Sex Difference and Smoking Effect of Lung Cancer Incidence in Asian Population
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Follow-Up
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Characteristics
3.2. Sex Difference in the Incidence of Lung Cancer
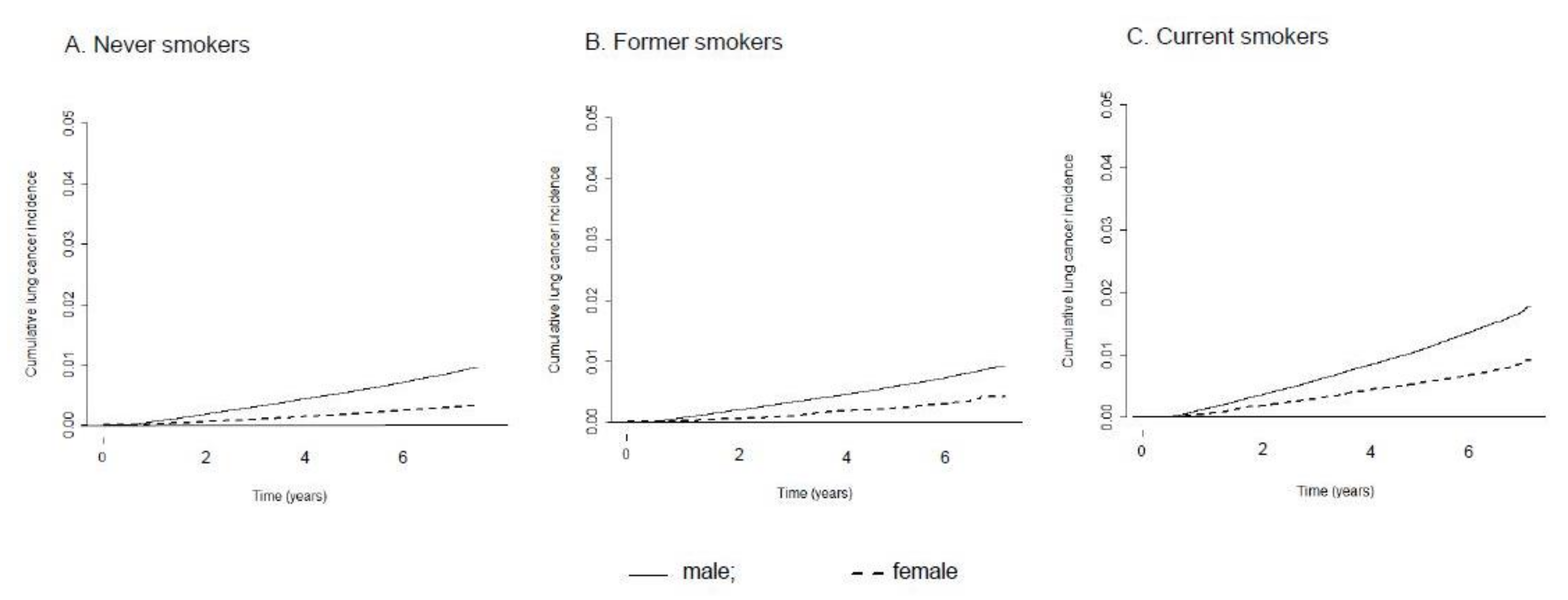
3.3. Sex Difference in the Effect of Smoking Status on Lung Cancer
3.4. Sex Difference in the Effect of Detailed Smoking History on Lung Cancer
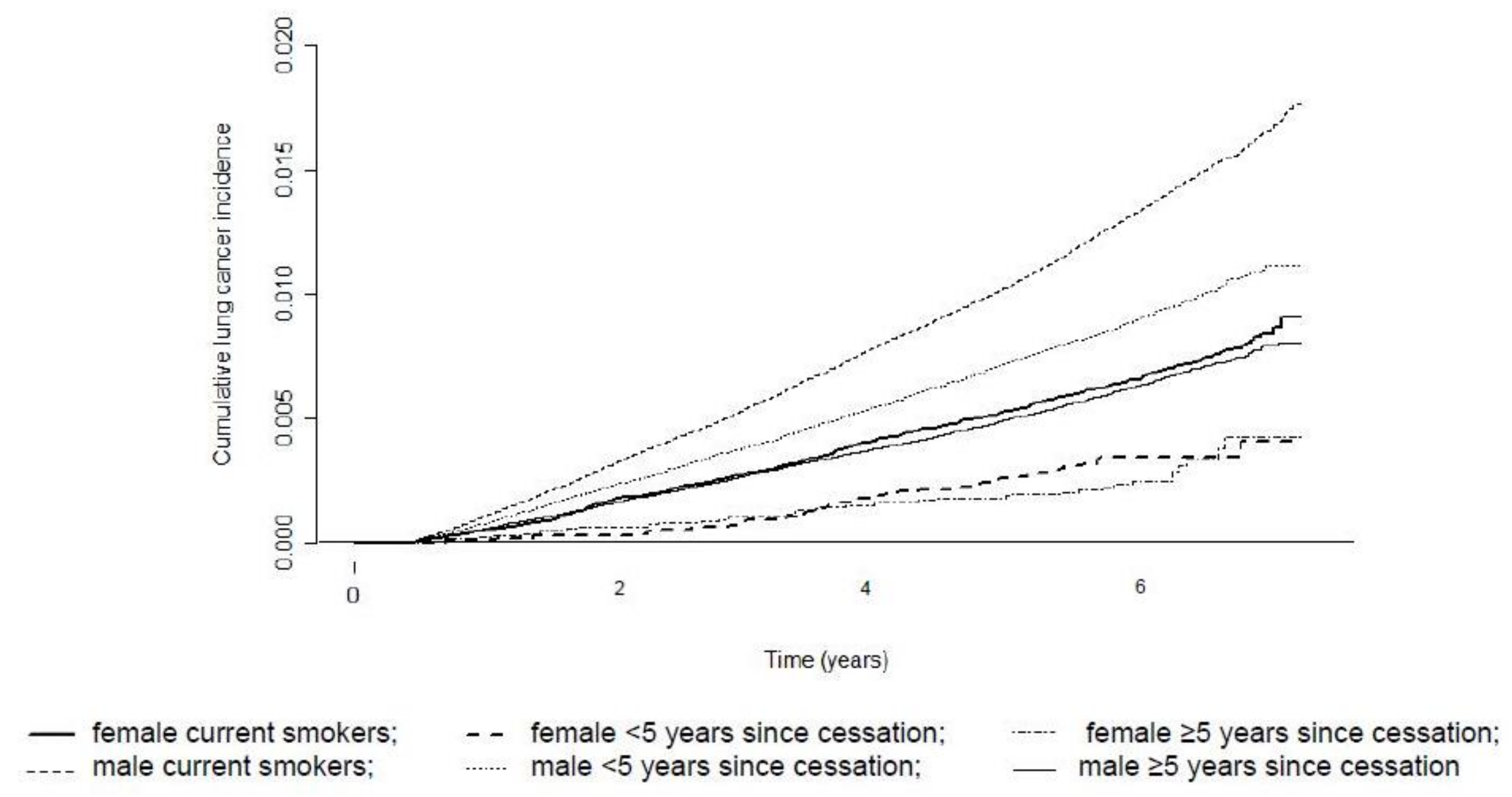
3.5. Sex Difference in the Effect of Smoking Cessation on Lung Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Wakelee, H.A.; Chang, E.T.; Gomez, S.L.; Keegan, T.H.M.; Feskanich, D.; Clarke, C.A.; Holmberg, L.; Yong, L.C.; Kolonel, L.N.; Gould, M.K.; et al. Lung Cancer Incidence in Never Smokers. J. Clin. Oncol. 2007, 25, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Gasperino, J. Gender Is a Risk Factor for Lung Cancer. Med. Hypotheses 2011, 76, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Couraud, S.; Zalcman, G.; Milleron, B.; Morin, F.; Souquet, P.-J. Lung Cancer in Never Smokers—A Review. Eur. J. Cancer 2012, 48, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, C. Lung Cancer in Never smokers—The East Asian Experience. Transl. Lung Cancer Res. 2018, 7, 450–463. [Google Scholar] [CrossRef]
- Reddy, P.P. Lung Cancer Incidence in Never Smokers: Genetic and Gender Basis. Gene Rep. 2016, 4, 198–207. [Google Scholar] [CrossRef]
- Korea Central Cancer Registry, National Cancer Center. Annual Report of Cancer Statistics in Korea in 2015; Ministry of Health and Welfare: Sejong, Korea, 2017.
- Freedman, N.D.; Leitzmann, M.F.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Cigarette Smoking and Subsequent Risk of Lung Cancer in Men and Women: Analysis of a Prospective Cohort Study. Lancet Oncol. 2008, 9, 649–656. [Google Scholar] [CrossRef]
- Bain, C.; Feskanich, D.; Speizer, F.E.; Thun, M.; Hertzmark, E.; Rosner, B.A.; Colditz, G.A. Lung Cancer Rates in Men and Women with Comparable Histories of Smoking. J. Natl. Cancer Inst. 2004, 96, 826–834. [Google Scholar] [CrossRef]
- Hansen, M.S.; Licaj, I.; Braaten, T.; Langhammer, A.; Le Marchand, L.; Gram, I.T. Sex Differences in Risk of Smoking-Associated Lung Cancer: Results from a Cohort of 600.000 Norwegians. Am. J. Epidemiol. 2017, 187, 971–981. [Google Scholar] [CrossRef]
- Prizment, A.E.; Yatsuya, H.; Lutsey, P.L.; Lubin, J.H.; Woodward, M.; Folsom, A.R.; Huxley, R.R. Smoking Behavior and Lung Cancer in a Biracial Cohort: The Atherosclerosis Risk in Communities Study. Am. J. Prev. Med. 2014, 46, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung Cancer in Never Smokers—A Different Disease. Nat. Rev. Cancer 2007, 7, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Sobue, T.; Yamamoto, S.; Hara, M.; Sasazuki, S.; Sasaki, S.; Tsugane, S.; The JPHC Study Group. Cigarette Smoking and Subsequent Risk of Lung Cancer by Histologic Type in Middle-Aged Japanese Men and Women: The JPHC Study. Int. J. Cancer 2002, 99, 245–251. [Google Scholar] [CrossRef]
- Yun, Y.D.; Back, J.H.; Ghang, H.; Jee, S.H.; Kim, Y.; Lee, S.M.; Samet, J.M.; Lee, K.S. Hazard Ratio of Smoking on Lung Cancer in Korea According to Histological Type and Gender. Lung 2015, 194, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Pampel, F.C. Global Patterns and Determinants of Sex Differences in Smoking. Int. J. Comp. Sociol. 2006, 47, 466–487. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R. Overview of Asbestos Issues in Korea. J. Korean Med. Sci. 2009, 24, 363–367. [Google Scholar] [CrossRef]
- Prescott, E.; Osler, M.; Hein, O.H.; Borch-Johnsen, K.; Lange, P.; Schnohr, P.; Vestbo, J. Gender and Smoking-Related Risk of Lung Cancer: The Copenhagen Center for Prospective Population Studies. Epidemiology 1998, 9, 79–83. [Google Scholar] [CrossRef]
- Freedman, N.D.; Abnet, C.C.; Caporaso, E.N.; Fraumeni, J.F.; Murphy, G.; Hartge, P.; Hollenbeck, A.R.; Park, Y.; Shiels, M.S.; Silverman, D.T. Impact of Changing US Cigarette Smoking Patterns on Incident Cancer: Risks of 20 Smoking-Related Cancers among the Women and Men of the NIH-AARP Cohort. Int. J. Epidemiol. 2015, 45, 846–856. [Google Scholar] [CrossRef]
- Lubin, J.H. Cigarette Smoking and Lung Cancer: Modeling Total Exposure and Intensity. Cancer Epidemiol. Biomark. Prev. 2006, 15, 517–523. [Google Scholar] [CrossRef]
- Tsai, Y.-W.; Tsai, T.-I.; Yang, C.-L.; Kuo, K.N. Gender Differences in Smoking Behaviors in an Asian Population. J. Womens Health 2008, 17, 971–978. [Google Scholar] [CrossRef]
- Fry, J.S.; Lee, P.N.; Forey, A.B.; Coombs, K.J. How Rapidly Does the Excess Risk of Lung Cancer Decline Following Quitting Smoking? A Quantitative Review Using the Negative Exponential Model. Regul. Toxicol. Pharmacol. 2013, 67, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Agudo, A.; Bonet, C.; Travier, N.; González, C.A.; Vineis, P.; Bueno-De-Mesquita, H.B.; Trichopoulos, D.; Boffetta, P.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; et al. Impact of Cigarette Smoking on Cancer Risk in the European Prospective Investigation into Cancer and Nutrition Study. J. Clin. Oncol. 2012, 30, 4550–4557. [Google Scholar] [CrossRef] [PubMed]
- Tindle, H.A.; Duncan, M.S.; Greevy, A.R.; Vasan, R.S.; Kundu, S.; Massion, P.P.; Freiberg, M.S. Lifetime Smoking History and Risk of Lung Cancer: Results from the Framingham Heart Study. J. Natl. Cancer Inst. 2018, 110, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, L.M.; Taylor, G.M.J.; Huxley, R.R.; Mitchell, P.; Woodward, M.; Peters, A.E.S. Smoking as a Risk Factor for Lung Cancer in Women and Men: A Systematic Review and Meta-Analysis. BMJ Open 2018, 8, e021611. [Google Scholar] [CrossRef]
- Shankar, A.; Yuan, J.-M.; Koh, W.-P.; Lee, H.-P.; Yu, M.C. Morbidity and Mortality in Relation to Smoking among Women and Men of Chinese Ethnicity: The Singapore Chinese Health Study. Eur. J. Cancer 2008, 44, 100–109. [Google Scholar] [CrossRef][Green Version]
- Park, S.; Jee, S.H.; Shin, H.R.; Park, E.H.; Shin, A.; Jung, K.W.; Hwank, S.S.; Cha, E.S.; Yun, Y.H.; Park, S.K. Attributable Fraction of Tobacco Smoking on Cancer Using Pop-Ulation-Based Nationwide Cancer Incidence and Mortality Data in Korea. BMC Cancer 2014, 14, 406. [Google Scholar]
- Jung, K.-W.; Won, Y.-J.; Kong, H.-J.; Lee, E.S. The Community of Population-Based Regional Cancer Registries Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2015. Cancer Res. Treat. 2018, 50, 303–316. [Google Scholar] [CrossRef]
- Choi, S.; Park, S.; Lee, J.; Kim, Y.; Oh, K.-W. Trends in Cigarette Smoking among Adolescents and Adults in Korea. Epidemiol. Health 2014, 36. [Google Scholar] [CrossRef]
- Myong, J.-P.; Shin, J.-Y.; Kim, S.-J. Factors Associated with Participation in Colorectal Cancer Screening in Korea: The Fourth Korean National Health and Nutrition Examination Survey (KNHANES IV). Int. J. Color. Dis. 2012, 27, 1061–1069. [Google Scholar] [CrossRef]
- Hahm, M.-I.; Choi, K.S.; Lee, H.-Y.; Jun, J.K.; Oh, D.; Park, E.-C. Who Participates in the Gastric Cancer Screening and on-Time Rescreening in the National Cancer Screening Program? A Population-Based Study in Korea. Cancer Sci. 2011, 102, 2241–2247. [Google Scholar] [CrossRef]
- Chang, Y.; Kang, H.-Y.; Lim, D.; Cho, H.-J.; Khang, Y.-H. Long-Term Trends in Smoking Prevalence and Its Socioeconomic Ine-Qualities in Korea, 1992–2016. Int. J. Equity Health 2019, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Nishino, Y.; Tanji, F.; Maemondo, M.; Takahashi, S.; Sato, I.; Kawai, M.; Minami, Y. Cigarette Smoking and Lung Cancer Risk According to Histologic Type in Japanese Men and Women. Cancer Sci. 2013, 104, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.M.; Kim, J.-E.; Kim, Y.; Lee, H.-H.; Kim, S.Y. Occupational Burden of Asbestos-Related Diseases in Korea, 1998–2013: Asbestosis, Mesothelioma, Lung Cancer, Laryngeal Cancer, and Ovarian Cancer. J. Korean Med. Sci. 2018, 33, e226. [Google Scholar] [CrossRef] [PubMed]
- Park, T.H.; Kang, D.R.; Park, S.H.; Yoon, D.K.; Lee, C.M. Indoor Radon Concentration in Korea Residential Environments. Environ. Sci. Pollut. Res. 2018, 25, 12678–12685. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Male | Female |
|---|---|---|
| Age, mean (SD) | 55.4 (10.3) | 54.8 (10.2) |
| Number of days of alcohol consumption | ||
| None | 1,039,557 (42.5) | 3,294,571 (79.9) |
| 1–2/week | 708,169 (28.9) | 396,165 (9.6) |
| ≥3/week | 622,680 (25.4) | 113,522 (2.8) |
| Missing | 77,530 (3.2) | 316,950 (7.7) |
| Number of days of sweating exercise | ||
| None | 809,073 (33.1) | 1,742,508 (42.3) |
| 1–2/week | 812,531 (33.2) | 1,166,595 (28.3) |
| ≥3/week | 815,661 (33.3) | 1,198,527 (29.1) |
| Missing | 10,671 (0.4) | 13,578 (0.3) |
| Body mass index | ||
| <18.5 kg/m2 | 36,558 (1.5) | 61,006 (1.5) |
| 18.5–24.9 kg/m2 | 1,055,025 (43.1) | 1,945,352 (47.2) |
| ≥25 kg/m2 | 657,009 (26.8) | 1,039,464 (25.2) |
| Missing | 699,344 (28.6) | 1,075,386 (26.1) |
| Family history of cancer | ||
| No | 1,991,495 (81.4) | 3,281,754 (79.6) |
| Yes | 456,441 (18.7) | 839,454 (20.4) |
| Missing | - | - |
| History of chronic pulmonary obstructive disease | ||
| No | 2,379,348 (97.2) | 4,052,245 (98.3) |
| Yes | 68,588 (2.8) | 68,963 (1.7) |
| History of pneumoconiosis | ||
| No | 2,446,322 (99.9) | 4,120,887 (100) |
| Yes | 1614 (0.1) | 321 (<0.1) |
| History of interstitial pulmonary disease | ||
| No | 2,445,434 (99.9) | 4,118,519 (99.9) |
| Yes | 2502 (0.1) | 2689 (0.1) |
| Male | Female | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PkY | N | N of PY | N of Events | CR 1 | ASR 2 | N | N of PY | N of Events | CR 1 | ASR 2 | RR 3 (95% CI) 3 | |
| Total | 244,7936 | 15,939,310 | 22,423 | 140.7 | 134.0 (132.2–135.8) | 4,121,208 | 27,586,676 | 11,147 | 40.4 | 39.7 (39.0–40.5) | 3.4 (3.3–3.4) | |
| Never | 115,7106 | 7,552,011 | 8561 | 113.4 | 92.5 (90.5–94.6) | 4,010,591 | 26,855,434 | 10,500 | 39.1 | 38.3 (37.5–39.1) | 2.4 (2.3–2.5) | |
| Past | 0–19 | 277,398 | 1,819,539 | 904 | 49.7 | 64.7 (59.9–69.6) | 23,250 | 153,742 | 52 | 33.8 | 50.3 (36.0–64.6) | 1.3 (0.9–1.7) |
| ≥20 | 236,362 | 1,528,950 | 2727 | 178.4 | 144.7 (138.7–150.7) | 2861 | 18,723 | 26 | 138.9 | 87.6 (51.5–123.6) | 1.7 (1.1–2.5) | |
| Current | 0–9 | 68,039 | 444,815 | 377 | 84.8 | 145.1 (128.9–161.2) | 45,751 | 303,737 | 156 | 51.4 | 81.4 (67.9–94.8) | 1.8 (1.5–2.2) |
| 10–19 | 203,575 | 1,328,750 | 1434 | 107.9 | 185.1 (174.7–195.5) | 24,063 | 158,746 | 200 | 126.0 | 118.0 (101.2–134.9) | 1.6 (1.3–1.8) | |
| 20–29 | 235,963 | 1,534,630 | 2347 | 152.9 | 219.7 (210.1–229.3) | 9188 | 60,411 | 98 | 162.2 | 126.2 (99.8–152.6) | 1.7 (1.4–2.1) | |
| ≥30 | 269,493 | 1,730,615 | 6073 | 350.9 | 305.3 (296.3–314.3) | 5504 | 35,883 | 115 | 320.5 | 188.4 (151.5–225.4) | 1.6 (1.3–2.0) | |
| Smoking Measures | Male | Female | Sex–Smoking Interaction | ||||
|---|---|---|---|---|---|---|---|
| N | Adjusted HR (95% CI) 1 | p-Value | N | Adjusted HR (95% CI) 1 | p-Value | ||
| Smoking status | |||||||
| Never | 1,157,106 | 1.00 (reference) | - | 4,010,591 | 1.00 (reference) | - | - |
| Former | 513,760 | 1.27 (1.23–1.33) | <0.001 | 26,111 | 1.43 (1.16–1.81) | <0.001 | 0.261 |
| Current | 777,070 | 2.71 (2.63–2.79) | <0.001 | 84,506 | 2.70 (2.48–2.94) | <0.001 | - |
| Duration of smoking | |||||||
| 1–9 years | 53,677 | 1.00 (reference) | - | 25,164 | 1.00 (reference) | - | - |
| 10–19 years | 186,724 | 0.95 (0.79–1.14) | 0.558 | 34,884 | 1.40 (0.95–2.06) | 0.093 | - |
| 20–29 years | 475,970 | 1.48 (1.26–1.75) | <0.001 | 29,250 | 2.09 (1.45–3.01) | <0.001 | <0.001 |
| ≥30 years | 574,023 | 3.07 (2.62–3.61) | <0.001 | 21,269 | 3.16 (2.20–4.54) | <0.001 | - |
| Duration of former smoking | |||||||
| 1–9 years | 41,386 | 1.00 (reference) | - | 9643 | 1.00 (reference) | - | - |
| 10–19 years | 129,515 | 1.09 (0.85–1.38) | 0.496 | 8331 | 2.39 (0.85–6.73) | 0.099 | - |
| 20–29 years | 169,258 | 1.52 (1.22–1.90) | <0.001 | 4849 | 5.49 (2.07–14.56) | <0.001 | 0.017 |
| ≥30 years | 173471 | 2.81 (2.27–3.49) | <0.001 | 3265 | 5.92 (2.17–16.15) | <0.001 | - |
| Duration of current smoking | |||||||
| 1–9 years | 12,291 | 1.00 (reference) | - | 15,521 | 1.00 (reference) | - | - |
| 10–19 years | 57,209 | 0.81 (0.60–1.08) | 0.151 | 26,553 | 1.14 (0.75–1.73) | 0.538 | - |
| 20–29 years | 306,712 | 1.08 (0.85–1.39) | 0.534 | 24,401 | 1.52 (1.02–2.25) | 0.038 | 0.019 |
| ≥30 years | 400,552 | 1.81 (1.42–2.31) | <0.001 | 18,004 | 2.36 (1.60–3.48) | <0.001 | - |
| Quantity of smoking | |||||||
| 1–10 cigarettes/day | 108,145 | 1.00 (reference) | - | 39,841 | 1.00 (reference) | - | - |
| 11–20 cigarettes/day | 491,187 | 1.39 (1.30–1.50) | <0.001 | 49,727 | 1.37 (1.14–1.65) | <0.001 | 0.143 |
| >20 cigarettes/day | 691,498 | 2.03 (1.89–2.17) | <0.001 | 21,049 | 1.69 (1.36–2.09) | <0.001 | - |
| Quantity of smoking in former smokers | |||||||
| 1–10 cigarettes/day | 48,946 | 1.00 (reference) | - | 11,361 | 1.00 (reference) | - | - |
| 11–20 cigarettes/day | 200,661 | 1.43 (1.23–1.66) | <0.001 | 10,334 | 1.56 (0.92–2.64) | 0.102 | 0.180 |
| >20 cigarettes/day | 264,153 | 2.17 (1.88–2.51) | <0.001 | 4416 | 1.49 (0.80–2.77) | 0.204 | |
| Quantity of smoking in current smokers | |||||||
| 1–10 cigarettes/day | 59,199 | 1.00 (reference) | - | 28,480 | 1.00 (reference) | - | - |
| 11–20 cigarettes/day | 290,526 | 1.43 (1.32–1.55) | <0.001 | 39,393 | 1.33 (1.09–1.62) | 0.005 | 0.208 |
| >20 cigarettes/day | 427,345 | 2.18 (2.03–2.36) | <0.001 | 16,633 | 1.74 (1.39–2.19) | <0.001 | - |
| Pack-year | |||||||
| 0–9 | 186,132 | 1.00 (reference) | - | 64,184 | 1.00 (reference) | - | - |
| 10–19 | 362,880 | 1.40 (1.28–1.53) | <0.001 | 28,880 | 1.70 (1.40–2.08) | <0.001 | - |
| 20–29 | 345,016 | 1.91 (1.76–2.07) | <0.001 | 10,844 | 1.89 (1.45–2.41) | <0.001 | 0.470 |
| ≥30 | 396,802 | 2.91 (2.69–3.15) | <0.001 | 6709 | 2.59 (2.05–3.28) | <0.001 | - |
| Pack-year in former smokers | |||||||
| 0–9 | 118,093 | 1.00 (reference) | - | 18,433 | 1.00 (reference) | - | - |
| 10–19 | 159,305 | 1.24 (1.08–1.42) | <0.001 | 4817 | 2.37 (1.35–4.14) | <0.001 | - |
| 20–29 | 109,053 | 1.75 (1.53–2.00) | <0.001 | 1656 | 3.15 (1.62–6.10) | <0.001 | 0.026 |
| ≥30 | 127,309 | 2.80 (2.48–3.16) | <0.001 | 1205 | 2.26 (1.08–4.76) | 0.042 | - |
| Pack-year in current smokers | |||||||
| 0–9 | 68,039 | 1.00 (reference) | - | 45,751 | 1.00 (reference) | - | - |
| 10–19 | 203,575 | 1.24 (1.10–1.39) | <0.001 | 24,063 | 1.54 (1.24–1.90) | <0.001 | |
| 20–29 | 235,963 | 1.50 (1.34–1.67) | <0.001 | 9188 | 1.66 (1.29–2.15) | <0.001 | 0.379 |
| ≥30 | 269,493 | 2.29 (2.06–2.54) | <0.001 | 5504 | 2.52 (1.96–3.23) | <0.001 | |
| Years since cessation in former smokers | |||||||
| Current smoker | 777,070 | 1.00 (reference) | - | 84,506 | 1.00 (reference) | - | - |
| 0–4 | 156,560 | 0.69 (0.65–0.73) | <0.001 | 11,981 | 0.65 (0.47–0.90) | 0.009 | 0.469 |
| ≥5 | 357,200 | 0.38 (0.36–0.40) | <0.001 | 14,130 | 0.46 (0.33–0.64) | <0.001 | - |
| PkY | Years Since Cessation | Male | Female | |||||
|---|---|---|---|---|---|---|---|---|
| N | Adjusted HR (95% CI) 1 | p-Value | N | Adjusted HR (95% CI) 1 | p-Value | |||
| Current | - | - | 1,157,106 | 1.00 (reference) | - | 4,010,591 | 1.00 (reference) | - |
| Past | <10 | <5 | 20,997 | 0.34 (0.26–0.45) | <0.001 | 7845 | 0.34 (0.18–0.63) | 0.001 |
| - | - | ≥5 | 97,096 | 0.24 (0.21–0.27) | <0.001 | 10,588 | 0.31 (0.18–0.51) | <0.001 |
| - | <20 | <5 | 44,599 | 0.47 (0.41–0.55) | <0.001 | 2530 | 0.83 (0.50–1.38) | 0.469 |
| - | - | ≥5 | 114,706 | 0.26 (0.24–0.29) | <0.001 | 2287 | 0.60 (0.34–1.06) | 0.080 |
| - | <30 | <5 | 39,048 | 0.57 (0.51–0.65) | <0.001 | 920 | 1.00 (0.50–2.02) | 0.994 |
| - | - | ≥5 | 70,005 | 0.37 (0.34–0.41) | <0.001 | 736 | 0.84 (0.40–1.78) | 0.649 |
| - | ≥30 | <5 | 51,916 | 0.87 (0.81–0.93) | <0.001 | 686 | 0.79 (0.37–1.67) | 0.535 |
| - | - | ≥5 | 75,393 | 0.55 (0.52–0.59) | <0.001 | 519 | 0.47 (0.18–1.26) | 0.135 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.; Kim, Y.; Lee, J.; Lee, N.; Jang, S.H. Sex Difference and Smoking Effect of Lung Cancer Incidence in Asian Population. Cancers 2021, 13, 113. https://doi.org/10.3390/cancers13010113
Park B, Kim Y, Lee J, Lee N, Jang SH. Sex Difference and Smoking Effect of Lung Cancer Incidence in Asian Population. Cancers. 2021; 13(1):113. https://doi.org/10.3390/cancers13010113
Chicago/Turabian StylePark, Boyoung, Yeol Kim, Jaeho Lee, Nayoung Lee, and Seung Hun Jang. 2021. "Sex Difference and Smoking Effect of Lung Cancer Incidence in Asian Population" Cancers 13, no. 1: 113. https://doi.org/10.3390/cancers13010113
APA StylePark, B., Kim, Y., Lee, J., Lee, N., & Jang, S. H. (2021). Sex Difference and Smoking Effect of Lung Cancer Incidence in Asian Population. Cancers, 13(1), 113. https://doi.org/10.3390/cancers13010113






