Thyroid Hürthle Cell Carcinoma: Clinical, Pathological, and Molecular Features
Abstract
:Simple Summary
Abstract
1. Introduction
2. Nomenclature and Definition
3. Clinical Features
3.1. Demographic Features
3.2. Laboratory Tests
3.3. Ultrasounds
3.4. Clinical Indicators
4. Treatment
5. Pathological Features
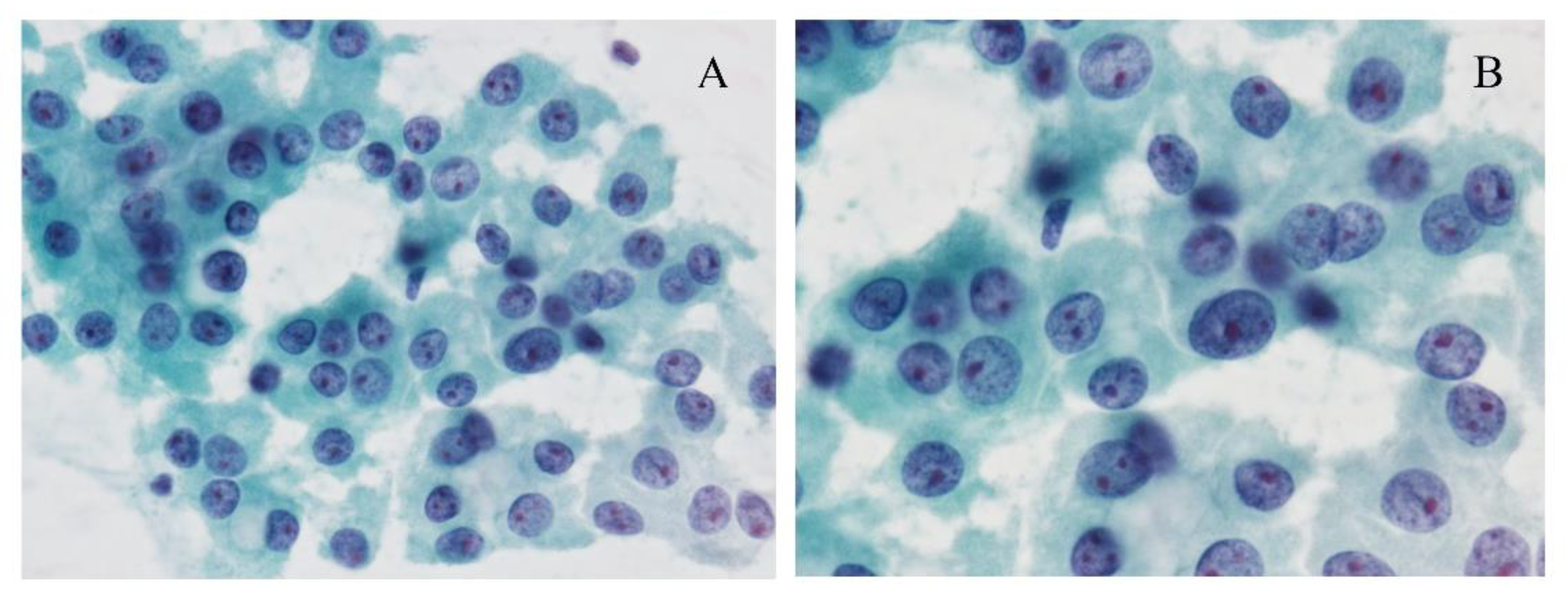
5.1. Cytological Features and Differential Diagnosis
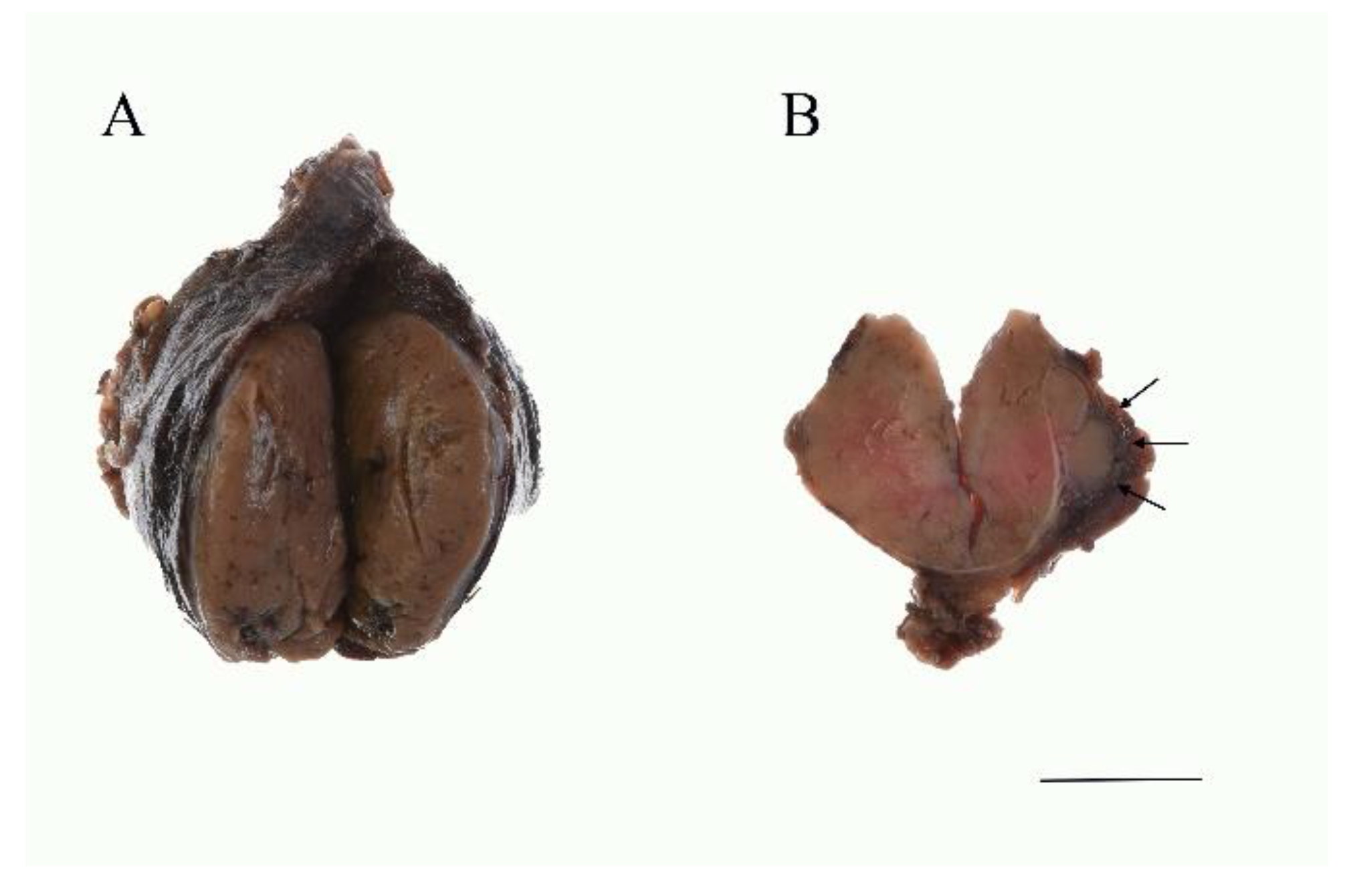
5.2. Macroscopic Features
5.3. Histological Features and Differential Diagnosis at Histology
5.4. Ultrastructure
6. Molecular Changes
6.1. Genetic Change in Genes Involved in RAS/RAF/MAPK and PIK3/Akt/mTOR Pathways
6.2. Genetic Rearrangements
6.3. TP53
6.4. TERT Promoter Mutations
6.5. Tumor Mutational Burden and Global Copy Number Diversity
6.6. Mutations in Mitochondrial DNA and Related Genes
6.7. Other Molecular Changes
7. Prognosis and Prognostic Factors
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Wei, S.; LiVolsi, V.A.; Montone, K.T.; Morrissette, J.J.D.; Baloch, Z.W. PTEN and TP53 Mutations in Oncocytic Follicular Carcinoma. Endocr. Pathol. 2015, 26, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2009, 19, 1167–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chindris, A.M.; Casler, J.D.; Bernet, V.J.; Rivera, M.; Thomas, C.; Kachergus, J.M.; Necela, B.M.; Hay, I.D.; Westphal, S.A.; Grant, C.S.; et al. Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J. Clin. Endocrinol. Metab. 2015, 100, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montone, K.T.; Baloch, Z.W.; LiVolsi, V.A. The thyroid Hürthle (oncocytic) cell and its associated pathologic conditions: A surgical pathology and cytopathology review. Arch. Pathol. Lab. Med. 2008, 132, 1241–1250. [Google Scholar]
- Tsybrovskyy, O.; Rossmann-Tsybrovskyy, M. Oncocytic versus mitochondrion-rich follicular thyroid tumours: Should we make a difference? Histopathology 2009, 55, 665–682. [Google Scholar] [CrossRef]
- Maximo, V.; Lima, J.; Prazeres, H.; Soares, P.; Sobrinho-Simoes, M. The biology and the genetics of Hurthle cell tumors of the thyroid. Endocr. Relat. Cancer 2012, 19, R131–R147. [Google Scholar] [CrossRef] [Green Version]
- Cavadas, B.; Pereira, J.B.; Correia, M.; Fernandes, V.; Eloy, C.; Sobrinho-Simões, M.; Soares, P.; Samuels, D.C.; Máximo, V.; Pereira, L. Genomic and transcriptomic characterization of the mitochondrial-rich oncocytic phenotype on a thyroid carcinoma background. Mitochondrion 2019, 46, 123–133. [Google Scholar] [CrossRef]
- Zito, G.; Richiusa, P.; Bommarito, A.; Carissimi, E.; Russo, L.; Coppola, A.; Zerilli, M.; Rodolico, V.; Criscimanna, A.; Amato, M.; et al. In vitro identification and characterization of CD133(pos) cancer stem-like cells in anaplastic thyroid carcinoma cell lines. PLoS ONE 2008, 3, e3544. [Google Scholar] [CrossRef] [Green Version]
- Hardin, H.; Montemayor-Garcia, C.; Lloyd, R.V. Thyroid cancer stem-like cells and epithelial-mesenchymal transition in thyroid cancers. Hum. Pathol. 2013, 44, 1707–1713. [Google Scholar] [CrossRef]
- Todaro, M.; Iovino, F.; Eterno, V.; Cammareri, P.; Gambara, G.; Espina, V.; Gulotta, G.; Dieli, F.; Giordano, S.; De Maria, R.; et al. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 2010, 70, 8874–8885. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Cui, D.; Xu, S.; Brabant, G.; Derwahl, M. Doxorubicin fails to eradicate cancer stem cells derived from anaplastic thyroid carcinoma cells: Characterization of resistant cells. Int. J. Oncol. 2010, 37, 307–315. [Google Scholar] [PubMed] [Green Version]
- Ohashi, R.; Kawahara, K.; Namimatsu, S.; Okamura, R.; Igarashi, T.; Sugitani, I.; Naito, Z. Expression of MRP1 and ABCG2 is associated with adverse clinical outcomes of papillary thyroid carcinoma with a solid component. Hum. Pathol. 2017, 67, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mitsutake, N.; Iwao, A.; Nagai, K.; Namba, H.; Ohtsuru, A.; Saenko, V.; Yamashita, S. Characterization of side population in thyroid cancer cell lines: Cancer stem-like cells are enriched partly but not exclusively. Endocrinology 2007, 148, 1797–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Reeb, A.N.; Sewell, W.A.; Elhomsy, G.; Lin, R.Y. Phenotypic characterization of metastatic anaplastic thyroid cancer stem cells. PLoS ONE 2013, 8, e65095. [Google Scholar] [CrossRef] [Green Version]
- Haigh, P.I.; Urbach, D.R. The treatment and prognosis of Hurthle cell follicular thyroid carcinoma compared with its non-Hurthle cell counterpart. Surgery 2005, 138, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Oluic, B.; Paunovic, I.; Loncar, Z.; Djukic, V.; Diklic, A.; Jovanovic, M.; Garabinovic, Z.; Slijepcevic, N.; Rovcanin, B.; Micic, D.; et al. Survival and prognostic factors for survival, cancer specific survival and disease free interval in 239 patients with Hurthle cell carcinoma: A single center experience. BMC Cancer 2017, 17, 371. [Google Scholar] [CrossRef] [Green Version]
- Santana, N.O.; Freitas, R.M.C.; Marcos, V.N.; Chammas, M.C.; Camargo, R.Y.A.; Schmerling, C.K.; Vanderlei, F.A.B.; Hoff, A.O.; Marui, S.; Danilovic, D.L.S. Diagnostic performance of thyroid ultrasound in Hurthle cell carcinomas. Arch. Endocrinol. Metab. 2019, 63, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, P.; Roman, S.A.; Sosa, J.A. Hurthle cell carcinoma: A population-level analysis of 3311 patients. Cancer 2013, 119, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Sugino, K.; Kameyama, K.; Ito, K.; Nagahama, M.; Kitagawa, W.; Shibuya, H.; Ohkuwa, K.; Uruno, T.; Akaishi, J.; Suzuki, A.; et al. Does Hurthle cell carcinoma of the thyroid have a poorer prognosis than ordinary follicular thyroid carcinoma? Ann. Surg. Oncol. 2013, 20, 2944–2950. [Google Scholar] [CrossRef]
- Petric, R.; Gazic, B.; Besic, N. Prognostic factors for disease-specific survival in 108 patients with Hürthle cell thyroid carcinoma: A single-institution experience. BMC Cancer 2014, 14, 777. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zheng, Z.; Chen, C.; Zhao, B.; Cao, H.; Li, T.; Liu, X.; Wang, W.; Li, Y. Clinical characteristics and prognostic factors of Hurthle cell carcinoma: A population based study. BMC Cancer 2020, 20, 407. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Penabad, L.; Chiu, A.C.; Hoff, A.O.; Schultz, P.; Gaztambide, S.; Ordonez, N.G.; Sherman, S.I. Prognostic factors in patients with Hurthle cell neoplasms of the thyroid. Cancer 2003, 97, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Heimann, A.; Moll, U. Spinal metastasis of a thyroglobulin-rich Hürthle cell carcinoma detected by fine needle aspiration. Light and electron microscopic study of an unusual case. Acta Cytol. 1989, 33, 639–644. [Google Scholar] [PubMed]
- Musholt, P.B.; Musholt, T.J.; Morgenstern, S.C.; Worm, K.; Sheu, S.Y.; Schmid, K.W. Follicular histotypes of oncocytic thyroid carcinomas do not carry mutations of the BRAF hot-spot. World J. Surg. 2008, 32, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Diagnosis and surgical indications of oxyphilic follicular tumors in Japan: Surgical specimens and cytology. Endocr. J. 2016, 63, 977–982. [Google Scholar] [CrossRef] [Green Version]
- Maizlin, Z.V.; Wiseman, S.M.; Vora, P.; Kirby, J.M.; Mason, A.C.; Filipenko, D.; Brown, J.A. Hurthle cell neoplasms of the thyroid: Sonographic appearance and histologic characteristics. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2008, 27, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Amino, N.; Yokozawa, T.; Ota, H.; Ohshita, M.; Murata, N.; Morita, S.; Kobayashi, K.; Miyauchi, A. Ultrasonographic Evaluation of Thyroid Nodules in 900 Patients: Comparison Among Ultrasonographic, Cytological, and Histological Findings. Thyroid Off. J. Am. Thyroid Assoc. 2007, 17, 1269–1276. [Google Scholar] [CrossRef]
- Wasvary, H.; Czako, P.; Poulik, J.; Lucas, R. Unilateral lobectomy for Hurthle cell adenoma. Am. Surg. 1998, 64, 729–732. [Google Scholar]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Nicol, T.L.; Zeiger, M.A.; Dooley, W.C.; Ladenson, P.W.; Cooper, D.S.; Ringel, M.; Parkerson, S.; Allo, M.; Udelsman, R. Hürthle cell neoplasms of the thyroid: Are there factors predictive of malignancy? Ann. Surg. 1998, 227, 542–546. [Google Scholar] [CrossRef]
- Mills, S.C.; Haq, M.; Smellie, W.J.; Harmer, C. Hurthle cell carcinoma of the thyroid: Retrospective review of 62 patients treated at the Royal Marsden Hospital between 1946 and 2003. Eur. J. Surg. Oncol. 2009, 35, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.F.; Clark, O.H. Hurthle Cell Carcinoma. Cancer Control J. Moffitt Cancer Cent. 1997, 4, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khafif, A.; Khafif, R.A.; Attie, J.N. Hürthle cell carcinoma: A malignancy of low-grade potential. Head Neck 1999, 21, 506–511. [Google Scholar] [CrossRef]
- Jillard, C.L. Radioactive Iodine Treatment Is Associated with Improved Survival for Patients with Hürthle Cell Carcinoma. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 959–964. [Google Scholar] [CrossRef]
- Anila, K.R.; Nayak, N.; George, P.S.; Jayasree, K. Cytomorphologic Spectrum of Hurthle Cell Lesions of Thyroid: A Study of 54 Cases. Gulf J. Oncol. 2018, 1, 6–10. [Google Scholar]
- Elliott, D.D.; Pitman, M.B.; Bloom, L.; Faquin, W.C. Fine-needle aspiration biopsy of Hurthle cell lesions of the thyroid gland: A cytomorphologic study of 139 cases with statistical analysis. Cancer 2006, 108, 102–109. [Google Scholar] [CrossRef]
- Doria, M.I., Jr.; Attal, H.; Wang, H.H.; Jensen, J.A.; DeMay, R.M. Fine needle aspiration cytology of the oxyphil variant of papillary carcinoma of the thyroid. A report of three cases. Acta Cytol. 1996, 40, 1007–1011. [Google Scholar] [CrossRef]
- Renshaw, A.A. Fine-needle aspirations of papillary carcinoma with oncocytic features: An expanded cytologic and histologic profile. Cancer Cytopathol. 2011, 119, 247–253. [Google Scholar] [CrossRef]
- Kaushal, S.; Iyer, V.K.; Mathur, S.R.; Ray, R. Fine needle aspiration cytology of medullary carcinoma of the thyroid with a focus on rare variants: A review of 78 cases. Cytopathology 2011, 22, 95–105. [Google Scholar] [CrossRef]
- Sams, S.B.; Tompkins, K.D.; Mayson, S.; Raeburn, C.D.; Mehrotra, S. Oncocytic variant of medullary thyroid carcinoma; a rare tumor with numerous diagnostic mimics by fine needle aspiration. Diagn. Cytopathol. 2017, 45, 1148–1152. [Google Scholar] [CrossRef]
- Canberk, S. Oncocytic Variant of Medullary Thyroid Carcinoma. Endocr. Pathol. 2015, 26, 320–323. [Google Scholar] [CrossRef] [PubMed]
- HARACH, H.R.; BERGHOLM, U. Medullary (C cell) carcinoma of the thyroid with features of follicular oxyphilic cell tumours. Histopathology 1988, 13, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, A.A. Hurthle cell carcinoma is a better gold standard than Hurthle cell neoplasm for fine-needle aspiration of the thyroid: Defining more consistent and specific cytologic criteria. Cancer 2002, 96, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Clouse, J.; Ren, R. Fine-needle aspiration cytology of Hurthle cell carcinoma of the thyroid. Diagn. Cytopathol. 2008, 36, 149–154. [Google Scholar] [CrossRef]
- Grani, G.; Lamartina, L.; Durante, C.; Filetti, S.; Cooper, D.S. Follicular thyroid cancer and Hürthle cell carcinoma: Challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018, 6, 500–514. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The Bethesda System for Reporting Thyroid Cytopathology, 2nd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 1341–1346. [Google Scholar] [CrossRef]
- Ren, Y. The Presence of Hürthle Cells Does Not Increase the Risk of Malignancy in Most Bethesda Categories in Thyroid Fine-Needle Aspirates. Thyroid Off. J. Am. Thyroid Assoc. 2020, 30, 425–431. [Google Scholar] [CrossRef]
- Conti, L.; Vatrano, S.; Bertero, L.; Masu, L.; Pacchioni, D.; Daniele, L.; De Rosa, G.; Cassoni, P.; Volante, M.; Papotti, M. Mitochondrial DNA “common deletion” in post–fine needle aspiration infarcted oncocytic thyroid tumors. Hum. Pathol. 2017, 69, 23–30. [Google Scholar] [CrossRef]
- Máximo, V.; Sobrinho-Simões, M. Hürthle cell tumours of the thyroid. A review with emphasis on mitochondrial abnormalities with clinical relevance. Virchows Arch. Int. J. Pathol. 2000, 437, 107–115. [Google Scholar] [CrossRef]
- Nesland, J.M.; Sobrinho-simões, M.A.; Holm, R.; Sambade, M.C.; Johannessen, J.V. Hürthle-Cell Lesions of the Thyroid: A Combined Study Using Transmission Electron Microscopy, Scanning Electron Microscopy, and Immunocytochemistry. Ultrastruct. Pathol. 1985, 8, 269–290. [Google Scholar] [CrossRef]
- Wang, D.; Feng, J.F.; Zeng, P.; Yang, Y.H.; Luo, J.; Yang, Y.W. Total oxidant/antioxidant status in sera of patients with thyroid cancers. Endocr. Relat. Cancer 2011, 18, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, M. Oxidative stress: A new risk factor for thyroid cancer. Endocr. Relat. Cancer 2012, 19, C7–C11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.C. Mitochondrial diseases in man and mouse. Science (N. Y.) 1999, 283, 1482–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller-Höcker, J. Immunoreactivity of p53, Ki-67, and Bcl-2 in oncocytic adenomas and carcinomas of the thyroid gland. Hum. Pathol. 1999, 30, 926–933. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Vivanco-Allende, B.; Alvarez-Marcos, C.; Suarez-Nieto, C. Multifocal papillary thyroid carcinoma associated with primary amyloid goiter. Auris Nasus Larynx 2012, 39, 549–551. [Google Scholar] [CrossRef]
- Munitiz, V.; Martinez-Barba, E.; Riquelme, J.; Rodriguez, J.M.; Pinero, A.; Parrilla, P. Elevated basal calcitonin levels in a patient with a hurthle-cell carcinoma of the thyroid and neuroendocrine differentiation: Report of a case. Surg. Today 2005, 35, 404–406. [Google Scholar] [CrossRef]
- Spaulding, S.L.; Ho, R.; Everest, S.; Chai, R.L. The role of molecular testing in the diagnosis of medullary thyroid cancer: A case report of oncocytic medullary thyroid carcinoma and review of the literature. Am. J. Otolaryngol. 2020, 41, 102312. [Google Scholar] [CrossRef]
- Donatini, G.; Beaulieu, A.; Castagnet, M.; Kraimps, J.L.; Levillain, P.; Fromont, G. Thyroid Hürthle cell tumors: Research of potential markers of malignancy. J. Endocrinol. Investig. 2016, 39, 153–158. [Google Scholar] [CrossRef]
- Ding, L.; Jiang, Y.; Yang, W. Approach the Invasive Potential with Hurthle Cell Tumors of Thyroid. Pathol. Oncol. Res. 2019, 25, 697–701. [Google Scholar] [CrossRef]
- Erickson, L.A.; Jin, L.; Goellner, J.R.; Lohse, C.; Pankratz, V.S.; Zukerberg, L.R.; Thompson, G.B.; van Heerden, J.A.; Grant, C.S.; Lloyd, R.V. Pathologic Features, Proliferative Activity, and Cyclin D1 Expression in Hurthle Cell Neoplasms of the Thyroid. Mod. Pathol. 2000, 13, 186–192. [Google Scholar] [CrossRef]
- Ambu, R.; Riva, A.; Lai, M.L.; Loffredo, F.; Riva, F.T.; Tandler, B. Scanning electron microscopy of the interior of cells in Hurthle cell tumors. Ultrastruct. Pathol. 2000, 24, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sobrinho-Simões, M.; Máximo, V.; Castro, I.V.; Fonseca, E.; Soares, P.; Garcia-Rostan, G.; Oliveira, M.C. Hürthle (oncocytic) cell tumors of thyroid: Etiopathogenesis, diagnosis and clinical significance. Int. J. Surg. Pathol. 2005, 13, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Tallini, G. Oncocytic tumours. Virchows Arch. Int. J. Pathol. 1998, 433, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Matias, C.; Nunes, J.F.; Sobrinho, L.G.; Soares, J. Giant mitochondria and intramitochondrial inclusions in benign thyroid lesions. Ultrastruct. Pathol. 1991, 15, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ganly, I.; Ricarte Filho, J.; Eng, S.; Ghossein, R.; Morris, L.G.; Liang, Y.; Socci, N.; Kannan, K.; Mo, Q.; Fagin, J.A.; et al. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. J. Clin. Endocrinol. Metab. 2013, 98, E962–E972. [Google Scholar] [CrossRef] [Green Version]
- Masood, S.; Auguste, L.-J.; Westerband, A.; Belluco, C.; Valderama, E.; Attie, J. Differential oncogenic expression in thyroid follicular and Hürthle cell carcinomas. Am. J. Surg. 1993, 166, 366–368. [Google Scholar] [CrossRef]
- Ganly, I.; McFadden, D.G. Short Review: Genomic Alterations in Hurthle Cell Carcinoma. Thyroid Off. J. Am. Thyroid Assoc. 2019, 29, 471–479. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Wald, A.I.; Roy, S.; Durso, M.B.; Nikiforov, Y.E. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J. Clin. Endocrinol. Metab. 2013, 98, E1852–E1860. [Google Scholar] [CrossRef] [Green Version]
- Tallini, G.; Hsueh, A.; Liu, S.; Garcia-Rostan, G.; Speicher, M.R.; Ward, D.C. Frequent chromosomal DNA unbalance in thyroid oncocytic (Hürthle cell) neoplasms detected by comparative genomic hybridization. Lab. Investig. J. Tech. Methods Pathol. 1999, 79, 547–555. [Google Scholar]
- Ganly, I.; Makarov, V.; Deraje, S.; Dong, Y.; Reznik, E.; Seshan, V.; Nanjangud, G.; Eng, S.; Bose, P.; Kuo, F.; et al. Integrated Genomic Analysis of Hurthle Cell Cancer Reveals Oncogenic Drivers, Recurrent Mitochondrial Mutations, and Unique Chromosomal Landscapes. Cancer Cell 2018, 34, 256–270.e5. [Google Scholar] [CrossRef] [Green Version]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Musholt, P.B.; Imkamp, F.; von Wasielewski, R.; Schmid, K.W.; Musholt, T.J. RET rearrangements in archival oxyphilic thyroid tumors: New insights in tumorigenesis and classification of Hurthle cell carcinomas? Surgery 2003, 134, 881–889, discussion 889. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Matsuse, M.; Saenko, V.; Nakao, T.; Yamanouchi, K.; Sakimura, C.; Yano, H.; Nishihara, E.; Hirokawa, M.; Suzuki, K.; et al. TERT mRNA Expression as a Novel Prognostic Marker in Papillary Thyroid Carcinomas. Thyroid Off. J. Am. Thyroid Assoc. 2019, 29, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Ritterhouse, L.L. Tumor mutational burden. Cancer Cytopathol. 2019, 127, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.K.; Kubler, K.; Calvo, S.E.; Polak, P.; Livitz, D.; Rosebrock, D.; Sadow, P.M.; Campbell, B.; Donovan, S.E.; Amin, S.; et al. Widespread Chromosomal Losses and Mitochondrial DNA Alterations as Genetic Drivers in Hurthle Cell Carcinoma. Cancer Cell 2018, 34, 242–255.e5. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2020, in press. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Y.; Cheng, X.; Teng, F.; Wang, C.; Han, S.; Chen, X.; Guo, W. Pathogenic Heteroplasmic Somatic Mitochondrial DNA Mutation Confers Platinum-Resistance and Recurrence of High-Grade Serous Ovarian Cancer. Cancer Manag. Res. 2020, 12, 11085–11093. [Google Scholar] [CrossRef]
- Máximo, V.; Soares, P.; Lima, J.; Cameselle-Teijeiro, J.; Sobrinho-Simões, M. Mitochondrial DNA somatic mutations (point mutations and large deletions) and mitochondrial DNA variants in human thyroid pathology: A study with emphasis on Hürthle cell tumors. Am. J. Pathol. 2002, 160, 1857–1865. [Google Scholar] [CrossRef]
- Gasparre, G.; Porcelli, A.M.; Bonora, E.; Pennisi, L.F.; Toller, M.; Iommarini, L.; Ghelli, A.; Moretti, M.; Betts, C.M.; Martinelli, G.N.; et al. Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 9001–9006. [Google Scholar] [CrossRef] [Green Version]
- Lufei, C.; Ma, J.; Huang, G.; Zhang, T.; Novotny-Diermayr, V.; Ong, C.T.; Cao, X. GRIM-19, a death-regulatory gene product, suppresses Stat3 activity via functional interaction. EMBO J. 2003, 22, 1325–1335. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Angell, J.E.; Lindner, D.J.; Shapiro, P.S.; Hofmann, E.R.; Kalvakolanu, D.V. Identification of GRIM-19, a novel cell death-regulatory gene induced by the interferon-beta and retinoic acid combination, using a genetic approach. J. Biol. Chem. 2000, 275, 33416–33426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Yang, M.; Hu, H.; Zhao, X.; Bao, L.; Huang, D.; Song, L.; Li, Y. Mitochondrial GRIM-19 as a potential therapeutic target for STAT3-dependent carcinogenesis of gastric cancer. Oncotarget 2016, 7, 41404–41420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chu, D.; Kawamura, T.; Tanaka, K.; He, S. GRIM-19 repressed hypoxia-induced invasion and EMT of colorectal cancer by repressing autophagy through inactivation of STAT3/HIF-1α signaling axis. J. Cell. Physiol. 2019, 234, 12800–12808. [Google Scholar] [CrossRef]
- Maximo, V.; Botelho, T.; Capela, J.; Soares, P.; Lima, J.; Taveira, A.; Amaro, T.; Barbosa, A.P.; Preto, A.; Harach, H.R.; et al. Somatic and germline mutation in GRIM-19, a dual function gene involved in mitochondrial metabolism and cell death, is linked to mitochondrion-rich (Hurthle cell) tumours of the thyroid. Br. J. Cancer 2005, 92, 1892–1898. [Google Scholar] [CrossRef]
- Rehling, P.; Brandner, K.; Pfanner, N. Mitochondrial import and the twin-pore translocase. Nat. Rev. Mol. Cell Biol. 2004, 5, 519–530. [Google Scholar] [CrossRef]
- Sheu, S.-Y.; Handke, S.; Bröcker-Preuss, M.; Görges, R.; Frey, U.; Ensinger, C.; Öfner, D.; Farid, N.; Siffert, W.; Schmid, K. The C allele of the GNB3 C825T polymorphism of the G protein β3-subunit is associated with an increased risk for the development of oncocytic thyroid tumours. J. Pathol. 2007, 211, 60–66. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S. Quantitative assessment of the association between GNB3 C825T polymorphism and cancer risk. J. BUON Off. J. Balk. Union Oncol. 2014, 19, 1092–1095. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kure, S.; Ohashi, R. Thyroid Hürthle Cell Carcinoma: Clinical, Pathological, and Molecular Features. Cancers 2021, 13, 26. https://doi.org/10.3390/cancers13010026
Kure S, Ohashi R. Thyroid Hürthle Cell Carcinoma: Clinical, Pathological, and Molecular Features. Cancers. 2021; 13(1):26. https://doi.org/10.3390/cancers13010026
Chicago/Turabian StyleKure, Shoko, and Ryuji Ohashi. 2021. "Thyroid Hürthle Cell Carcinoma: Clinical, Pathological, and Molecular Features" Cancers 13, no. 1: 26. https://doi.org/10.3390/cancers13010026




