Prognostic and Predictive Value of Tumor-Infiltrating Immune Cells in Urothelial Cancer of the Bladder
Abstract
Simple Summary
Abstract
1. Introduction
2. Prognostic Value of Tumor-Infiltrating Immune Cells
2.1. CD3+ and CD8+ T Cells
2.2. B Cells
2.3. Dendritic Cells
2.4. Natural Killer Cells
2.5. Tregs
2.6. TAMs
2.7. MDSCs
2.8. Neutrophils
2.9. Eosinophils
2.10. Mast Cells
2.11. Immune Checkpoint Molecules
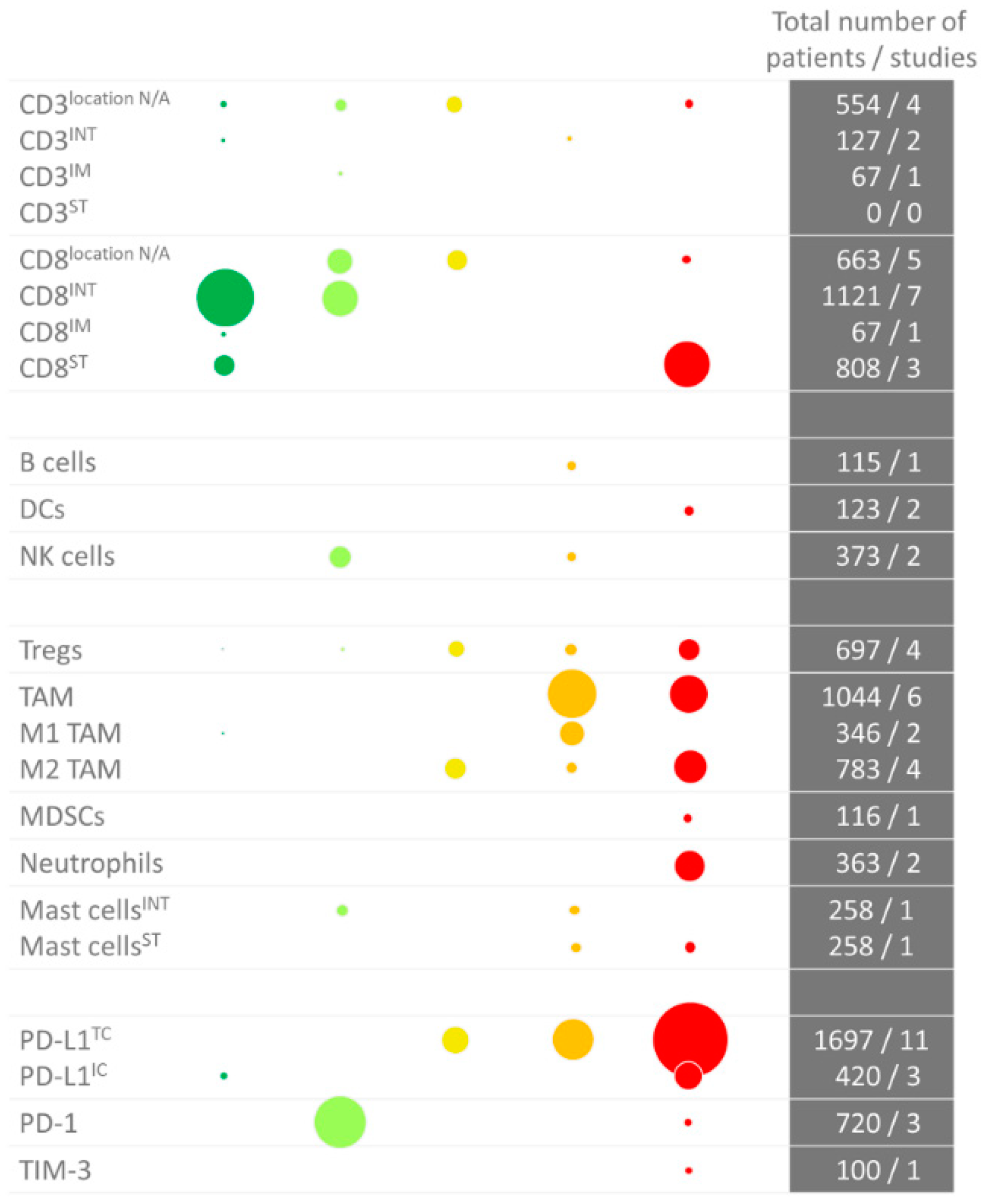
2.12. Summary
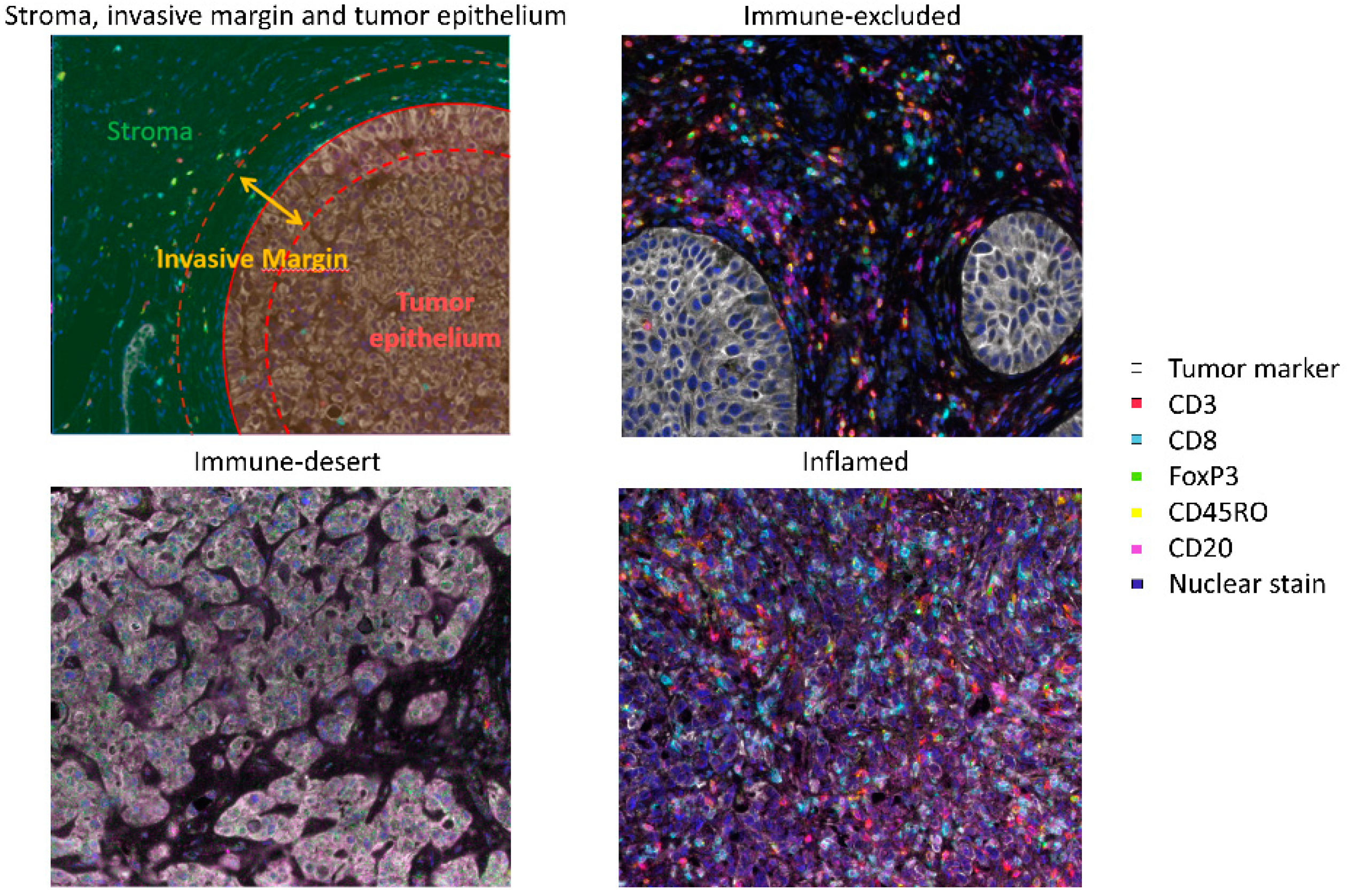
- Immunohistochemistry: A common method to quantify tumor-infiltrating immune cells is immunohistochemistry (IHC). Most studies included in this review used single-marker IHC. An advantage of IHC is the ability to study immune cells in their spatial context, which makes it possible to distinguish between immune cells located in the tumor epithelium, invasive margin or surrounding stroma. A disadvantage of single-marker IHC is that it utilizes only one marker per test, whereas, for the phenotypic characterization of some cell types (i.e., MDSCs), multiple markers are needed. However, recent advances in multiplex immunohistochemistry and multispectral imaging now enable the simultaneous analysis of multiple tissue markers. Another disadvantage of single-marker IHC is that it is laborious and has a low throughput. Although advances are made in the automated analysis of IHC images, stainings are still often visually assessed by pathologists. Most studies included in this review used either 1.0-mm tissue microarrays (TMAs) or selected a limited number of fields from whole slides for analyses (mostly 0.07 mm2/field). It is questionable whether these small regions accurately reflect the tumor immune infiltrate. A recent study in NMIBC reported that two to six 0.6-mm TMAs are needed to provide a correct sampling of NMIBC tumors because of spatial heterogeneity [26].
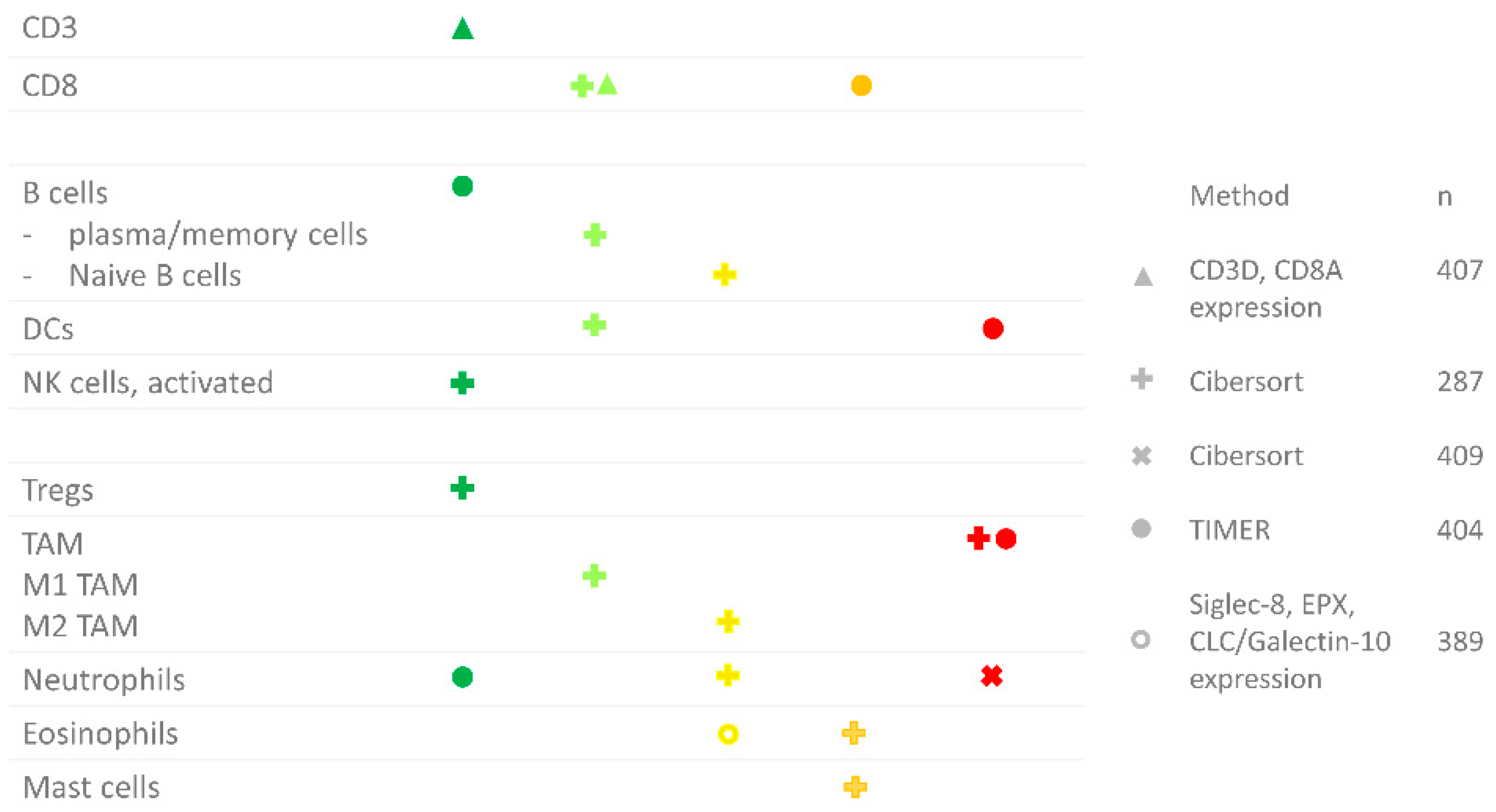
- RNA sequencing: Various computational tools have been developed to estimate the relative abundance of immune cells in the tumor using bulk RNA data. Studies included in this review have used CIBERSORT, TIMER or measured CD8A and CD3D gene expression. Where CIBERSORT determines the fraction of 22 immune cell types relative to the total immune cell content, TIMER measures the abundance of six immune cell types. Currently available computational tools have shown good correlations for CD8+ T cells, B cell, NK cells and macrophages. However, nonregulatory and regulatory CD4+ cells are hard to distinguish. Moreover, DCs cannot be accurately quantified, and none of the current methods take MDSCs into account [33]. A disadvantage of the use of bulk RNA data for immune cell quantification is that it is not possible to study the location of the immune cells.
3. The Predictive Value of Tumor-Infiltrating Immune Cells for Response to Therapy
3.1. Immune Checkpoint Inhibitors
3.2. Chemotherapy
4. Conclusions
Funding
Conflicts of Interest
References
- Fridman, W.H.; Zitvogel, L.; Sautès–Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, V.; Chin, J.L.; Izawa, J.I. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. J. Can. Urol. Assoc. 2009, 3, S193. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.E.N.; Kates, M.R.; Bivalacqua, T.J. Identification of Candidates for Salvage Therapy: The Past, Present, and Future of Defining Bacillus Calmette-Guérin Failure. Urol. Clin. 2020, 47, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Joshi, M.; Meijer, R.P.; Glantz, M.; Holder, S.; Harvey, H.A.; Kaag, M.; Fransen van de Putte, E.E.; Horenblas, S.; Drabick, J.J. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist 2016, 21, 708–715. [Google Scholar] [CrossRef]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2018, 36, JCO1801148. [Google Scholar] [CrossRef]
- Powles, T.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van der Heijden, M.S.; Font Pous, A.; Maillet, D. A phase II study investigating the safety and efficacy of neoadjuvant atezolizumab in muscle invasive bladder cancer (ABACUS). Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.J.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 2019, 30, 970–976. [Google Scholar] [CrossRef]
- Sjödahl, G.; Lövgren, K.; Lauss, M.; Chebil, G.; Patschan, O.; Gudjonsson, S.; Månsson, W.; Fernö, M.; Leandersson, K.; Lindgren, D.; et al. Infiltration of CD3+ and CD68+ cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 791–797. [Google Scholar] [CrossRef]
- Li, X.-D.; Huang, C.-W.; Liu, Z.-F.; Jiang, L.-J.; Chen, J.-W.; Xie, D.; Zhou, F.-J.; Lu, H.-M.; Liu, Z.-W. Prognostic Role of the Immunoscore for Patients with Urothelial Carcinoma of the Bladder Who Underwent Radical Cystectomy. Ann. Surg. Oncol. 2019. [Google Scholar] [CrossRef]
- Krpina, K.; Babarović, E.; Jonjić, N. Correlation of tumor-infiltrating lymphocytes with bladder cancer recurrence in patients with solitary low-grade urothelial carcinoma. Virchows Arch. 2015, 467, 443–448. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, K.; Wang, L.; Sun, E. The prognostic values of tumor-infiltrating neutrophils, lymphocytes and neutrophil/lymphocyte rates in bladder urothelial cancer. Pathol. Res. Pract. 2018, 214, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Severson, E.; Pignon, J.-C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.J.; Meng, X.Y.; Wu, Q.J.; Zhou, X.H. High CD3D/CD4 ratio predicts better survival in muscle-invasive bladder cancer. Cancer Manag. Res. 2019, 11, 2987–2995. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pan, W.; Yang, M.; Yang, W.; He, W.; Chen, X.; Bi, J.; Jiang, N.; Huang, J.; Lin, T. Programmed death ligand-1 is associated with tumor infiltrating lymphocytes and poorer survival in urothelial cell carcinoma of the bladder. Cancer Sci. 2019, 110, 489–498. [Google Scholar] [CrossRef]
- Horn, T.; Laus, J.; Seitz, A.K.; Maurer, T.; Schmid, S.C.; Wolf, P.; Haller, B.; Winkler, M.; Retz, M.; Nawroth, R.; et al. The prognostic effect of tumour-infiltrating lymphocytic subpopulations in bladder cancer. World J. Urol. 2016, 34, 181–187. [Google Scholar] [CrossRef]
- Wang, B.; Lin, J.; Yu, H.; Zeng, H.; Lin, T. Distribution and prognostic significance of CD8+ T cells in urothelial cell carcinoma of the bladder. Chin. J. Urol. 2015. [Google Scholar] [CrossRef]
- Asano, T.; Ohnishi, K.; Shiota, T.; Motoshima, T.; Sugiyama, Y.; Yatsuda, J.; Kamba, T.; Ishizaka, K.; Komohara, Y. CD169-positive sinus macrophages in the lymph nodes determine bladder cancer prognosis. Cancer Sci. 2018, 109, 1723–1730. [Google Scholar] [CrossRef]
- Yu, A.; Mansure, J.J.; Solanki, S.; Siemens, D.R.; Koti, M.; Dias, A.B.T.; Burnier, M.M.; Brimo, F.; Kassouf, W. Presence of lymphocytic infiltrate cytotoxic T lymphocyte CD3+, CD8+, and immunoscore as prognostic marker in patients after radical cystectomy. PLoS ONE 2018, 13, e0205746. [Google Scholar] [CrossRef]
- Otto, W.; Denzinger, S.; Wieland, W.F.; Hartmann, A. First analysis of immune cell infiltration in stage pT1 urothelial bladder carcinoma: CD3 positivity as a prognostic marker for cancer-specific survival. World J. Urol. 2012, 30, 875–877. [Google Scholar] [CrossRef]
- Winerdal, M.E.; Marits, P.; Winerdal, M.; Hasan, M.; Rosenblatt, R.; Tolf, A.; Selling, K.; Sherif, A.; Winqvist, O. FOXP3 and survival in urinary bladder cancer. BJU Int. 2011, 108, 1672–1678. [Google Scholar] [CrossRef]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef] [PubMed]
- Faraj, S.F.; Munari, E.; Guner, G.; Taube, J.; Anders, R.; Hicks, J.; Meeker, A.; Schoenberg, M.; Bivalacqua, T.; Drake, C.; et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology 2015, 85, 703. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fu, H.; Liu, Z.; Zhang, J.; Ye, D. Immune-desert, immune-excluded and inflamed phenotypes predict survival and adjuvant chemotherapy response in patients with MIBC. Eur. Urol. Suppl. 2018, 17, 128–130. [Google Scholar] [CrossRef]
- Fu, H.; Zhu, Y.; Wang, Y.; Liu, Z.; Zhang, J.; Xie, H.; Fu, Q.; Dai, B.; Ye, D.; Xu, J. Identification and Validation of Stromal Immunotype Predict Survival and Benefit from Adjuvant Chemotherapy in Patients with Muscle-Invasive Bladder Cancer. Clin. Cancer Res. 2018, 24, 3069–3078. [Google Scholar] [CrossRef]
- Wang, B.; Wu, S.; Zeng, H.; Liu, Z.; Dong, W.; He, W.; Chen, X.; Dong, X.; Zheng, L.; Lin, T.; et al. CD103+ Tumor Infiltrating Lymphocytes Predict a Favorable Prognosis in Urothelial Cell Carcinoma of the Bladder. J. Urol. 2015, 194, 556–562. [Google Scholar] [CrossRef]
- Masson-Lecomte, A.; Maillé, P.; Pineda, S.; Soyeux, P.; Sagrera, A.; Rava, M.; Lopez De Maturana, E.; Márquez, M.; Tardón, A.; Carrato, A.; et al. CD8+ Cytotoxic Immune Infiltrate in Non-Muscle Invasive Bladder Cancer: A Standardized Methodology to Study Association with Clinico-Pathological Features and Prognosis. Bladder Cancer 2019, 5, 159–169. [Google Scholar] [CrossRef]
- Zhang, Q.; Hao, C.; Cheng, G.; Wang, L.; Wang, X.; Li, C.; Qiu, J.; Ding, K. High CD4+ T cell density is associated with poor prognosis in patients with non-muscle-invasive bladder cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11510–11516. [Google Scholar]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Ayari, C.; Larue, H.; Hovington, H.; Caron, A.; Bergeron, A.; Têtu, B.; Fradet, V.; Fradet, Y. High level of mature tumor-infiltrating dendritic cells predicts progression to muscle invasion in bladder cancer. Hum. Pathol. 2013, 44, 1630–1637. [Google Scholar] [CrossRef]
- Ayari, C.; LaRue, H.; Hovington, H.; Decobert, M.; Harel, F.; Bergeron, A.; Têtu, B.; Lacombe, L.; Fradet, Y. Bladder tumor infiltrating mature dendritic cells and macrophages as predictors of response to bacillus Calmette-Guérin immunotherapy. Eur. Urol. 2009, 55, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ju, X.; Silveira, P.A.; Abadir, E.; Hsu, W.H.; Hart, D.N.J.; Clark, G.J. CD83: Activation marker for antigen presenting cells and its therapeutic potential. Front. Immunol. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.; Finotello, F.; Petitprez, F.; Zhang, J.D.; Baumbach, J.; Fridman, W.H.; List, M.; Aneichyk, T. Comprehensive evaluation of transcriptome-based cell-type quantification methods for immuno-oncology. Bioinformatics 2019, 35, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Ji, N.; Hurez, V.; Curiel, T.J.; Montgomery, M.O.; Braun, A.J.; Nicolas, M.; Aguilera, M.; Kaushik, D.; Liu, Q.; et al. Intratumoral CD56bright natural killer cells are associated with improved survival in bladder cancer. Oncotarget 2018, 9, 36492–36502. [Google Scholar] [CrossRef]
- Michel, T.; Poli, A.; Cuapio, A.; Briquemont, B.; Iserentant, G.; Ollert, M.; Zimmer, J. Human CD56 bright NK Cells: An Update. J. Immunol. 2016, 196, 2923–2931. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.J.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Komohara, Y.; Jinushi, M.; Takeya, M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014, 105, 1–8. [Google Scholar] [CrossRef]
- Asano, K.; Nabeyama, A.; Miyake, Y.; Qiu, C.H.; Kurita, A.; Tomura, M.; Kanagawa, O.; Shin-ichiro, F.; Tanaka, M. CD169-Positive Macrophages Dominate Antitumor Immunity by Crosspresenting Dead Cell-Associated Antigens. Immunity 2011, 34, 85–95. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Dong, X.; Wu, S.; Zeng, H.; Liu, Z.; Wan, D.; Dong, W.; He, W.; Chen, X.; et al. High CD204+ tumor-infiltrating macrophage density predicts a poor prognosis in patients with urothelial cell carcinoma of the bladder. Oncotarget 2015, 6, 20204–202014. [Google Scholar] [CrossRef]
- Takeuchi, H.; Tanaka, M.; Tanaka, A.; Tsunemi, A.; Yamamoto, H. Predominance of M2-polarized macrophages in bladder cancer affects angiogenesis, tumor grade and invasiveness. Oncol. Lett. 2016, 11, 3403–3408. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, L.; Liu, M.; Liu, Q.; Duan, X.; Bo, J. CD163+ macrophages predict a poor prognosis in patients with primary T1 high-grade urothelial carcinoma of the bladder. World J. Urol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Chang, Y.; Wang, Z.; Chen, L.; Kong, Y.; Zhang, P.; Liu, Z.; Zhou, Q.; Chen, Y.; Wang, J.; et al. Tumor-associated macrophages expressing galectin-9 identify immunoevasive subtype muscle-invasive bladder cancer with poor prognosis but favorable adjuvant chemotherapeutic response. Cancer Immunol. Immunother. 2019, 68, 2067–2080. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.; Tian, J.; Man, H.; Li, P.; Shan, B. High expression of B7-H3 and CD163 in cancer tissues indicates malignant clinicopathological status and poor prognosis of patients with urothelial cell carcinoma of the bladder. Oncol. Lett. 2018, 15, 6519–6526. [Google Scholar] [CrossRef] [PubMed]
- Boström, M.M.; Irjala, H.; Mirtti, T.; Taimen, P.; Kauko, T.; Ålgars, A.; Jalkanen, S.; Boström, P.J. Tumor-Associated Macrophages Provide Significant Prognostic Information in Urothelial Bladder Cancer. PLoS ONE 2015, 10, e0133552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ye, Y.-L.; Li, M.-X.; Ye, S.-B.; Huang, W.-R.; Cai, T.-T.; He, J.; Peng, J.-Y.; Duan, T.-H.; Cui, J.; et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene 2017, 36, 2095–2104. [Google Scholar] [CrossRef]
- Ornstein, M.C.; Diaz-Montero, C.M.; Rayman, P.; Elson, P.; Haywood, S.; Finke, J.H.; Kim, J.S.; Pavicic, P.G.; Lamenza, M.; Devonshire, S.; et al. Myeloid-derived suppressors cells (MDSC) correlate with clinicopathologic factors and pathologic complete response (pCR) in patients with urothelial carcinoma (UC) undergoing cystectomy. Urol. Oncol. 2018, 36, 405–412. [Google Scholar] [CrossRef]
- Yang, G.; Shen, W.; Zhang, Y.; Liu, M.; Zhang, L.; Liu, Q.; Lu, H.H.; Bo, J. Accumulation of myeloid-derived suppressor cells (MDSCs) induced by low levels of IL-6 correlates with poor prognosis in bladder cancer. Oncotarget 2017, 8, 38378–38388. [Google Scholar] [CrossRef]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salomé, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-Pereira, S.; Dartiguenave, F.; Fritschi, A.-S.; et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Investig. 2017, 127, 2916–2929. [Google Scholar] [CrossRef]
- Shen, M.; Hu, P.; Donskov, F.; Wang, G.; Liu, Q.; Du, J. Tumor-associated neutrophils as a new prognostic factor in cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e98259. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, L.; Chen, L.; Fu, Q.; Liu, Z.; Chang, Y.; Lin, Z.; Xu, J. Tumor-infiltrating neutrophils predict benefit from adjuvant chemotherapy in patients with muscle invasive bladder cancer. Oncoimmunology 2017, 6, 1293211. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavonid, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2018, 7, 1393134. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.; Calli, A.; Cakalagaoglu, F.; Altınboga, A.A.; Bal, K. Association of mast cells with microvessel density in urothelial carcinomas of the urinary bladder. Ann. Diagn. Pathol. 2012, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Chen, Y.; Yeh, C.-R.; Ding, J.; Li, L.; Chang, C.; Yeh, S. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERβ/CCL2/CCR2 EMT/MMP9 signals. Oncotarget 2016, 7, 7842–7855. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sun, J.; Wang, L.; Li, Z.; Wang, L.; Li, Z. Prognostic and Clinicopathological Significance of PD-L1 in Patients With Bladder Cancer: A Meta-Analysis. Front. Pharmacol. 2019, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Xylinas, E.; Robinson, B.D.; Kluth, L.A.; Volkmer, B.G.; Hautmann, R.; Küfer, R.; Zerbib, M.; Kwon, E.; Thompson, R.H.; Boorjian, S.A.; et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur. J. Surg. Oncol. 2014, 40, 121–127. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Sheinin, Y.; Crispen, P.L.; Farmer, S.A.; Lohse, C.M.; Kuntz, S.M.; Leibovich, B.C.; Kwon, E.D.; Frank, I. T-Cell coregulatory molecule expression in urothelial cell carcinoma: Clinicopathologic correlations and association with survival. Clin. Cancer Res. 2008, 14, 4800–4808. [Google Scholar] [CrossRef]
- Pichler, R.; Fritz, J.; Lackner, F.; Sprung, S.; Brunner, A.; Horninger, W.; Loidl, W.; Pircher, A.; Heidegger, I. Prognostic Value of Testing PD-L1 Expression After Radical Cystectomy in High-risk Patients. Clin. Genitourin. Cancer 2018, 16, 1015–1024. [Google Scholar] [CrossRef]
- Bellmunt, J.; Mullane, S.A.; Werner, L.; Fay, A.P.; Callea, M.; Leow, J.J.; Taplin, M.E.; Choueiri, T.K.; Hodi, F.S.; Freeman, G.J.; et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann. Oncol. 2015, 26, 812–817. [Google Scholar] [CrossRef]
- Yang, M.; Yu, Q.; Liu, J.; Fu, W.; Cao, Y.; Yu, L.; Shao, S.; Wang, X.; Niu, H.; Wang, Y. T-cell immunoglobulin mucin-3 expression in bladder urothelial carcinoma: Clinicopathologic correlations and association with survival. J. Surg. Oncol. 2015, 112, 430–435. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556. [Google Scholar] [CrossRef] [PubMed]
- De Wit, R.; Kulkarni, G.S.; Uchio, E.M.; Krieger, L.E.M.; Boormans, J.L.; Roumiguié, M.; Singer, E.A.; Bajorin, D.F.; Kamat, A.M.; Grivas, P.; et al. Pembrolizumab (pembro) for patients (pts) with high-risk (HR) non–muscle invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guérin (BCG): Updated follow-up from KEYNOTE-057. J. Clin. Oncol. 2019, 37, 4530. [Google Scholar] [CrossRef]
- Rijnders, M.; van der Veldt, A.A.M.; Zuiverloon, T.C.M.; Grünberg, K.; Thunnissen, E.; de Wit, R.; van Leenders, G.J.L.H. PD-L1 Antibody Comparison in Urothelial Carcinoma. Eur. Urol. 2019, 75, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Gu, T.-T.; Pan, H.-F.; Zhang, H.-Z. Evaluation of PD-L1 biomarker for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatments for urothelial carcinoma patients: A meta-analysis. Int. Immunopharmacol. 2019, 67, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Durán, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Wang, L.; Saci, A.; Szabo, P.M.; Gong, Y.; Zhu, J. Epithelial-mesenchymal transition (EMT), T cell infiltration, and outcomes with nivolumab (nivo) in urothelial cancer (UC). Ann. Oncol. 2017, 28. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Powles, T.; Nickles, D.; Van Allen, E.; Chappey, C.; Zou, W.; Kowanetz, M.; Kadel, E.; Denker, M.; Boyd, Z.; Vogelzang, N.; et al. Immune biomarkers associated with clinical benefit from atezolizumab (MPDL3280a; anti-PD-L1) in advanced urothelial bladder cancer (UBC). J. Immunother. Cancer 2015, 3, 83. [Google Scholar] [CrossRef]
- Xiao, Y.; Rabe, C.; Kowanetz, M.; Powles, T.; Vogelzang, N.J.; Petrylak, D.P.; Loriot, Y.; Denker, M.; Nakamura, R.; Wu, Q.J.; et al. Myeloid cell biology and inhibition of anti-tumor immune responses by MPDL3280A in urothelial bladder cancer. J. Immunother. Cancer 2014, 2, 131. [Google Scholar] [CrossRef][Green Version]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Baron, A.; Necchi, A.; Plimack, E.R.; Pal, S.K.; Bedke, J.; Arranz, J.A.; Vaena, D.; Grimm, M.-O.; Bracarda, S.; et al. Abstract CT178: Nivolumab monotherapy in patients with advanced platinum-resistant urothelial carcinoma: Efficacy and safety update and association between biomarkers and overall survival in CheckMate 275. In Cancer Research; American Association for Cancer Research (AACR): Philadelphia, PA, USA, 2018; p. 178. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullén, A.; et al. Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN Bladder 100 phase III interim analysis. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Wakita, D.; Iwai, T.; Harada, S.; Suzuki, M.; Yamamoto, K.; Sugimoto, M. Cisplatin augments antitumor T-cell responses leading to a potent therapeutic effect in combination with PD-L1 blockade. Anticancer Res. 2019, 39, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, X.; Huang, G.; Chen, Y.; Chen, L.; Song, H. Preconditioning chemotherapy with cisplatin enhances the antitumor activity of cytokine-induced killer cells in a murine melanoma model. Cancer Biother. Radiopharm. 2012, 27, 210–220. [Google Scholar] [CrossRef]
- Markasz, L.; Skribek, H.; Uhlin, M.; Otvos, R.; Flaberg, E.; Eksborg, S.; Olah, E.; Stuber, G.; Szekely, L. Effect of frequently used chemotherapeutic drugs on cytotoxic activity of human cytotoxic T-lymphocytes. J. Immunother. 2008, 31, 283–293. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Y.; Lv, C.; Huang, M.; Li, M.; Dong, B.; Liu, X.; An, G.; Zhang, W.; Zhang, J.; et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 2016, 107, 1563–1571. [Google Scholar] [CrossRef]
- Peng, J.; Hamanishi, J.; Matsumura, N.; Abiko, K.; Murat, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Hosoe, Y.; Murphy, S.K.; et al. Chemotherapy Induces Programmed Cell Death-Ligand 1 Overexpression via the Nuclear Factor-κB to Foster an Immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer Res. 2015, 75, 5034–5045. [Google Scholar] [CrossRef]
- Wu, K.; Tan, M.Y.; Jiang, J.T.; Mu, X.Y.; Wang, J.R.; Zhou, W.J.; Wang, X.; Li M qing He, Y.Y.; Liu, Z.H. Cisplatin inhibits the progression of bladder cancer by selectively depleting G-MDSCs: A novel chemoimmunomodulating strategy. Clin. Immunol. 2018, 193, 60–69. [Google Scholar] [CrossRef]
- Suzuki, E.; Kapoor, V.; Jassar, A.S.; Kaiser, L.R.; Albelda, S.M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b + myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 2005, 11, 6713–6721. [Google Scholar] [CrossRef]
- Le, H.K.; Graham, L.; Cha, E.; Morales, J.K.; Manjili, M.H.; Bear, H.D. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int. Immunopharmacol. 2009, 9, 900–909. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Z.; Chen, D.; Zhang, B.; Wang, Z.; Le, H. Suppressive effects of gemcitabine plus cisplatin chemotherapy on regulatory T cells in nonsmall-cell lung cancer. J. Int. Med. Res. 2015, 43, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Taniguchi, K.; Nakazawa, M.; Matsuyama, R.; Mori, R.; Takeda, K.; Ichikawa, Y.; Tanaka, K.; Endo, I. Changes in the immune cell population and cell proliferation in peripheral blood after gemcitabine-based chemotherapy for pancreatic cancer. Clin. Transl. Oncol. 2014, 16, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Mitchem, J.B.; Brennan, D.J.; Knolhoff, B.L.; Belt, B.A.; Zhu, Y.; Sanford, D.E.; Belaygorod, L.; Carpenter, D.; Collins, L.; Piwnica-Worms, D.; et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013, 73, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Krantz, D.; Hartana, C.A.; Winerdal, M.E.; Johansson, M.; Alamdari, F.; Jakubczyk, T.; Huge, Y.; Aljabery, F.; Palmqvist, K.; Zirakzadeh, A.A.; et al. Neoadjuvant Chemotherapy Reinforces Antitumour T cell Response in Urothelial Urinary Bladder Cancer. Eur. Urol. 2018, 74, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Baras, A.S.; Drake, C.; Liu, J.-J.; Gandhi, N.; Kates, M.; Hoque, M.O.; Meeker, A.; Hahn, N.; Taube, J.M.; Schoenberg, M.P.; et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology 2016, 5, e1134412. [Google Scholar] [CrossRef] [PubMed]
- Erlmeier, F.; Seitz, A.K.; Hatzichristodoulou, G.; Stecher, L.; Retz, M.; Gschwend, J.E.; Weichert, W.; Kübler, H.R.; Horn, T. The Role of PD-L1 Expression and Intratumoral Lymphocytes in Response to Perioperative Chemotherapy for Urothelial Carcinoma. Bladder Cancer 2016, 2, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, Y.; Xu, L.; Zhang, J.; Xie, H.; Fu, H.; Zhou, Q.; Chang, Y.; Dai, B.; Xu, J. Tumor stroma-infiltrating mast cells predict prognosis and adjuvant chemotherapeutic benefits in patients with muscle invasive bladder cancer. Oncoimmunology 2018, 7, e1474317. [Google Scholar] [CrossRef]
- Enninga, E.A.L.; Nevala, W.K.; Holtan, S.G.; Leontovich, A.A.; Markovic, S.N. Galectin-9 modulates immunity by promoting Th2/M2 differentiation and impacts survival in patients with metastatic melanoma. Melanoma Res. 2016, 26, 429–441. [Google Scholar] [CrossRef]
| Ref | N | Stage | UC only | Methods | Relation with Survival | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Parameter | Immune Marker | HR 1 | 95% CI | p | Adjusted for | |||||
| [10] | 115 | Low-grade NMIBC | Yes | IHC | RFS | CD3 | 5.83 2 | 1.52–22.36 | 0.010 | Tumor size, T stage, GZMB and CD4,20,56,68 |
| CD8 | 5.33 2 | 1.51–18.74 | 0.009 | |||||||
| [25] | 302 | NMIBC: 212 MIBC: 90 | Yes | IHC | OS | CD8 INT | 0.87 | 0.46–1.65 | 0.67 | Age, T and N stages, tumor size and grade |
| RFS | CD8 INT | 0.68 | 0.46–1.01 | 0.057 | - | |||||
| [21] | 69 | NMIBC: 38 MIBC: 31 | Yes | IHC | OS | CD8 INT | 0.3 | 0.09–0.96 | 0.042 | T stage |
| DFS | CD8 INT | 0.79 | 0.27–2.33 | 0.67 | ||||||
| [8] | 253 | NMBIC: 201 MIBC: 52 | Yes | IHC | PFS - NMIBC | CD3, CD8 | NR | NR | ns | - |
| CSS - MIBC | CD3 | 0.60 | 0.41–0.89 | 0.009 | ||||||
| CD8 | 0.82 | 0.57–1.16 | 0.26 | |||||||
| [18] | 67 | NMIBC: 5 MIBC: 62 | No | IHC | OS | CD3 INT | 2.91 | 0.85–10.0 | 0.09 | Stage, LVI, surgical margins, prior BCG and (N)AC |
| CD3 IM | 0.57 | 0.12–2.63 | 0.47 | |||||||
| CD8 INT | 0.32 | 0.09–1.11 | 0.07 | |||||||
| CD8 IM | 0.33 | 0.12–0.88 | 0.03 | |||||||
| DFS | CD3 INT | 1.27 | 0.46–3.53 | 0.64 | ||||||
| CD3 IM | 0.60 | 0.16–2.17 | 0.43 | |||||||
| CD8 INT | 0.55 | 0.18–1.65 | 0.29 | |||||||
| CD8 IM | 0.35 | 0.14–0.86 | 0.02 | |||||||
| [11] | 102 | NMIBC: 53 MIBC: 49 | Yes | IHC | OS | CD8 | 0.66 | 0.28–1.58 | 0.35 | T stage, tumor size, grade, NLR and neutrophils |
| [19] | 60 | T1 | Yes | IHC | PFS | CD3 INT | NR | NR | 0.69 | - |
| CSS | CD3 INT | NR 3 | NR | 0.045 | ||||||
| [20] | 37 | NMIBC: 4 MIBC: 33 | Yes | IHC | OS | CD3 INT/IM | 0.42 | 0.18–1.00 | 0.049 | Age, gender, T and M stages, and (N)AC |
| PFS | CD3 INT/IM | 0.09 | 0.02–0.48 | 0.004 | ||||||
| [22] | 56 | NMIBC: 11 MIBC: 45 | Yes | IHC | OS | CD8 INT | 0.1 | 0.01–0.69 | 0.02 | T and N stages, and LVI |
| CSS | CD8 INT | 0.05 | 0.01–0.62 | 0.02 | ||||||
| [12] | 404 | MIBC 4 | Yes | RNA-seq, TIMER | OS | CD8 | 7.70 | 1.60–36.97 | 0.12 | Age, stage, B cells, DCs, neutrophils and TAMs |
| [24] | 287 | MIBC 4 | Yes | RNA-seq, CIBERSORT | OS | CD8 | 0.15 | 0.014–1.75 | 0.13 | - |
| 118 | MIBC | No | IHC | OS | CD8 INT | 0.91 | 0.84–0.98 | 0.016 | ||
| CD8 ST | 0.97 | 0.94–0.99 | 0.003 | |||||||
| 140 | MIBC | No | IHC | OS | CD8 INT | 0.94 | 0.90–0.99 | 0.015 | ||
| CD8 ST | 0.98 | 0.97–0.99 | 0.001 | |||||||
| [13] | 407 | MIBC 4 | Yes | RNA-seq | OS | CD3D | NR 5 | NR | 0.032 | Age, gender and T stage |
| CD8A | NR 5 | NR | 0.06 | |||||||
| [14] | 248 | NMIBC: 129 MIBC: 119 | Yes | IHC | OS | CD8 ST | NR 6 | NR | <0.01 | - |
| RFS | CD8 ST | NR | NR | 0.99 | ||||||
| [15] | 149 | NMIBC: 18 MIBC: 131 | Yes | IHC | OS | CD3 | 0.84 | 0.68–1.04 | 0.11 | - |
| CD8 | 0.80 | 0.63–1.02 | 0.07 | |||||||
| CSS | CD3 | 0.81 | 0.63-1.03 | 0.089 | ||||||
| CD8 | 0.75 | 0.56-1.01 | 0.56 | |||||||
| [16]7 | 302 | NMIBC: 212 MIBC: 90 | Yes | IHC | OS | CD8 INT | 0.43 | NR | 0.003 | NR |
| CD8 ST | 2.21 | NR | 0.009 | |||||||
| [17] | 44 | NMIBC: 5 MIBC: 39 | No | IHC | CSS | CD8 | 0.95 | 0.20–3.55 | 0.947 | T and N stages, and CD169+ cells |
| [26] | 67 | T1 | Yes | IHC | OS | CD8 INT | 0.36 | 0.07–1.40 | 0.13 | Tumor size and multifocality |
| [9] | 221 | NMIBC: 43 MIBC: 178 | Yes | IHC | OS | Immunoscore | 2.01 1 | 1.20–3.36 | 0.008 | T and N stages, and vascular invasion |
| Ref | N | Stage | UC only | Methods | Relation with survival | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Parameter | Immune Marker | HR 1 | 95% CI | p | Adjusted for | |||||
| [8] | 253 | NMBIC: 201 MIBC: 52 | Yes | IHC | PFS-NMIBC | CD68 | 1.52 | 1.08–2.14 | 0.016 | - |
| CD163 | NR | NR | NS | |||||||
| CSS-MIBC | CD68 | 3.88 | 1.00–15.08 | 0.051 | ||||||
| CD163 | 0.99 | 0.69–1.41 | 0.95 | |||||||
| CD68/CD3 > 1 | 7.73 | 3.13–19.10 | 9.5 × 10-6 | T stage | ||||||
| [42] | 94 | T1, high grade | Yes | IHC | RFS | CD163 | 2.11 | 1.04–4.28 | 0.038 | Gender, age, tumor size, multifocality, CIS, bladder instillations |
| PFS | CD163 | 6.69 | 1.99–22.50 | 0.002 | ||||||
| CSS | CD163 | 5.86 | 1.41–24.30 | 0.015 | ||||||
| [40] | 302 | NMIBC: 212 MIBC: 90 | Yes | IHC | RFS | CD68 INT/ST CD204 INT/ST CD169 INT/ST | NR | NR | NS | - |
| OS | CD68 INT | 1.38 | 1.88–2.18 | 0.162 | ||||||
| CD68 ST | 0.91 | 0.53–1.57 | 0.735 | Age, tumor size, T and N stages, grade | ||||||
| CD204 INT | 1.04 | 0.59–1.84 | 0.887 | |||||||
| CD204 ST | 1.981 | 1.10–3.56 | 0.022 | |||||||
| CD169 INT | 1.51 | 0.91–2.52 | 0.11 | |||||||
| CD169 ST | 1.60 | 0.94–2.73 | 0.08 | |||||||
| [17] | 44 | NMIBC: 5 MIBC: 39 | No | IHC | DSS | CD169 | 0.13 | 0.01–0.76 | 0.021 | T and N stages, CD8+ T cells |
| [12] | 404 | MIBC | Yes | RNA-seq, TIMER | OS | TAMs | 17.35 | 5.86–51.33 | 0.009 | Age, stage, CD4+ and CD8+ T cells, neutrophils, TAMs and DCs |
| [24] | 405 | MIBC | Yes | RNA-seq 6, CIBERSORT | OS | TAMs | 16.57 | 4.98–55.17 | <0.000 | - |
| M1 TAMs | 0.72 | 0.04–13.68 | 0.83 | |||||||
| M2 TAMs | 0.94 | 0.16–5.65 | 0.95 | |||||||
| 118 | MIBC | No | IHC | OS | CD68 INT | 1.03 | 1.01–1.05 | 0.005 | ||
| CD68 ST | 1.02 | 1.01–1.02 | <0.000 | |||||||
| 140 | MIBC | No | IHC | OS | CD68 INT | 1.04 | 1.02–1.05 | <0.000 | ||
| CD68 ST | 1.01 | 1.01–1.02 | <0.000 | |||||||
| [10] | 115 | Low-grade NMIBC | Yes | IHC | RFS | CD68 | 3.88 2 | 1.00–15.08 | 0.051 | Tumor size, stage, GZMB and CD3,4,8,20,56 |
| [43] | 141 | MIBC | No | IHC | OS | CD68 | 1.23 | 0.76–1.99 | 0.40 | T stage, grade, LVI and AC |
| RFS | CD68 | 1.40 | 0.78–2.52 | 0.26 | ||||||
| [30] | 93 | Ta: 69 T1: 24 | No | IHC | RFS | CD68 | 1.19 | 0.56–2.54 | 0.65 | Gender, smoking status, age, stage, grade and multifocality |
| [44] | 134 | NMIBC: 19 MIBC: 115 | Yes | IHC | OS | CD163 | NR 3 | NR | 0.074 | - |
| PFS | CD163 | NR 3 | NR | 0.090 | ||||||
| [45] | 184 | NMIBC: 81 MIBC: 11 | Yes | IHC | OS | CD68 | 1.01 | 0.99–1.01 | 0.19 | Grade, T stage, age and multifocality |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Wilpe, S.; Gerretsen, E.C.F.; van der Heijden, A.G.; de Vries, I.J.M.; Gerritsen, W.R.; Mehra, N. Prognostic and Predictive Value of Tumor-Infiltrating Immune Cells in Urothelial Cancer of the Bladder. Cancers 2020, 12, 2692. https://doi.org/10.3390/cancers12092692
van Wilpe S, Gerretsen ECF, van der Heijden AG, de Vries IJM, Gerritsen WR, Mehra N. Prognostic and Predictive Value of Tumor-Infiltrating Immune Cells in Urothelial Cancer of the Bladder. Cancers. 2020; 12(9):2692. https://doi.org/10.3390/cancers12092692
Chicago/Turabian Stylevan Wilpe, Sandra, Eveline C. F. Gerretsen, Antoine G. van der Heijden, I. Jolanda M. de Vries, Winald R. Gerritsen, and Niven Mehra. 2020. "Prognostic and Predictive Value of Tumor-Infiltrating Immune Cells in Urothelial Cancer of the Bladder" Cancers 12, no. 9: 2692. https://doi.org/10.3390/cancers12092692
APA Stylevan Wilpe, S., Gerretsen, E. C. F., van der Heijden, A. G., de Vries, I. J. M., Gerritsen, W. R., & Mehra, N. (2020). Prognostic and Predictive Value of Tumor-Infiltrating Immune Cells in Urothelial Cancer of the Bladder. Cancers, 12(9), 2692. https://doi.org/10.3390/cancers12092692






