |
| 3 | 7 | 5 | |
| 4 | 1 | 2 | |
| ER status | |||
| Negative | 71 | 67 | 0.62 |
| Positive | 62 | 66 | |
| HER2 status | |||
| Negative | 73 | 81 | 0.38 |
| Positive | 60 | 52 | |
| NG | |||
| 1 | 31 | 33 | 0.18 |
| 2 | 35 | 47 | |
| 3 | 67 | 53 | |
| Type of surgery | |||
| Partial mastectomy | 61 | 70 | 0.33 |
| Mastectomy | 72 | 63 | |
| Pre and Postoperative Chemotherapy | |||
| No | 0 | 61 | <0.01 |
| Anthracycline | 5 | 63 | |
| Taxane | 2 | 3 | |
| Anthracycline followed by taxane | 126 | 3 | |
| Others | 0 | 3 | |
| Pre and Postoperative Trastuzumab for HER2-positive disease | |||
| Yes | 58 | 38 | 0.02 |
| No | 75 | 95 | |
| Hormone therapy after surgery for ER-positive disease | |||
| Yes | 61 | 52 | 0.39 |
| No | 72 | 81 | |
| Radiotherapy after surgery | |||
| Yes | 88 | 38 | <0.01 |
| No | 45 | 95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inari, H.; Teruya, N.; Kishi, M.; Horii, R.; Akiyama, F.; Takahashi, S.; Ito, Y.; Ueno, T.; Iwase, T.; Ohno, S. Survival in Cytologically Proven Node-Positive Breast Cancer Patients with Nodal Pathological Complete Response after Neoadjuvant Chemotherapy. Cancers 2020, 12, 2633. https://doi.org/10.3390/cancers12092633
Inari H, Teruya N, Kishi M, Horii R, Akiyama F, Takahashi S, Ito Y, Ueno T, Iwase T, Ohno S. Survival in Cytologically Proven Node-Positive Breast Cancer Patients with Nodal Pathological Complete Response after Neoadjuvant Chemotherapy. Cancers. 2020; 12(9):2633. https://doi.org/10.3390/cancers12092633
Chicago/Turabian StyleInari, Hitoshi, Natsuki Teruya, Miki Kishi, Rie Horii, Futoshi Akiyama, Shunji Takahashi, Yoshinori Ito, Takayuki Ueno, Takuji Iwase, and Shinji Ohno. 2020. "Survival in Cytologically Proven Node-Positive Breast Cancer Patients with Nodal Pathological Complete Response after Neoadjuvant Chemotherapy" Cancers 12, no. 9: 2633. https://doi.org/10.3390/cancers12092633
APA StyleInari, H., Teruya, N., Kishi, M., Horii, R., Akiyama, F., Takahashi, S., Ito, Y., Ueno, T., Iwase, T., & Ohno, S. (2020). Survival in Cytologically Proven Node-Positive Breast Cancer Patients with Nodal Pathological Complete Response after Neoadjuvant Chemotherapy. Cancers, 12(9), 2633. https://doi.org/10.3390/cancers12092633