Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Overview on Approved Immune Checkpoint Inhibitors for Metastatic Bladder Cancer
2.1. Atezolizumab
2.2. Pembrolizumab
2.3. Durvalumab
2.4. Nivolumab
2.5. Avelumab
3. Immune Checkpoint Inhibitors for Bladder Cancer: The Adjuvant Setting
4. Immune Checkpoint Inhibitors for Bladder Cancer: The Neoadjuvant Setting
5. Is There Any Role of Combination Immunotherapy in Bladder Cancer?
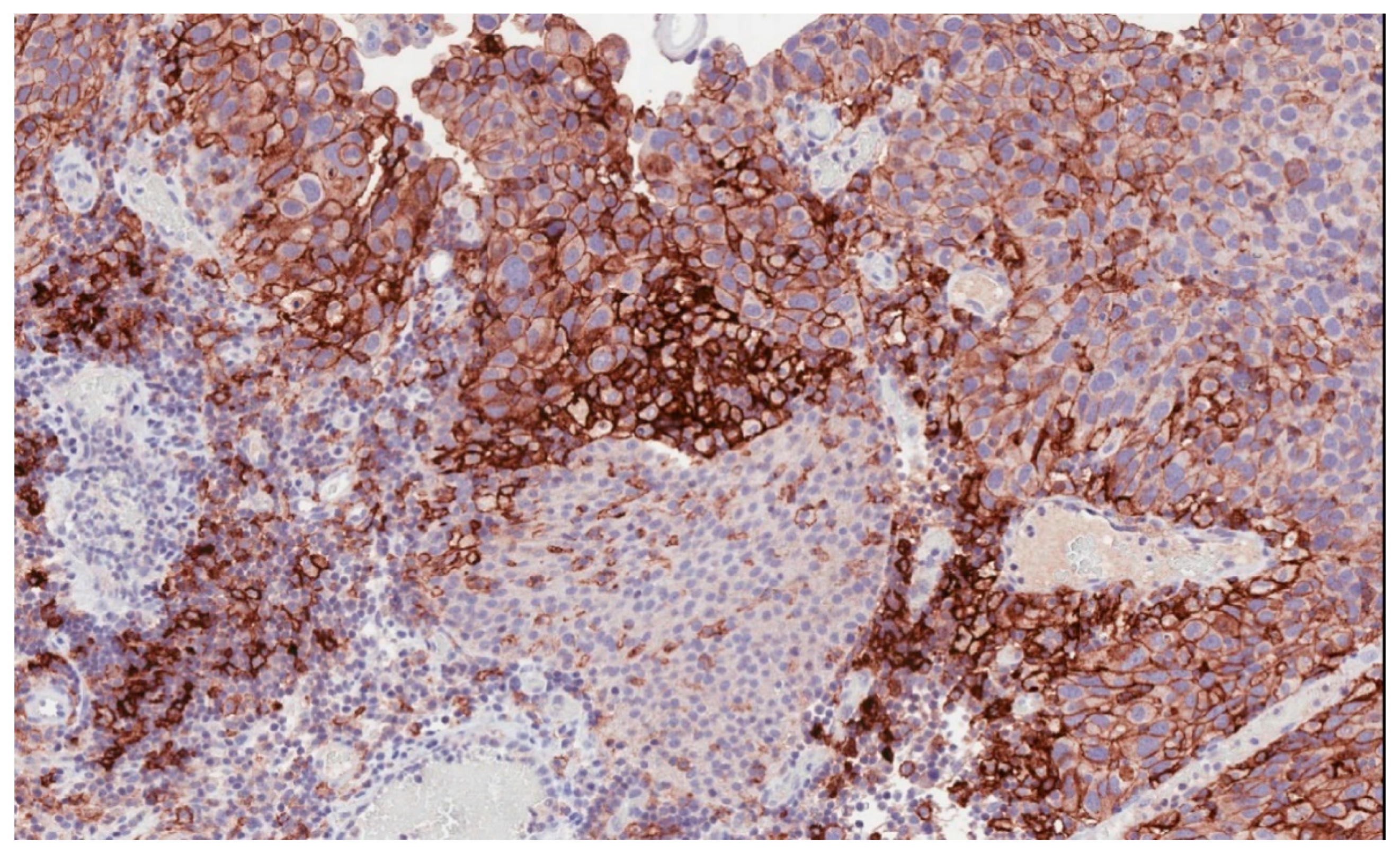
6. Biomarkers for PD-1/PD-L1 Blockade in Bladder Cancer
6.1. PD-L1 Expression
6.2. Molecular Subtype of Bladder Cancer
6.3. Tumor Mutational Burden
6.4. Immune-Gene Expression Profiling
6.5. Other Potential Biomarkers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Polo, S.H.; Gonzalez del Alba, A.; Perez-Valderrama, B.; Villa Guzman, J.C.; Climent, M.A.; Lainez, N.; Font, A.; Duran, I.; Mellado, B.; Castellano, D.; et al. Vinflunine maintenance therapy versus best supportive care after platinum combination in advanced bladder cancer: A phase II, randomized, open label, study (MAJA study, SOGUG 2011-02)—Interim analysis on safety. J. Clin. Oncol. 2014, 32, 359. [Google Scholar] [CrossRef]
- Bellmunt, J.; Théodore, C.; Demkov, T.; Komyakov, B.; Sengelov, L.; Daugaard, G.; Caty, A.; Carles, J.; Jagiello-Gruszfeld, A.; Karyakin, O.; et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J. Clin. Oncol. 2009, 27, 4454–4461. [Google Scholar] [CrossRef] [PubMed]
- Oing, C.; Rink, M.; Oechsle, K.; Seidel, C.; Von Amsberg, G.; Bokemeyer, C. Second line chemotherapy for advanced and metastatic urothelial carcinoma: Vinflunine and beyond-a comprehensive review of the current Literature. J. Urol. 2016, 195, 254–263. [Google Scholar] [CrossRef]
- Bellmunt, J.; Powles, T.; Vogelzang, N.J. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: The future is now. Cancer Treat. Rev. 2017, 54, 58–67. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for pd-l1–positive non–small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Bellmunt, J.; Mullane, S.A.; Werner, L.; Fay, A.P.; Callea, M.; Leow, J.J.; Taplin, M.E.; Choueiri, T.K.; Hodi, F.S.; Freeman, G.J.; et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann. Oncol. 2015, 26, 812–817. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Zibelman, M.; Ramamurthy, C.; Plimack, E.R. Emerging role of immunotherapy in urothelial carcinoma—advanced disease. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 538–547. [Google Scholar] [CrossRef]
- Pierantoni, F.; Maruzzo, M.; Gardi, M.; Bezzon, E.; Gardiman, M.P.; Porreca, A.; Basso, U.; Zagonel, V. Immunotherapy and urothelial carcinoma: An overview and future prospectives. Crit. Rev. Oncol. Hematol. 2019, 143, 46–55. [Google Scholar] [CrossRef]
- Nakanishi, J.; Wada, Y.; Matsumoto, K.; Azuma, M.; Kikuchi, K.; Ueda, S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 2007, 56, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Inman, B.A.; Sebo, T.J.; Frigola, X.; Dong, H.; Bergstralh, E.J.; Frank, I.; Fradet, Y.; Lacombe, L.; Kwon, E.D. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: Associations with localized stage progression. Cancer 2007, 109, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Aggen, D.H.; Drake, C.G. Biomarkers for immunotherapy in bladder cancer: A moving target. J. Immunother. Cancer 2017, 5, 94. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; Van Der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Powles, T.; Durán, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Sharma, P.; Callahan, M.K.; Bono, P.; Kim, J.; Spiliopoulou, P.; Calvo, E.; Pillai, R.N.; Ott, P.A.; de Braud, F.; Morse, M.; et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): A multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016, 17, 1590–1598. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Ciccarese, C.; Iacovelli, R.; Bria, E.; Mosillo, C.; Bimbatti, D.; Fantinel, E.; Bisogno, I.; Brunelli, M.; Tortora, G. Second-line therapy for metastatic urothelial carcinoma: Defining the best treatment option among immunotherapy, chemotherapy, and antiangiogenic targeted therapies. A systematic review and meta-analysis. Semin. Oncol. 2019, 46, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line Pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Plimack, E.R.; Bellmunt, J.; Gupta, S.; Berger, R.; Chow, L.Q.M.; Juco, J.; Lunceford, J.; Saraf, S.; Perini, R.F.; O’Donnell, P.H. Safety and activity of Pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): A non-randomised, open-label, phase 1b study. Lancet Oncol. 2017, 18, 212–220. [Google Scholar] [CrossRef]
- Massard, C.; Gordon, M.S.; Sharma, S.; Rafii, S.; Wainberg, Z.A.; Luke, J.; Curiel, T.J.; Colon-Otero, G.; Hamid, O.; Sanborn, R.E.; et al. Safety and efficacy of Durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 2016, 34, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Infante, J.R.; Balmanoukian, A.; Patel, M.R.; Wang, D.; Kelly, K.; Mega, A.E.; Britten, C.D.; Ravaud, A.; Mita, A.C.; et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: Results from a multicenter, Phase Ib study. J. Clin. Oncol. 2017, 35, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Gaule, P.; Smithy, J.W.; Toki, M.; Rehman, J.; Patell-Socha, F.; Cougot, D.; Collin, P.; Morrill, P.; Neumeister, V.; Rimm, D.L. A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol. 2017, 3, 256–259. [Google Scholar] [CrossRef]
- Rijnders, M.; van der Veldt, A.A.M.; Zuiverloon, T.C.M.; Grünberg, K.; Thunnissen, E.; de Wit, R.; van Leenders, G.J.L.H. PD-L1 Antibody Comparison in Urothelial Carcinoma. Eur. Urol. 2019, 75, 538–540. [Google Scholar] [CrossRef]
- Hodgson, A.; Slodkowska, E.; Jungbluth, A.; Liu, S.K.; Vesprini, D.; Enepekides, D.; Higgins, K.; Katabi, N.; Xu, B.; Downes, M.R. PD-L1 Immunohistochemistry Assay Concordance in Urothelial Carcinoma of the Bladder and Hypopharyngeal Squamous Cell Carcinoma. Am. J. Surg. Pathol. 2018, 42, 1059–1066. [Google Scholar] [CrossRef]
- Gevaert, T.; Cimadamore, A.; Eckstein, M.; Scarpelli, M.; Lopez-Beltran, A.; Cheng, L.; Montironi, R. Predictive biomarkers for immunotherapy in the treatment of advanced urothelial carcinoma: Where we stand and where we go. Future Oncol. 2019, 15, 2199–2202. [Google Scholar] [CrossRef]
- Eckstein, M.; Cimadamore, A.; Hartmann, A.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Montironi, R.; Gevaert, T. PD-L1 assessment in urothelial carcinoma: A practical approach. Ann. Transl. Med. 2019, 7, 690. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Erben, P.; Kriegmair, M.C.; Worst, T.S.; Weiß, C.A.; Wirtz, R.M.; Wach, S.; Stoehr, R.; Sikic, D.; Geppert, C.I.; et al. Performance of the Food and Drug Administration/EMA-approved programmed cell death ligand-1 assays in urothelial carcinoma with emphasis on therapy stratification for first-line use of Atezolizumab and Pembrolizumab. Eur. J. Cancer 2019, 106, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Walker, J.; Andrew Williams, J.; Bellmunt, J. The evolving role of PD-L1 testing in patients with metastatic urothelial carcinoma. Cancer Treat. Rev. 2020, 82, 101925. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.-T.; Friedlander, T.W.; Hoimes, C.; Lee, J.-L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Updated efficacy and tolerability of Durvalumab in locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 2017, 35, 286. [Google Scholar] [CrossRef]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and safety of Durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017, 3, e172411. [Google Scholar] [CrossRef]
- Patel, M.R.; Ellerton, J.; Infante, J.R.; Agrawal, M.; Gordon, M.; Aljumaily, R.; Britten, C.D.; Dirix, L.; Lee, K.W.; Taylor, M.; et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018, 19, 51–64. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Bednova, O.; Leyton, J.V. Targeted molecular therapeutics for bladder cancer—a new option beyond the mixed fortunes of immune checkpoint inhibitors? Int. J. Mol. Sci. 2020, 21, 1–25. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija, J.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet (London, England) 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.L.; De Giorgi, U.; et al. Durvalumab alone and Durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588. [Google Scholar] [CrossRef]
- Yu, S.S.; Ballas, L.K.; Skinner, E.C.; Dorff, T.B.; Sadeghi, S.; Quinn, D.I. Immunotherapy in urothelial cancer, part 2: Adjuvant, neoadjuvant, and adjunctive treatment. Clin. Adv. Hematol. Oncol. 2017, 15, 543–551. [Google Scholar] [PubMed]
- Massari, F.; Santoni, M.; di Nunno, V.; Cheng, L.; Lopez-Beltran, A.; Cimadamore, A.; Gasparrini, S.; Scarpelli, M.; Battelli, N.; Montironi, R. Adjuvant and neoadjuvant approaches for urothelial cancer: Updated indications and controversies. Cancer Treat. Rev. 2018, 68, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Di Nunno, V.; Cubelli, M.; Santoni, M.; Fiorentino, M.; Montironi, R.; Cheng, L.; Lopez-Beltran, A.; Battelli, N.; Ardizzoni, A. Immune checkpoint inhibitors for metastatic bladder cancer. Cancer Treat. Rev. 2018, 64, 11–20. [Google Scholar] [CrossRef]
- Rouanne, M.; Bajorin, D.F.; Hannan, R.; Galsky, M.D.; Williams, S.B.; Necchi, A.; Sharma, P.; Powles, T. Rationale and outcomes for neoadjuvant immunotherapy in urothelial carcinoma of the bladder. Eur. Urol. Oncol. 2020, 3, 728–738. [Google Scholar] [CrossRef]
- Powles, T.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Font Pous, A.; Gravis, G.; Anido Herranz, U.; Protheroe, A.; Ravaud, A.; et al. A phase II study investigating the safety and efficacy of neoadjuvant Atezolizumab in muscle invasive bladder cancer (ABACUS). J. Clin. Oncol. 2018, 36, 4506. [Google Scholar] [CrossRef]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): An open-label, single-arm, phase II study. J. Clin. Oncol. 2018, 36, 3353–3360. [Google Scholar] [CrossRef]
- Powles, T.; Gschwend, J.E.; Loriot, Y.; Bellmunt, J.; Geczi, L.; Vulsteke, C.; Abdelsalam, M.; Gafanov, R.; Bae, W.K.; Revesz, J.; et al. Phase 3 KEYNOTE-361 trial: Pembrolizumab (pembro) with or without chemotherapy versus chemotherapy alone in advanced urothelial cancer. J. Clin. Oncol. 2017, 35, TPS4590. [Google Scholar] [CrossRef]
- Kamat, A.M.; Bellmunt, J.; Choueiri, T.K.; Nam, K.; De Santis, M.; Dreicer, R.; Hahn, N.M.; Perini, R.F.; Siefker-Radtke, A.O.; Sonpavde, G.; et al. KEYNOTE-057: Phase 2 study of Pembrolizumab for patients (pts) with Bacillus Calmette Guerin (BCG)-unresponsive, high-risk non-muscle-invasive bladder cancer (NMIBC). J. Clin. Oncol. 2016, 34, TPS4576. [Google Scholar] [CrossRef]
- Apolo, A.B.; Nadal, R.; Girardi, D.M.; Niglio, S.A.; Ley, L.; Cordes, L.M.; Steinberg, S.M.; Ortiz, O.S.; Cadena, J.; Diaz, C.; et al. Phase I Study of cabozantinib and nivolumab alone or with ipilimumab for advanced or metastatic urothelial carcinoma and other genitourinary tumors. J. Clin. Oncol. 2020, 38, 3672–3684. [Google Scholar] [CrossRef]
- Rebola, J.; Aguiar, P.; Blanca, A.; Montironi, R.; Cimadamore, A.; Cheng, L.; Henriques, V.; Lobato-Faria, P.; Lopez-Beltran, A. Predicting outcomes in non-muscle invasive (Ta/T1) bladder cancer: The role of molecular grade based on luminal/basal phenotype. Virchows Arch. 2019, 475, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Black, P.C.; Catherine, T.; Lerner, S.P.; McConkey, D.J.; Lucia, M.S.; Woods, M.; Bivalacqua, T.; Kassouf, W.; Bangs, R.C.; Plets, M.; et al. S1605: Phase II trial of Atezolizumab in BCG-unresponsive nonmuscle invasive bladder cancer. J. Clin. Oncol. 2018, 36, TPS527. [Google Scholar] [CrossRef]
- Emens, L.A.; Middleton, G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Correale, P.; Del Vecchio, M.T.; La Placa, M.; Montagnani, F.; Di Genova, G.; Savellini, G.G.; Terrosi, C.; Mannucci, S.; Giorgi, G.; Francini, G.; et al. Chemotherapeutic drugs may be used to enhance the killing efficacy of human tumor antigen peptide-specific CTLs. J. Immunother. 2008, 31, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Gómez de Liaño Lista, A.; van Dijk, N.; de Velasco Oria de Rueda, G.; Necchi, A.; Lavaud, P.; Morales-Barrera, R.; Alonso Gordoa, T.; Maroto, P.; Ravaud, A.; Durán, I.; et al. Clinical outcome after progressing to frontline and second-line Anti–PD-1/PD-L1 in advanced urothelial cancer[Formula presented]. Eur. Urol. 2020, 77, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Hayashi, H.; Chiba, Y.; Miyawaki, E.; Shimizu, J.; Ozaki, T.; Fujimoto, D.; Toyozawa, R.; Nakamura, A.; Kozuki, T.; et al. Propensity score-weighted analysis of chemotherapy after PD-1 inhibitors versus chemotherapy alone in patients with non-small cell lung cancer (WJOG10217L). J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Narits, J.; Tamm, H.; Jaal, J. PD-L1 induction in tumor tissue after hypofractionated thoracic radiotherapy for non-small cell lung cancer. Clin. Transl. Radiat. Oncol. 2020, 22, 83–87. [Google Scholar] [CrossRef]
- Jamal, S.; Hudson, M.; Fifi-Mah, A.; Ye, C. Immune-related adverse events associated with cancer immunotherapy: A review for the practicing rheumatologist. J. Rheumatol. 2020, 47, 166–175. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Sweis, R.F.; Spranger, S.; Bao, R.; Paner, G.P.; Stadler, W.M.; Steinberg, G.; Gajewski, T.F. Molecular drivers of the non- T-cell-inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunol. Res. 2016, 4, 563–568. [Google Scholar] [CrossRef]
- Sharma, P.; Siefker-Radtke, A.; de Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab alone and with Ipilimumab in previously treated metastatic urothelial carcinoma: CheckMate 032 Nivolumab 1 mg/kg plus Ipilimumab 3 mg/kg expansion cohort results. J. Clin. Oncol. 2019, 37, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Wang, H.; Hahn, N.M.; Twardowski, P.; Pal, S.K.; Albany, C.; Fleming, M.T.; Starodub, A.; Hauke, R.J.; Yu, M.; et al. Phase 2 trial of gemcitabine, cisplatin, plus ipilimumab in patients with metastatic urothelial cancer and impact of dna damage response gene mutations on outcomes. Eur. Urol. 2018, 73, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Allard, D.; Chrobak, P.; Allard, B.; Messaoudi, N.; Stagg, J. Targeting the CD73-adenosine axis in immuno-oncology. Immunol. Lett. 2019, 205, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Onesti, C.E.; Zizzari, I.; Cerbelli, B.; Sciattella, P.; Occhipinti, M.; Roberto, M.; Di Pietro, F.; Bonifacino, A.; Ghidini, M.; et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget 2017, 8, 99336–99346. [Google Scholar] [CrossRef] [PubMed]
- Faraj, S.F.; Munari, E.; Guner, G.; Taube, J.; Anders, R.; Hicks, J.; Meeker, A.; Schoenberg, M.; Bivalacqua, T.; Drake, C.; et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology 2015, 85, 703.e1–703.e6. [Google Scholar] [CrossRef]
- Cimadamore, A.; Scarpelli, M.; Santoni, M.; Massari, F.; Tartari, F.; Cerqueti, R.; Lopez-Beltran, A.; Cheng, L.; Montironi, R. Genitourinary tumors: Update on molecular biomarkers for diagnosis, prognosis and prediction of response to therapy. Curr. Drug Metab. 2019, 20, 305–312. [Google Scholar] [CrossRef]
- Reis, H.; Serrette, R.; Posada, J.; Lu, V.; Chen, Y.B.; Gopalan, A.; Fine, S.W.; Tickoo, S.K.; Sirintrapun, S.J.; Iyer, G.; et al. PD-L1 Expression in urothelial carcinoma with predominant or pure variant histology: Concordance among 3 commonly used and commercially available antibodies. Am. J. Surg. Pathol. 2019, 43, 920–927. [Google Scholar] [CrossRef]
- Gevaert, T.; Montironi, R.; Lopez-Beltran, A.; Van Leenders, G.; Allory, Y.; De Ridder, D.; Claessens, F.; Kockx, M.; Akand, M.; Joniau, S.; et al. Genito-urinary genomics and emerging biomarkers for immunomodulatory cancer treatment. Semin. Cancer Biol. 2018, 52, 216–227. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Santoni, M.; Massari, F.; Ciccarese, C.; Tortora, G.; Cheng, L.; Moch, H.; Scarpelli, M.; Reymundo, C.; Montironi, R. Bladder cancer: Molecular determinants of personalized therapy. Curr. Drug Targets 2015, 16, 115–124. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017, 171, 540–556. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Akbani, R.; Broom, B.M.; Wang, W.; Verhaak, R.G.W.; McConkey, D.; Lerner, S.; Morgan, M.; Creighton, C.J.; Smith, C.; et al. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Cimadamore, A.; Gasparrini, S.; Santoni, M.; Cheng, L.; Lopez-Beltran, A.; Battelli, N.; Massari, F.; Giunchi, F.; Fiorentino, M.; Scarpelli, M.; et al. Biomarkers of aggressiveness in genitourinary tumors with emphasis on kidney, bladder, and prostate cancer. Expert Rev. Mol. Diagn. 2018, 18, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Catalano, M.; Nobili, S.; Santi, R.; Mini, E.; Nesi, G. Focus on biochemical and clinical predictors of response to immune checkpoint inhibitors in metastatic urothelial carcinoma: Where do we stand? Int. J. Mol. Sci. 2020, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ma, J.; Liu, X.; Liu, Z. Identification of four immune subtypes in bladder cancer based on immune gene Sets. Front. Oncol. 2020, 10, 544610. [Google Scholar] [CrossRef]
- Lemery, S.; Keegan, P.; Pazdur, R. First FDA approval agnostic of cancer site—when a biomarker defines the Indication. N. Engl. J. Med. 2017, 377, 1409–1412. [Google Scholar] [CrossRef]
- Teo, M.Y.; Seier, K.; Ostrovnaya, I.; Regazzi, A.M.; Kania, B.E.; Moran, M.M.; Cipolla, C.K.; Bluth, M.J.; Chaim, J.; Al-Ahmadie, H.; et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J. Clin. Oncol. 2018, 36, 1685–1694. [Google Scholar] [CrossRef]
- Phelan, A.; Lopez-Beltran, A.; Montironi, R.; Zhang, S.; Raspollini, M.R.; Cheng, M.; Kaimakliotis, H.Z.; Koch, M.O.; Cheng, L. Inherited forms of bladder cancer: A review of Lynch syndrome and other inherited conditions. Futur. Oncol. 2018, 14, 277–290. [Google Scholar] [CrossRef]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef]
- Vidotto, T.; Nersesian, S.; Graham, C.; Siemens, D.R.; Koti, M. DNA damage repair gene mutations and their association with tumor immune regulatory gene expression in muscle invasive bladder cancer subtypes. J. Immunother. Cancer 2019, 7, 148. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Blanca, A.; Cheng, L.; Montironi, R.; Moch, H.; Massari, F.; Fiorentino, M.; Raspollini, M.R.; Scarpelli, M.; Lopez-Beltran, A. Mirna expression in bladder cancer and their potential role in clinical practice. Curr. Drug Metab. 2017, 18, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, C.; Massari, F.; Blanca, A.; Tortora, G.; Montironi, R.; Cheng, L.; Scarpelli, M.; Raspollini, M.R.; Vau, N.; Fonseca, J.; et al. Tp53 and its potential therapeutic role as a target in bladder cancer. Expert Opin. Ther. Targets 2017, 21, 401–414. [Google Scholar] [CrossRef] [PubMed]
| Trial (Year) | Phase | Treatment Arms | N | Disease Stage | Line of Therapy | Characteristics |
|---|---|---|---|---|---|---|
| IMvigor210 (2016) [16] | 2 | Atezolizumab monotherapy in c-DDP unfit | 123 | mMIBC | 1st | ORR: 23% (9% cRR) mPFS: 2.7 mo mOS: 15.9 mo |
| IMvigor130 (2020) [39] | 3 | Atezolizumab + platinum-based chemo (A) vs. Atezolizumab monotherapy (B) vs. platinum-based chemo (C) | 1213 | mMIBC | 1st | mPFS: 8.2 vs. 6.3 mo (A vs. C) (p = 0.007) mOS: 16 vs. 13.4 mo (A vs C) (p = 0.027) mOS: 15.7 vs. 13.1 mo (B vs. C) |
| JAVELIN Bladder 100 (2020) [37] | 3 | Avelumab (A) maintenance + BSC vs. BSC | 700 | mMIBC | 1st | mOS: 21.4 vs. 14.3 mo (A vs. BSC, all pts) (p = 0.001) mOS: NE vs. 17.1 mo (A vs. BSC, PD-L1 ≥ 5% TC+) (p < 0.001) |
| DANUBE (2020) [40] | 3 | Durvalumab (D) monotherapy vs. D + Tremelimumab (T) vs. platinum-based chemo | 1032 | mMIBC | 1st | mOS: 14.4 vs. 12.1 mo (D vs. chemo, PD-L1 +) (p = 0.30) mOS: 15.1 vs. 12.1 (D + T vs. chemo, all pts) (p = 0.075) |
| CheckMate275 (2017) [20] | 2 | Nivolumab after platinum-based chemo | 270 | mMIBC | 2nd | ORR: 19.6% (52/265). Paters of responses unrelated to PD-L1 status |
| KEYNOTE-045 (2017) [26] | 3 | Pembrolizumab (P) vs. chemo-regimen (TXT or PTX or vinflunine) | 542 | mMIBC | 2nd | mOS: 10.3 vs. 7.4 (P vs chemo, all pts) (p = 0.002) mOS: 8.0 vs. 5.2 (P vs chemo, PD-L1 status CPS ≥ 10%) (p = 0.005) |
| Trial Identifier | Phase | N | Line of Therapy | Characteristics |
|---|---|---|---|---|
| NCT02632409 | 3 | 640 | After surgery and/or neoadjuvant chemotherapy | Adjuvant Nivolumab (CheckMate 274) |
| NCT02450331 | 3 | 700 | After surgery and/or neoadjuvant chemotherapy | Adjuvant Atezolizumab (IMvigor 010/WO29636) |
| NCT02736266 | 2 | 90 | Neoadjuvant prior to chemoradiation | Neoadjuvant Pembrolizumab for muscle invasive bladder cancer (PURE-01) |
| NCT02365766 | 1/2 | 81 | Neoadjuvant | Neoadjuvant Pembrolizumab + gemcitabine vs Pembrolizumab + gemcitabine/cisplatin |
| NCT02845323 | 2 | 44 | Neoadjuvant | Neoadjuvant Nivolumab + urelumab vs Nivolumab monotherapy |
| NCT02690558 | 2 | 39 | Neoadjuvant | Neoadjuvant Pembrolizumab + gemcitabine/cisplatin |
| NCT02662309 | 2 | 85 | Neoadjuvant | Neoadjuvant Atezolizumab (ABACUS) |
| Properties | Avelumab | Durvalumab | Atezolizumab | Nivolumab | Pembrolizumab | |||
|---|---|---|---|---|---|---|---|---|
| PD-L1 assay (antibody) | Dako 73-10 | Ventana SP263 | Ventana SP142 | Dako 28.8 | Dako 22C3 | |||
| Cell types scored for UC | TC | IC and TC | IC | TC | TC and IC | |||
| PD-L1 cut-offs: High/positive Low/negative | ≥5% TC No visible staining | ≥25% TC or IC <25% TC and IC | ≥5% IC <1% IC | ≥5% IC <1% IC | ≥1% TC <1% TC | ≥1%, ≥5% TC <1% TC | ≥10% CPS NA | ≥10% CPS <10% CPS |
| Study (Phase) | JAVELIN - UC cohort (phase 1b) | Study 1108- UC cohort (phase 1/2) | IMvigor 210 (phase 2) | IMvigor 210 (phase 2) | CM-032 UC cohort (phase 1/2) | CM-275 (phase 2) | KN-045 (phase 3) | KN-052 (phase 2) |
| Line of therapy | ≥2L | ≥1L | ≥2L | 1L | ≥2L | ≥2L | 2L | 1L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Beltran, A.; Cimadamore, A.; Blanca, A.; Massari, F.; Vau, N.; Scarpelli, M.; Cheng, L.; Montironi, R. Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer. Cancers 2021, 13, 131. https://doi.org/10.3390/cancers13010131
Lopez-Beltran A, Cimadamore A, Blanca A, Massari F, Vau N, Scarpelli M, Cheng L, Montironi R. Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer. Cancers. 2021; 13(1):131. https://doi.org/10.3390/cancers13010131
Chicago/Turabian StyleLopez-Beltran, Antonio, Alessia Cimadamore, Ana Blanca, Francesco Massari, Nuno Vau, Marina Scarpelli, Liang Cheng, and Rodolfo Montironi. 2021. "Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer" Cancers 13, no. 1: 131. https://doi.org/10.3390/cancers13010131
APA StyleLopez-Beltran, A., Cimadamore, A., Blanca, A., Massari, F., Vau, N., Scarpelli, M., Cheng, L., & Montironi, R. (2021). Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer. Cancers, 13(1), 131. https://doi.org/10.3390/cancers13010131








