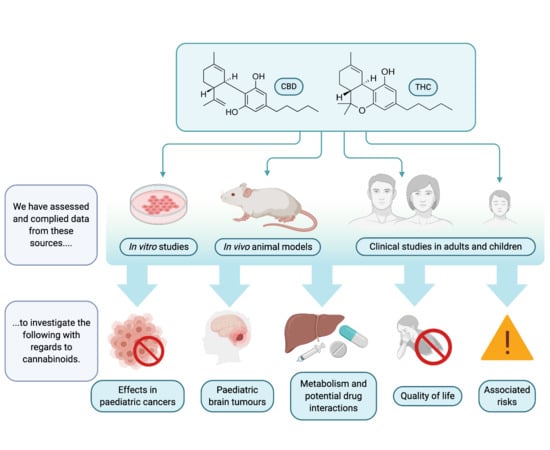
The Role of Cannabinoids as Anticancer Agents in Pediatric Oncology
Abstract
:Simple Summary
Abstract
1. Introduction
2. Effects of Cannabinoids in Pediatric Cancer
3. Cannabinoids and Pediatric Brain Tumors
4. Clinical Evidence of the Effect of Cannabinoids in Pediatric Brain Cancer
5. Cannabinoid Metabolism and Potential Interactions with Other Cancer Therapeutics
6. Role for Cannabinoids to Improve Quality of Life for Pediatric Cancer Patients
7. Risk of Cannabinoid Use in Children
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvi, L.; Pentimalli, D.; Panseri, S.; Giupponi, L.; Gelmini, F.; Beretta, G.; Vitali, D.; Bruno, M.; Zilio, E.; Pavlovic, R.; et al. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC-MS and LC-HRMS (q-exactive orbitrap®) approach. J. Pharm. Biomed. Anal. 2018, 150, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936. [Google Scholar] [CrossRef] [Green Version]
- Atwood, B.K.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Castillo, A.; Tolón, M.R.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and Endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 637–659. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Chiurchiù, V.; Díaz-Alonso, J.; Bari, M.; Guzmán, M.; Maccarrone, M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res. 2013, 52, 633–650. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Anticancer mechanisms of cannabinoids. Curr. Oncol. 2016, 23, S23–S32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, I.M.; Wright, A.; Peteet, J.; Meyer, F.L.; Yuppa, D.P.; Bolcic-Jankovic, D.; LeBlanc, J.; Chang, Y.; Yu, L.; Nayak, M.M.; et al. Medical Oncologists’ Beliefs, Practices, and Knowledge Regarding Marijuana Used Therapeutically: A Nationally Representative Survey Study. J. Clin. Oncol. 2018, 36, 1957–1962. [Google Scholar] [CrossRef]
- Zylla, D.; Steele, G.; Eklund, J.; Mettner, J.; Arneson, T. Oncology Clinicians and the Minnesota Medical Cannabis Program: A Survey on Medical Cannabis Practice Patterns, Barriers to Enrollment, and Educational Needs. Cannabis Cannabinoid Res. 2018, 3, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkie, G.; Sakr, B.; Rizack, T. Medical Marijuana Use in Oncology: A Review. JAMA Oncol. 2016, 2, 670–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, J.N.; Colbert, J.A. Clinical decisions. Medicinal use of marijuana--polling results. N. Engl. J. Med. 2013, 368, e30. [Google Scholar] [CrossRef]
- Ryan, J.E.; Smeltzer, S.C.; Sharts-Hopko, N.C. Parents’ experiences using medical cannabis for their child. Nurs. Outlook 2020. [Google Scholar] [CrossRef]
- Ananth, P.; Reed-Weston, A.; Wolfe, J. Medical marijuana in pediatric oncology: A review of the evidence and implications for practice. Pediatr. Blood Cancer 2018, 65. [Google Scholar] [CrossRef]
- Ramer, R.; Hinz, B. Cannabinoids as Anticancer Drugs. Adv. Pharmacol. 2017, 80, 397–436. [Google Scholar] [CrossRef]
- Lombard, C.; Nagarkatti, M.; Nagarkatti, P.S. Targeting cannabinoid receptors to treat leukemia: Role of cross-talk between extrinsic and intrinsic pathways in Delta9-tetrahydrocannabinol (THC)-induced apoptosis of Jurkat cells. Leuk. Res. 2005, 29, 915–922. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-Lopez, L.; Valle-Reyes, J.S.; Hernandez-Cruz, A.; Pottosin, I.; Dobrovinskaya, O. Cannabidiol directly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell Death Dis. 2019, 10, 779. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Hegde, V.L.; Singh, N.P.; Sisco, D.; Grant, S.; Nagarkatti, M.; Nagarkatti, P.S. Delta9-tetrahydrocannabinol-induced apoptosis in Jurkat leukemia T cells is regulated by translocation of Bad to mitochondria. Mol. Cancer Res. 2006, 4, 549–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, B.; Carracedo, A.; Diez-Zaera, M.; Gómez del Pulgar, T.; Guzmán, M.; Velasco, G. The CB2 cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Exp. Cell Res. 2006, 312, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Soto-Mercado, V.; Mendivil-Perez, M.; Jimenez-Del-Rio, M.; Fox, J.E.; Velez-Pardo, C. Cannabinoid CP55940 selectively induces apoptosis in Jurkat cells and in ex vivo T-cell acute lymphoblastic leukemia through H(2)O(2) signaling mechanism. Leuk. Res. 2020, 95, 106389. [Google Scholar] [CrossRef]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Tiedra, S.; Fabriàs, G.; Dávila, D.; Salanueva, Í.J.; Casas, J.; Montes, L.R.; Antón, Z.; García-Taboada, E.; Salazar-Roa, M.; Lorente, M.; et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy 2016, 12, 2213–2229. [Google Scholar] [CrossRef] [Green Version]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, D., Jr.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef]
- Kalenderoglou, N.; Macpherson, T.; Wright, K.L. Cannabidiol Reduces Leukemic Cell Size—But Is It Important? Front. Pharmacol. 2017, 8, 144. [Google Scholar] [CrossRef] [Green Version]
- Salazar, M.; Carracedo, A.; Salanueva, Í.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [Green Version]
- Vara, D.; Salazar, M.; Olea-Herrero, N.; Guzmán, M.; Velasco, G.; Díaz-Laviada, I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011, 18, 1099–1111. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.M.; Scott, K.A.; Shamash, J.; Joel, S.; Powles, T.B. Enhancing the in vitro cytotoxic activity of Delta9-tetrahydrocannabinol in leukemic cells through a combinatorial approach. Leuk. Lymphoma 2008, 49, 1800–1809. [Google Scholar] [CrossRef]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. Int. J. Oncol. 2017, 51, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Oesch, S.; Walter, D.; Wachtel, M.; Pretre, K.; Salazar, M.; Guzman, M.; Velasco, G.; Schafer, B.W. Cannabinoid receptor 1 is a potential drug target for treatment of translocation-positive rhabdomyosarcoma. Mol. Cancer Ther. 2009, 8, 1838–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carracedo, A.; Gironella, M.; Lorente, M.; Garcia, S.; Guzmán, M.; Velasco, G.; Iovanna, J.L. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res. 2006, 66, 6748–6755. [Google Scholar] [CrossRef] [Green Version]
- Notaro, A.; Sabella, S.; Pellerito, O.; Di Fiore, R.; De Blasio, A.; Vento, R.; Calvaruso, G.; Giuliano, M. Involvement of PAR-4 in cannabinoid-dependent sensitization of osteosarcoma cells to TRAIL-induced apoptosis. Int. J. Biol. Sci. 2014, 10, 466–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, T.; Golan, H.; Schiby, G.; PriChen, S.; Smoum, R.; Moshe, I.; Peshes-Yaloz, N.; Castiel, A.; Waldman, D.; Gallily, R.; et al. In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Curr. Oncol. 2016, 23, S15–S22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Liu, Y.; Liu, Y.; Alexandrov, L.B.; Edmonson, M.N.; Gawad, C.; Zhou, X.; Li, Y.; Rusch, M.C.; Easton, J.; et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature 2018, 555, 371–376. [Google Scholar] [CrossRef]
- Borrell, B. How accurate are cancer cell lines? Nature 2010, 463, 858. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- López-Valero, I.; Saiz-Ladera, C.; Torres, S.; Hernández-Tiedra, S.; García-Taboada, E.; Rodríguez-Fornés, F.; Barba, M.; Dávila, D.; Salvador-Tormo, N.; Guzmán, M.; et al. Targeting Glioma Initiating Cells with A combined therapy of cannabinoids and temozolomide. Biochem. Pharmacol. 2018, 157, 266–274. [Google Scholar] [CrossRef]
- Torres, S.; Lorente, M.; Rodríguez-Fornés, F.; Hernández-Tiedra, S.; Salazar, M.; García-Taboada, E.; Barcia, J.; Guzmán, M.; Velasco, G. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol. Cancer Ther. 2011, 10, 90–103. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. The combination of cannabidiol and Delta9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol. Cancer Ther. 2014, 13, 2955–2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellert-Miklaszewska, A.; Grajkowska, W.; Gabrusiewicz, K.; Kaminska, B.; Konarska, L. Distinctive pattern of cannabinoid receptor type II (CB2) expression in adult and pediatric brain tumors. Brain Res. 2007, 1137, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Sredni, S.T.; Huang, C.C.; Suzuki, M.; Pundy, T.; Chou, P.; Tomita, T. Spontaneous involution of pediatric low-grade gliomas: High expression of cannabinoid receptor 1 (CNR1) at the time of diagnosis may indicate involvement of the endocannabinoid system. Childs Nerv. Syst. 2016, 32, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Poppleton, H.; Fuller, C.; Su, X.; Liu, Y.; Jensen, P.; Magdaleno, S.; Dalton, J.; Calabrese, C.; Board, J.; et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell 2005, 8, 323–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vladoiu, M.C.; El-Hamamy, I.; Donovan, L.K.; Farooq, H.; Holgado, B.L.; Sundaravadanam, Y.; Ramaswamy, V.; Hendrikse, L.D.; Kumar, S.; Mack, S.C.; et al. Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature 2019, 572, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Petersen, G.; Moesgaard, B.; Schmid, P.C.; Schmid, H.H.; Broholm, H.; Kosteljanetz, M.; Hansen, H.S. Endocannabinoid metabolism in human glioblastomas and meningiomas compared to human non-tumour brain tissue. J. Neurochem. 2005, 93, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, L.; Zhang, X.; Li, L.; Jiang, C.; Qiu, Y.; Huang, R.; Xie, B.; Lin, Z.; Ren, J.; et al. Alteration of endocannabinoid system in human gliomas. J. Neurochem. 2012, 120, 842–849. [Google Scholar] [CrossRef]
- Maccarrone, M.; Attinà, M.; Cartoni, A.; Bari, M.; Finazzi-Agrò, A. Gas chromatography-mass spectrometry analysis of endogenous cannabinoids in healthy and tumoral human brain and human cells in culture. J. Neurochem. 2001, 76, 594–601. [Google Scholar] [CrossRef]
- De Jesús, M.L.; Hostalot, C.; Garibi, J.M.; Sallés, J.; Meana, J.J.; Callado, L.F. Opposite changes in cannabinoid CB1 and CB2 receptor expression in human gliomas. Neurochem. Int. 2010, 56, 829–833. [Google Scholar] [CrossRef]
- Foroughi, M.; Hendson, G.; Sargent, M.A.; Steinbok, P. Spontaneous regression of septum pellucidum/forniceal pilocytic astrocytomas--possible role of Cannabis inhalation. Childs Nerv. Syst. 2011, 27, 671–679. [Google Scholar] [CrossRef]
- Kenyon, J.; Liu, W.; Dalgleish, A. Report of Objective Clinical Responses of Cancer Patients to Pharmaceutical-grade Synthetic Cannabidiol. Anticancer Res. 2018, 38, 5831–5835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suraev, A.; Lintzeris, N.; Stuart, J.; Kevin, R.C.; Blackburn, R.; Richards, E.; Arnold, J.C.; Ireland, C.; Todd, L.; Allsop, D.J.; et al. Composition and Use of Cannabis Extracts for Childhood Epilepsy in the Australian Community. Sci. Rep. 2018, 8, 10154. [Google Scholar] [CrossRef]
- Matsunaga, T.; Iwawaki, Y.; Watanabe, K.; Yamamoto, I.; Kageyama, T.; Yoshimura, H. Metabolism of delta 9-tetrahydrocannabinol by cytochrome P450 isozymes purified from hepatic microsomes of monkeys. Life Sci. 1995, 56, 2089–2095. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome p450 and chemical toxicology. Chem. Res. Toxicol. 2008, 21, 70–83. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Queiroz, R.H.; Zuardi, A.W.; Crippa, J.A. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Huang, Z.; Roy, P.; Waxman, D.J. Role of human liver microsomal CYP3A4 and CYP2B6 in catalyzing N-dechloroethylation of cyclophosphamide and ifosfamide. Biochem. Pharmacol. 2000, 59, 961–972. [Google Scholar] [CrossRef]
- Baumhäkel, M.; Kasel, D.; Rao-Schymanski, R.A.; Böcker, R.; Beckurts, K.T.; Zaigler, M.; Barthold, D.; Fuhr, U. Screening for inhibitory effects of antineoplastic agents on CYP3A4 in human liver microsomes. Int. J. Clin. Pharmacol. Ther. 2001, 39, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Bebin, E.M.; Cutter, G.R.; Liu, Y.; Szaflarski, J.P.; Program, U.C. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 2017, 58, 1586–1592. [Google Scholar] [CrossRef] [Green Version]
- Krishna, D.R.; Klotz, U. Extrahepatic metabolism of drugs in humans. Clin. Pharm. 1994, 26, 144–160. [Google Scholar] [CrossRef]
- Ferguson, C.S.; Tyndale, R.F. Cytochrome P450 enzymes in the brain: Emerging evidence of biological significance. Trends Pharmacol. Sci. 2011, 32, 708–714. [Google Scholar] [CrossRef] [Green Version]
- McLeod, H.L.; Relling, M.V.; Crom, W.R.; Silverstein, K.; Groom, S.; Rodman, J.H.; Rivera, G.K.; Crist, W.M.; Evans, W.E. Disposition of antineoplastic agents in the very young child. Br. J. Cancer Suppl. 1992, 18, S23–S29. [Google Scholar] [PubMed]
- Zane, N.R.; Chen, Y.; Wang, M.Z.; Thakker, D.R. Cytochrome P450 and flavin-containing monooxygenase families: Age-dependent differences in expression and functional activity. Pediatr. Res. 2018, 83, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Wheless, J.W.; Dlugos, D.; Miller, I.; Oh, D.A.; Parikh, N.; Phillips, S.; Renfroe, J.B.; Roberts, C.M.; Saeed, I.; Sparagana, S.P.; et al. Pharmacokinetics and Tolerability of Multiple Doses of Pharmaceutical-Grade Synthetic Cannabidiol in Pediatric Patients with Treatment-Resistant Epilepsy. CNS Drugs 2019, 33, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Sallan, S.E.; Zinberg, N.E.; Frei, E., 3rd. Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. N. Engl. J. Med. 1975, 293, 795–797. [Google Scholar] [CrossRef]
- Chang, A.E.; Shiling, D.J.; Stillman, R.C.; Goldberg, N.H.; Seipp, C.A.; Barofsky, I.; Simon, R.M.; Rosenberg, S.A. Delata-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. Ann. Intern. Med. 1979, 91, 819–824. [Google Scholar] [CrossRef]
- Sallan, S.E.; Cronin, C.; Zelen, M.; Zinberg, N.E. Antiemetics in patients receiving chemotherapy for cancer: A randomized comparison of delta-9-tetrahydrocannabinol and prochlorperazine. N. Engl. J. Med. 1980, 302, 135–138. [Google Scholar] [CrossRef]
- Chang, A.E.; Shiling, D.J.; Stillman, R.C.; Goldberg, N.H.; Seipp, C.A.; Barofsky, I.; Rosenberg, S.A. A prospective evaluation of delta-9-tetrahydrocannabinol as an antiemetic in patients receiving adriamycin and cytoxan chemotherapy. Cancer 1981, 47, 1746–1751. [Google Scholar] [CrossRef]
- Skrypek, M.M.; Bostrom, B.C.; Bendel, A.E. Medical Cannabis Certification in a Large Pediatric Oncology Center. Children 2019, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- Udaka, Y.T.; Packer, R.J. Pediatric Brain Tumors. Neurol. Clin. 2018, 36, 533–556. [Google Scholar] [CrossRef]
- Wong, S.S.; Wilens, T.E. Medical Cannabinoids in Children and Adolescents: A Systematic Review. Pediatrics 2017, 140, e20171818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, P.P.; Bebin, E.M.; Nabors, L.B.; Szaflarski, J.P. The use of cannabidiol for seizure management in patients with brain tumor-related epilepsy. Neurocase 2017, 23, 287–291. [Google Scholar] [CrossRef]
- Ofir, R.; Bar-Sela, G.; Weyl Ben-Arush, M.; Postovsky, S. Medical marijuana use for pediatric oncology patients: Single institution experience. Pediatr. Hematol. Oncol. 2019, 36, 255–266. [Google Scholar] [CrossRef]
- Borgelt, L.M.; Franson, K.L.; Nussbaum, A.M.; Wang, G.S. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 2013, 33, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorris, K.; Channell, J.; Hemenway, M.; Baroffio, A.; Ellison, M.; Brionse, N.; Griesinger, A.; Donson, A.; Madden, J.; Van Essen, C.; et al. QOL-52. Use of Cannabinoids in the Pediatric Central Nervous System Tumor Population. Neuro-Oncol. 2018, 20, i168. [Google Scholar] [CrossRef] [Green Version]
- Meyer, H.C.; Lee, F.S.; Gee, D.G. The Role of the Endocannabinoid System and Genetic Variation in Adolescent Brain Development. Neuropsychopharmacology 2018, 43, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Harkany, T.; Guzmán, M.; Galve-Roperh, I.; Berghuis, P.; Devi, L.A.; Mackie, K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 2007, 28, 83–92. [Google Scholar] [CrossRef]
- Ahmed, K.T.; Amin, M.R.; Shah, P.; Ali, D.W. Motor neuron development in zebrafish is altered by brief (5-hr) exposures to THC ((9)-tetrahydrocannabinol) or CBD (cannabidiol) during gastrulation. Sci. Rep. 2018, 8, 10518. [Google Scholar] [CrossRef] [Green Version]
- Dalterio, S.L. Perinatal or adult exposure to cannabinoids alters male reproductive functions in mice. Pharmacol. Biochem. Behav. 1980, 12, 143–153. [Google Scholar] [CrossRef]
- Navarro, M.; Rodríguez de Fonseca, F.; Hernández, M.L.; Ramos, J.A.; Fernández-Ruiz, J.J. Motor behavior and nigrostriatal dopaminergic activity in adult rats perinatally exposed to cannabinoids. Pharmacol. Biochem. Behav. 1994, 47, 47–58. [Google Scholar] [CrossRef]
- de Salas-Quiroga, A.; Díaz-Alonso, J.; García-Rincón, D.; Remmers, F.; Vega, D.; Gómez-Cañas, M.; Lutz, B.; Guzmán, M.; Galve-Roperh, I. Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proc. Natl. Acad. Sci. USA 2015, 112, 13693–13698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dow-Edwards, D.; Silva, L. Endocannabinoids in brain plasticity: Cortical maturation, HPA axis function and behavior. Brain Res. 2017, 1654, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadland, S.E.; Harris, S.K. Youth marijuana use: State of the science for the practicing clinician. Curr. Opin. Pediatr. 2014, 26, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse health effects of marijuana use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef] [Green Version]
- Meier, M.H.; Caspi, A.; Danese, A.; Fisher, H.L.; Houts, R.; Arseneault, L.; Moffitt, T.E. Associations between adolescent cannabis use and neuropsychological decline: A longitudinal co-twin control study. Addiction 2018, 113, 257–265. [Google Scholar] [CrossRef]
- Jackson, N.J.; Isen, J.D.; Khoddam, R.; Irons, D.; Tuvblad, C.; Iacono, W.G.; McGue, M.; Raine, A.; Baker, L.A. Impact of adolescent marijuana use on intelligence: Results from two longitudinal twin studies. Proc. Natl. Acad. Sci. USA 2016, 113, E500–E508. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Ryan, N.; Pingault, J.B.; Parent, S.; Vitaro, F.; Tremblay, R.E.; Seguin, J.R. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: A longitudinal study from early adolescence to young adulthood. Dev. Psychopathol. 2017, 29, 1253–1266. [Google Scholar] [CrossRef] [Green Version]
- Nabbout, R.; Thiele, E.A. The role of cannabinoids in epilepsy treatment: A critical review of efficacy results from clinical trials. Epileptic Disord. 2020, 22, 23–28. [Google Scholar] [CrossRef]
- Golan, H.; Fisher, T.; Toren, A. The Role of Cannabinoids in the Treatment of Cancer in Pediatric Patients. ISR Med. Assoc. J. 2017, 19, 89–94. [Google Scholar] [PubMed]
- Ammerman, S.; Ryan, S.; Adelman, W.P. Committee on Substance Abuse, t.C.o.A. The impact of marijuana policies on youth: Clinical, research, and legal update. Pediatrics 2015, 135, e769–e785. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andradas, C.; Truong, A.; Byrne, J.; Endersby, R. The Role of Cannabinoids as Anticancer Agents in Pediatric Oncology. Cancers 2021, 13, 157. https://doi.org/10.3390/cancers13010157
Andradas C, Truong A, Byrne J, Endersby R. The Role of Cannabinoids as Anticancer Agents in Pediatric Oncology. Cancers. 2021; 13(1):157. https://doi.org/10.3390/cancers13010157
Chicago/Turabian StyleAndradas, Clara, Alexandra Truong, Jacob Byrne, and Raelene Endersby. 2021. "The Role of Cannabinoids as Anticancer Agents in Pediatric Oncology" Cancers 13, no. 1: 157. https://doi.org/10.3390/cancers13010157