Clinical Performance and Future Potential of Magnetic Resonance Thermometry in Hyperthermia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Minimum Recommended Clinical MRT Performance
3. Methods
3.1. Literature Search
3.2. Categories and Classification
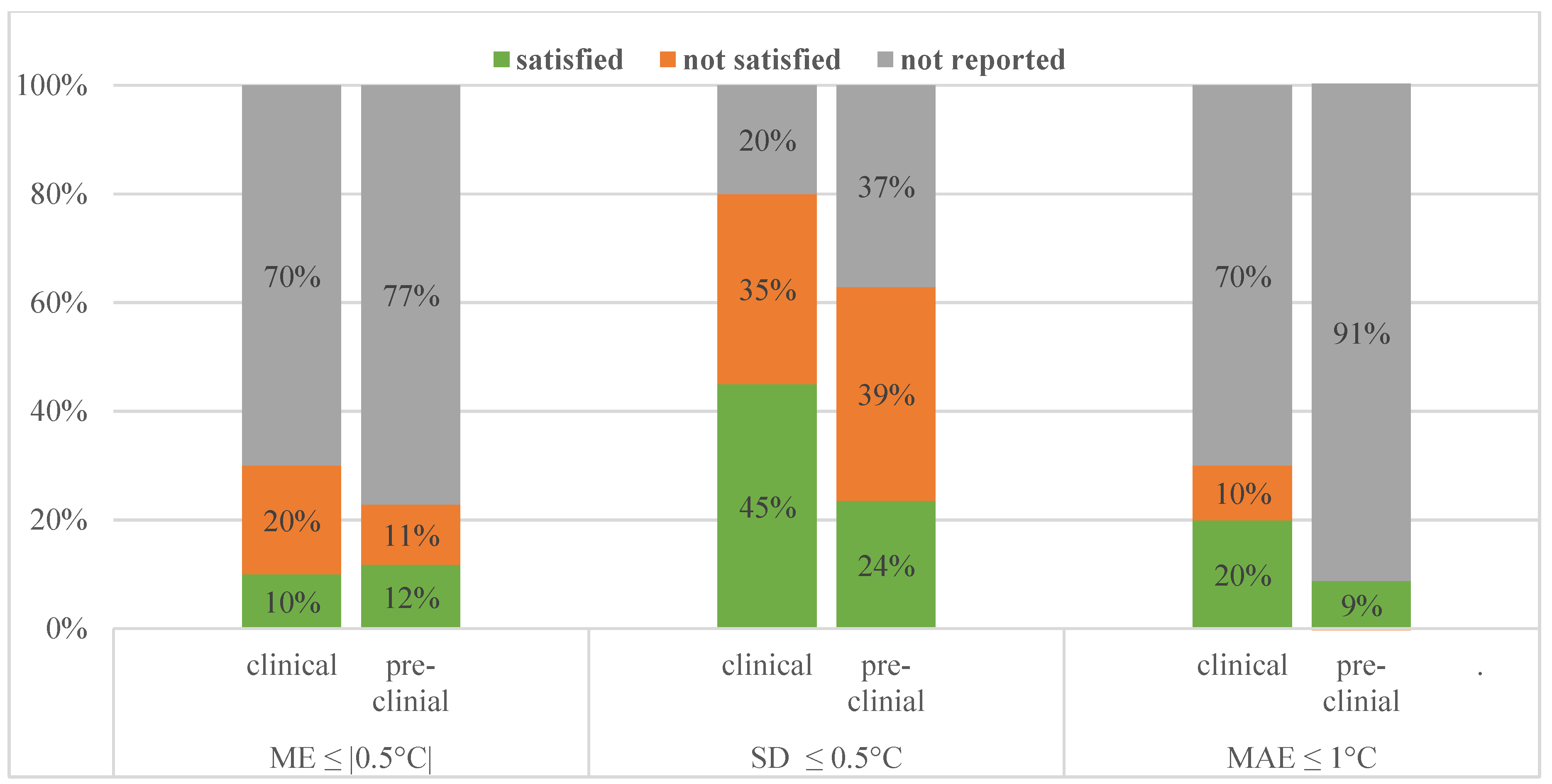
4. MRT Performance in Clinical Studies
4.1. Status
4.2. Exclusion of Data
4.3. Pre-Clinical Status—How Does It Compare?
4.4. New Techniques and Their Improvement
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FOV | Field of view |
| MAE | Mean absolute error |
| ME | Mean error |
| MR-HIFU | MR-high intensity focused ultrasound |
| MRT | Magnetic resonance thermometry |
| MSE | Mean squared error |
| NEX | Number of excitations |
| PRFS | Proton resonance frequency shift |
| RF | Radiofrequency |
| RMSE | Root mean squared error |
| ROI | Region of interest |
| SD | Standard deviation |
Appendix A. Detailed Search Strings
| DATABASE | Number of Results | Number of Results after Removing Duplicates |
|---|---|---|
| Embase (1971–) | 1292 | 1345 |
| Medline ALL Ovid (1946–) | 723 | 113 |
| Web of Science Core Collection (1975–) | 693 | 55 |
| Cochrane CENTRAL register of trials (1992–) | 21 | 15 |
| Google scholar | 200 | 147 |
| Total | 2929 | 1675 |
Appendix A.1. Embase.com (1971–) 1292
Appendix A.2. Medline ALL Ovid (1946–) 723
Appendix A.3. Web of Science Core Collection (1975–) 693
Appendix A.4. Cochrane CENTRAL Register of Trials (1992–) 21
Appendix A.5. Google Scholar 200
References
- Van den Tempel, N.; Horsman, M.R.; Kanaar, R. Improving efficacy of hyperthermia in oncology by exploiting biological mecha-nisms. Int. J Hyperth. 2016, 32, 446–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, N.R.; Kok, H.P.; Crezee, H.; Gaipl, U.S.; Bodis, S. Integrating Loco-Regional Hyperthermia Into the Current Oncology Practice: SWOT and TOWS Analyses. Front. Oncol. 2020, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.; Kampmann, E.; Kanaar, R.; Lindner, L.H. Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: Translation into clinical application. Int. J. Hyperth. 2016, 32, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, N.; Ordóñez, S.G.; Gaipl, U.; Paulides, M.M.; Crezee, J.; Gellermann, J.; Marder, D.; Puric, E.; Bodis-Wollner, I. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Van der Zee, J.; González, D.G.; Rhoon, G.C.; Van Dijk, J.D.P.; Van Putten, W.L.J.; Hart, A.A.M. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A pro-spective, randomised, multicentre trial. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef]
- Kroesen, M.; Mulder, H.T.; Van Holthe, J.M.; Aangeenbrug, A.A.; Mens, J.W.M.; Van Doorn, H.C.; Paulides, M.M.; Hoop, E.O.-D.; Vernhout, R.M.; Lutgens, L.C.; et al. Confirmation of thermal dose as a predictor of local control in cervical carcinoma patients treated with state-of-the-art radiation therapy and hyperthermia. Radiother. Oncol. 2019, 140, 150–158. [Google Scholar] [CrossRef]
- Franckena, M.; Fatehi, D.; De Bruijne, M.; Canters, R.A.M.; Van Norden, Y.; Mens, J.W.; Van Rhoon, G.C.; Van Der Zee, J. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur. J. Cancer 2009, 45, 1969–1978. [Google Scholar] [CrossRef]
- Jones, E.L.; Oleson, J.R.; Prosnitz, L.R.; Samulski, T.V.; Vujaskovic, Z.; Yu, D.; Sanders, L.L.; Dewhirst, M.W. Randomized Trial of Hyperthermia and Radiation for Superficial Tumors. J. Clin. Oncol. 2005, 23, 3079–3085. [Google Scholar] [CrossRef] [Green Version]
- Fotopoulou, C.; Cho, C.-H.; Kraetschell, R.; Gellermann, J.; Wust, P.; Lichtenegger, W.; Sehouli, J. Regional abdominal hyperthermia combined with systemic chemotherapy for the treatment of patients with ovari—An cancer relapse: Results of a pilot study. Int. J. Hyperth. 2010, 26, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Rau, B.; Wust, P.; Tilly, W.; Gellermann, J.; Harder, C.; Riess, H.; Budach, V.; Felix, R.; Schlag, P.M. Preoperative radio-chemotherapy in locally advanced recurrent rectal cancer: Regional radiofrequency hyperthermia correlates with clinical parameters. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 381–391. [Google Scholar] [CrossRef]
- Kapp, D.S.; Cox, R.S. Thermal treatment parameters are most predictive of outcome in patients with single tumor nodules per treat-ment field in recurrent adenocarcinoma of the breast. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 887–899. [Google Scholar] [CrossRef]
- Oleson, J.R.; Samulski, T.V.; Leopold, K.A.; Clegg, S.T.; Dewhirst, M.W.; Dodge, R.K.; George, S.L. Sensitivity of hyperthermia trial outcomes to temperature and time: Implications for thermal goals of treatment. Int. J. Radiat. Oncol. 1993, 25, 289–297. [Google Scholar] [CrossRef]
- Van Der Zee, J.; Peer-Valstar, J.N.; Rietveld, P.J.; De Graaf-Strukowska, L.; Van Rhoon, G.C. Practical Limitations of Interstitial Thermometry During Deep Hyperthermia. Int. J. Radiat. Oncol. 1998, 40, 1205–1212. [Google Scholar] [CrossRef]
- Sneed, P.K.; Dewhirst, M.W.; Samulski, T.; Blivin, J.; Prosnitz, L.R. Should interstitial thermometry be used for deep hyperthermia? Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 1015–1017. [Google Scholar] [PubMed]
- Unsoeld, M.; Lamprecht, U.; Traub, F.; Hermes, B.; Scharpf, M.O.; Potkrajcic, V.; Zips, D.; Paulsen, F.; Eckert, F. MR Thermometry Data Correlate with Pathological Response for Soft Tissue Sarcoma of the Lower Extremity in a Single Center Analysis of Prospectively Registered Patients. Cancers 2020, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Rieke, V.; Pauly, K.B. MR thermometry. J. Magn. Reson. Imaging 2008, 27, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Oberacker, E.; Paul, K.; Ji, Y.; Oezerdem, C.; Ghadjar, P.; Thieme, A.; Budach, V.; Wust, P.; Niendorf, T. Magnetic resonance thermometry: Methodology, pitfalls and practical solutions. Int. J. Hyperth. 2015, 32, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüdemann, L.; Wlodarczyk, W.; Nadobny, J.; Weihrauch, M.; Gellermann, J.; Wust, P. Non-invasive magnetic resonance thermography during regional hyperthermia. Int. J. Hyperth. 2010, 26, 273–282. [Google Scholar] [CrossRef]
- Gellermann, J.; Hildebrandt, B.; Issels, R.; Ganter, H.; Wlodarczyk, W.; Budach, V.; Felix, R.; Tunn, P.-U.; Reichardt, P.; Wust, P. Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: Correlation with response and direct thermometry. Cancer 2006, 107, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Sherar, M.; Liu, F.-F.; Pintilie, M.; Levin, W.; Hunt, J.; Hill, R.; Hand, J.; Vernon, C.; Van Rhoon, G.; Van Der Zee, J.; et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: Data from a phase III trial. Int. J. Radiat. Oncol. 1997, 39, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Canters, R.A.M.; Paulides, M.M.; Franckena, M.F.; Van Der Zee, J.; Van Rhoon, G.C. Implementation of treatment planning in the routine clinical procedure of regional hyperthermia treatment of cervical cancer: An overview and the Rotterdam experience. Int. J. Hyperth. 2012, 28, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.P.; Wust, P.; Stauffer, P.R.; Bardati, F.; Van Rhoon, G.C.; Crezee, J. Current state of the art of regional hyperthermia treatment planning: A review. Radiat. Oncol. 2015, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Walther, B.A.; Moore, J.L. The concepts of bias, precision and accuracy, and their use in testing the performance of species richness estimators, with a literature review of estimator performance. Ecography 2005, 28, 815–829. [Google Scholar] [CrossRef]
- Curto, S.; Aklan, B.; Mulder, H.T.; Mils, O.; Schmidt, M.; Lamprecht, U.; Peller, M.; Wessalowski, R.; Lindner, L.H.; Fietkau, R.; et al. Quantitative, Multi-institutional Evaluation of MR Thermometry Accuracy for Deep-Pelvic MR-Hyperthermia Systems Operating in Multi-vendor MR-systems Using a New Anthropomorphic Phantom. Cancers 2019, 11, 1709. [Google Scholar] [CrossRef] [Green Version]
- Bramer, W.M.; Milic, J.; Mast, F. Reviewing retrieved references for inclusion in systematic reviews using EndNote. J. Med. Libr. Assoc. 2017, 105, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Peller, M.; Reinl, H.M.; Weigel, A.; Meininger, M.; Issels, R.D.; Reiser, M. T1 relaxation time at 0.2 Tesla for monitoring regional hyperthermia: Feasibility study in muscle and adipose tissue. Magn. Reson. Med. 2002, 47, 1194–1201. [Google Scholar] [CrossRef]
- Yuan, J.; Panych, L.P.; McDannold, N.J.; Madore, B.; Mei, C.-S. Towards fast and accurate temperature mapping with proton resonance frequency-based MR thermometry. Quant. Imaging Med. Surg. 2012, 2, 21–32. [Google Scholar]
- Stauffer, P.R.; Craciunescu, O.I.; Maccarini, P.F.; Wyatt, C.; Arunachalam, K.; Arabe, O.; Stakhursky, V.; Li, Z.; Soher, B.; MacFall, J.R.; et al. Clinical utility of magnetic resonance thermal imaging (MRTI) for realtime guidance of deep hyperthermia. Proc. SPIE 2009, 7181, 71810I. [Google Scholar] [CrossRef] [Green Version]
- Craciunescu, O.I.; Stauffer, P.R.; Soher, B.J.; Wyatt, C.R.; Arabe, O.; Maccarini, P.; Das, S.K.; Cheng, K.-S.; Wong, T.Z.; Jones, E.L.; et al. Accuracy of real time noninvasive temperature measurements using magnetic resonance thermal imaging in patients treated for high grade extremity soft tissue sarcomas. Med. Phys. 2009, 36, 4848–4858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadakova, T.; Gellermann, J.; Voigt, O.; Korvink, J.G.; Pavlina, J.M.; Hennig, J.; Bock, M. Fast PRF-based MR thermometry using double-echo EPI: In vivo comparison in a clinical hyperthermia setting. Magma Magn. Reson. Mater. Phys. Biol. Med. 2014, 28, 305–314. [Google Scholar] [CrossRef]
- Craciunescu, O.I.; Das, S.K.; McCauley, R.L.; MacFall, J.R.; Samulski, T.V. 3D numerical reconstruction of the hyperthermia induced temperature distribution in human sarcomas using DE-MRI measured tissue perfusion: Validation against non-invasive MR temperature measurements. Int. J. Hyperth. 2001, 17, 221–239. [Google Scholar] [CrossRef]
- Wu, M.; Mulder, H.T.; Baron, P.; Coello, E.; Menzel, M.I.; Van Rhoon, G.C.; Haase, A. Correction of motion-induced susceptibility artifacts and B 0 drift during proton resonance frequency shift-based MR thermometry in the pelvis with background field removal methods. Magn. Reson. Med. 2020, 84, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.L.; MacFall, J.R.; Clegg, S.T.; Wan, X.; Prescott, D.M.; Charles, H.C.; Samulski, T.V. Magnetic Resonance Thermometry during Hyperthermia for Human High-Grade Sarcoma. Int. J. Radiat. Oncol. 1998, 40, 815–822. [Google Scholar] [CrossRef]
- Gellermann, J.; Wlodarczyk, W.; Hildebrandt, B.; Ganter, H.; Nicolau, A.; Rau, B.; Tilly, W.; Fähling, H.; Nadobny, J.; Felix, R.; et al. Noninvasive Magnetic Resonance Thermography of Recurrent Rectal Carcinoma in a 1.5 Tesla Hybrid System. Cancer Res. 2005, 65, 5872–5880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, C.J.; Bartels, L.W.; Van Der Velden, T.A.; Grüll, H.; Heijman, E.; Moonen, C.T.W.; Bos, C. Field drift correction of proton resonance frequency shift temperature mapping with multichannel fast alternating nonselective free induction decay readouts. Magn. Reson. Med. 2019, 83, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Bing, C.; Cheng, B.; Staruch, R.M.; Nofiele, J.; Staruch, M.W.; Szczepanski, D.; Farrow-Gillespie, A.; Yang, A.; Laetsch, T.W.; Chopra, R. Breath-hold MR-HIFU hyperthermia: Phantom and in vivo feasibility. Int. J. Hyperth. 2019, 36, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- Odéen, H.; Parker, D.L. Magnetic resonance thermometry and its biological applications—Physical principles and practical consider-ations. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 110, 34–61. [Google Scholar] [CrossRef]
- Tan, J.; Mougenot, C.; Pichardo, S.; Drake, J.M.; Waspe, A.C. Motion compensation using principal component analysis and projection onto dipole fields for abdominal magnetic reso-nance thermometry. Magn. Reson. Med. 2019, 81, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Mulder, H.T.; Zur, Y.; Lechner-Greite, S.; Menzel, M.I.; Paulides, M.M.; Van Rhoon, G.C.; Haase, A. A phase-cycled temperature-sensitive fast spin echo sequence with conductivity bias correction for monitoring of mild RF hyperthermia with PRFS. Magma Magn. Reson. Mater. Phys. Biol. Med. 2018, 32, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lam, D.; Pacia, C.P.; Gach, H.M.; Partanen, A.; Talcott, M.R.; Greco, S.C.; Zoberi, I.; Hallahan, D.E.; Chen, H.; et al. Feasibility and safety assessment of magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU)-mediated mild hyperthermia in pelvic targets evaluated using an in vivo porcine model. Int. J. Hyperth. 2019, 36, 1147–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonathan, S.V.; Grissom, W.A. Volumetric MRI thermometry using a three-dimensional stack-of-stars echo-planar imaging pulse sequence. Magn. Reson. Med. 2018, 79, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, S.V.V.N. Evaluation and selection of anatomic sites for magnetic resonance imaging-guided mild hyperthermia thera-py: A healthy volunteer study. Int. J. Hyperth. 2018, 34, 1381–1389. [Google Scholar] [CrossRef]
- Chu, W. Magnetic resonance-guided high-intensity focused ultrasound hyperthermia for recurrent rectal cancer: MR thermome-try evaluation and preclinical validation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Svedin, B.T.; Payne, A.; Parker, D.L. Respiration artifact correction in three-dimensional proton resonance frequency MR ther-mometry using phase navigators. Magn. Reson. Med. 2016, 76, 206–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillander, M.; Hokland, S.; Koskela, J.; Dam, H.; Andersen, N.P.; Ylihautala, M.; Pedersen, M.; Tanderup, K.; Köhler, M.O. High intensity focused ultrasound induced in vivo large volume hyperthermia under 3D MRI temperature control. Med. Phys. 2016, 43, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Boulant, N. FID navigator-based MR thermometry method to monitor small temperature changes in the brain of ventilated animals. NMR Biomed. 2015, 28, 101–107. [Google Scholar] [CrossRef] [Green Version]
- De Senneville, B.D.; El Hamidi, A.; Moonen, C. A Direct PCA-Based Approach for Real-Time Description of Physiological Organ Deformations. IEEE Trans. Med. Imaging 2015, 34, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.; Grissom, W.A. Accelerated MRI thermometry by direct estimation of temperature from undersampled k-space data. Magn. Reson. Med. 2015, 73, 1914–1925. [Google Scholar] [CrossRef] [Green Version]
- Marx, M.; Plata, J.; Pauly, K.B. Toward Volumetric MR Thermometry with the MASTER Sequence. IEEE Trans. Med. Imaging 2015, 34, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.-S.; Chu, R.; Hoge, W.S.; Panych, L.P.; Madore, B. Accurate field mapping in the presence of B0 inhomogeneities, applied to MR thermometry. Magn. Reson. Med. 2014, 73, 2142–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichardo, S.; Kohler, M.; Lee, J.; Hynnyen, K. In vivo optimisation study for multi-baseline MR-based thermometry in the context of hyperthermia using MR-guided high intensity focused ultrasound for head and neck applications. Int. J. Hyperth. 2014, 30, 579–592. [Google Scholar] [CrossRef]
- Shi, C.; Xie, G.; Song, Y.; Zou, C.; Liu, X.; Zhou, S. Referenceless PRFS MR Thermometry Using Partial Separability Model. Appl. Magn. Reson. 2013, 45, 93–108. [Google Scholar] [CrossRef]
- Minalga, E.; Payne, A.; Merrill, R.; Todd, N.; Vijayakumar, S.; Kholmovski, E.; Parker, D.L.; Hadley, J.R. An 11-channel radio frequency phased array coil for magnetic resonance guided high-intensity focused ultrasound of the breast. Magn. Reson. Med. 2013, 69, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, E.; Mougenot, C.; Köhler, M.; Bronskill, M.; Klotz, L.; Haider, M.A.; Chopra, R. MR thermometry in the human prostate gland at 3.0T for transurethral ultrasound therapy. J. Magn. Reson. Imaging 2013, 38, 1564–1571. [Google Scholar] [CrossRef] [Green Version]
- Kickhefel, A.; Roland, J.; Weiss, C.; Schick, F. Accuracy of real-time MR temperature mapping in the brain: A comparison of fast sequences. Phys. Med. 2010, 26, 192–201. [Google Scholar] [CrossRef]
- Wyatt, C.; Soher, B.J.; MacFall, J.R. Correction of breathing-induced errors in magnetic resonance thermometry of hyperthermia using multiecho field fitting techniques. Med. Phys. 2010, 37, 6300–6309. [Google Scholar] [CrossRef] [Green Version]
- Roujol, S.; De Senneville, B.D.; Vahala, E.; Sørensen, T.S.; Moonen, C.; Ries, M. Online real-time reconstruction of adaptive TSENSE with commodity CPU/GPU hardware. Magn. Reson. Med. 2009, 62, 1658–1664. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, C.; Soher, B.; Maccarini, P.F.; Charles, H.C.; Stauffer, P.R.; MacFall, J. Hyperthermia MRI temperature measurement: Evaluation of measurement stabilisation strategies for extremity and breast tumours. Int. J. Hyperth. 2009, 25, 422–433. [Google Scholar] [CrossRef]
- Silcox, C.E.; Smith, R.C.; King, R.; McDannold, N.; Bromley, P.; Walsh, K.; Hynynen, K. MRI-guided ultrasonic heating allows spatial control of exogenous luciferase in canine prostate. Ultrasound Med. Biol. 2005, 31, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Collins, C.M.; Schiano, J.L.; Smith, M.B.; Smith, N.B. Adaptive Real-Time Closed-Loop Temperature Control for Ultrasound Hyperthermia Using Magnetic Resonance Ther-mometry. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2005, 27, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Il’yasov, K.A.; Hennig, J. Single-shot diffusion-weighted RARE sequence: Application for temperature monitoring during hyperthermia session. J. Magn. Reson. Imaging 1998, 8, 1296–1305. [Google Scholar] [CrossRef]
- Corbett, R.; Laptook, A.; Weatherall, P. Noninvasive Measurements of Human Brain Temperature Using Volume-Localized Proton Magnetic Resonance Spectroscopy. Br. J. Pharmacol. 1997, 17, 363–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacFall, J.R.; Prescott, D.M.; Charles, H.C.; Samulski, T.V. 1 H MRI phase thermometry in vivo in canine brain, muscle, and tumor tissue. Med. Phys. 1996, 23, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- De Poorter, J.; De Wagter, C.; De Deene, Y.; Thomsen, C.; Ståhlberg, F.; Achten, E. Noninvasive MRI Thermometry with the Proton Resonance Frequency (PRF) Method:In Vivo Results in Human Muscle. Magn. Reson. Med. 1995, 33, 74–81. [Google Scholar] [CrossRef]
- MacFall, J.; Prescott, D.M.; Fullar, E.; Samulski, T.V. Temperature dependence of canine brain tissue diffusion coefficient measured in vivo with magnetic resonance echo-planar imaging. Int. J. Hyperth. 1995, 11, 73–86. [Google Scholar] [CrossRef]
- Young, I.R.; Hand, J.W.; Oatridge, A.; Prior, M.V. Modeling and observation of temperature changes in vivo using MRI. Magn. Reson. Med. 1994, 32, 358–369. [Google Scholar] [CrossRef]
- Hall, A.S.; Prior, M.V.; Hand, J.W.; Young, I.R.; Dickinson, R.J. Observation by MR Imaging of In Vivo Temperature Changes Induced by Radio Frequency Hyperthermia. J. Comput. Assist. Tomogr. 1990, 14, 430–436. [Google Scholar] [CrossRef]
- Odéen, H.; Parker, D.L. Improved MR thermometry for laser interstitial thermotherapy. Lasers Surg. Med. 2019, 51, 286–300. [Google Scholar] [CrossRef]
- Kothapalli, S.V.V.N.; Partanen, A.; Zhu, L.; Altman, M.B.; Gach, H.M.; Hallahan, D.E.; Chen, H. A convenient, reliable, and fast acoustic pressure field measurement method for magnetic resonance-guided high-intensity focused ultrasound systems with phased array transducers. J. Ther. Ultrasound 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Paulides, M.M.; Drizdal, T.; Van Rhoon, G.C.; Yeo, D. Novel Applicator Design for MR Guided RF Hyperthermia in Head and Neck Cancers: Heating Performance and RF Coupling; International Society for Magnetic Resonance in Medicine (ISMRM): Paris, France, 2018. [Google Scholar]
- Nakagawa, Y.; Kokuryo, D.; Kaihara, T.; Fujii, N.; Kumamoto, E. Image reconstruction method with compressed sensing for high-speed MR temperature measurement of abdominal organs. IEEE Conf. Proc. Eng. Med. Biol. Soc. 2019, 2019, 2731–2735. [Google Scholar]
- Todd, N.; Diakite, M.; Payne, A.; Parker, D.L. In vivo evaluation of multi-echo hybrid PRF/T1 approach for temperature monitoring during breast MR-guided focused ultrasound surgery treatments. Magn. Reson. Med. 2014, 72, 793–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
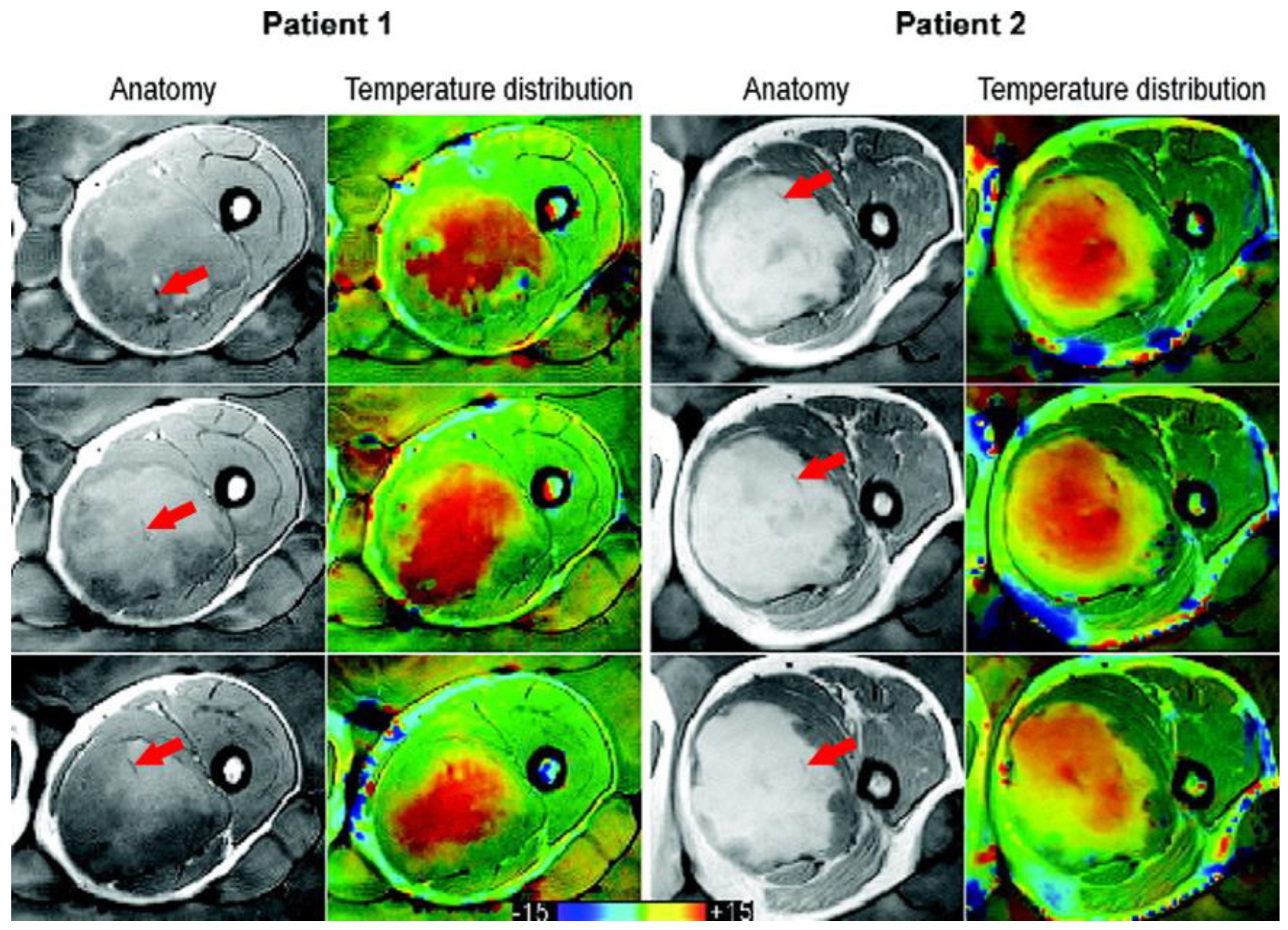

| Parameter | Definition | Minimum |
|---|---|---|
| Spatial resolution | In-plane resolution times slice width (2D) or through-plane resolution (3D) | 125 mm3 |
| Temporal resolution | Time needed to acquire one MRT slice | 20 s |
| Measure | Metric | Definition | Minimum |
|---|---|---|---|
| Bias | Mean error (ME) | ≤|0.5 °C| | |
| Spatial temperature precision | Spatial temperature standard deviation (SD) | ≤0.5 °C | |
| Temporal temperature precision | Temporal temperature standard deviation (SD) | Variability at different time points over 90 min | ≤0.5 °C |
| Accuracy | Mean absolute error (MAE) | ≤1 °C |
| Author (year) | Body Part | Sequence | Spatial Res (mm3) | Temporal Res (s) | ME (°C) | Spatial Temperature Precision (°C) | Temporal Temperature Precision (°C) | MAE (°C) |
|---|---|---|---|---|---|---|---|---|
| Carter (1998) [35] | E | GRE | 8.8 | - | - | 0.50 | - | - |
| Craciunescu (2009) [31] | E | GRE | 27.4 | 10 | - | 0.52 | - | 0.74 |
| Craciunescu (2001) [33] † | E | GRE | 11.9 | - | - | 0.49~ | - | - |
| E | GRE | 13.7 | - | - | 0.56~ | - | - | |
| EPI | 156.0 | 1 | ||||||
| Dadakova (2015) [32] | E,P | EPI | 67.6 | 1.08 | −0.04 | 0.55 | - | 0.40 |
| GRE | 152.1 | 3.12 | ||||||
| Gellermann (2005) [36] | P | GRE | 146.8 | 3.12 | - | 2.10 | - | 1.50 |
| Gellermann (2006) [21] | E,P | GRE | 146.8 | 3.12 | 1.10 | 0.70 | - | - |
| Peller (2002) [28] *,^ | E,P | GRE | 96.1 | 64 | - | 0.10 | - | - |
| Stauffer (2009) [30] | E | GRE | 21.1 | 15 | 0.85 | - | - | - |
| Unsoeld (2020) [17] | E | not stated | - | - | - | 0.21 | - | - |
| Wu (2020) [34] | P | GRE | 152.1 | 3.32 | - | - | - | - |
| Author | All Data included? | Size of Study | What Was Excluded? | Why? |
|---|---|---|---|---|
| Carter (1998) [35] | No | 4 patients 5 treatments | Not stated | Artefacts |
| Craciunescu (2001) [33] | Yes | 2 patients | - | - |
| Craciunescu (2009) [31] | No | 10 patients 40 treatments | 4 patients 12 treatments | Lack of MR information in HT treatments performed outside the MR scanner, image/motion artefacts, uncorrectable drift, impossibility to localize the fiber optic probes, missing/corrupted data files |
| Dadakova (2015) [32] | No | 3 patients 20 treatments | 1 patient 1 treatment | Susceptibility artefact in the ROI from air in rectum |
| Gellermann (2005) [36] | No | 15 patients | Everything but 1 best session per patient | MR data sets incomplete and/or disturbed by technical reasons |
| Gellermann (2006) [21] | No | 9 patients 30 treatments | 15 treatments | Breakdown or malfunction of applicator, restlessness of the patient |
| Peller (2002) [28] | No | 1 patient | “Data sets” | Artefacts |
| Stauffer (2009) [30] | No | 10 patients | 3 patients All except 12 treatments | Uncorrected field drift or inability to locate or correlate sensor positions or significant patient position shift early in treatment |
| Unsoeld (2020) [17] | No | 24 patients | 13 patients: 11 patients with abdominal and pelvic tumors; 1 patient with different time course of therapy; 1 patient without surgery | Breathing and intestinal motion artefacts in MRT data; pathological response is not comparable; lack of information on pathological response |
| Wu (2020) [34] | No | 4 patients | 2 patients | Bulk motion due to discomfort during treatment, ROI contained too much gas |
| Author | Year | Technique/Method Investigated | Improvement | Main Aim |
|---|---|---|---|---|
| Wu [34] | 2020 | Correction of motion-induced susceptibility artifacts | TNR improvement | B0 changes and image gaps due to motion, B0 drift |
| Ferrer [37] | 2020 | Different B0 drift corrections | IQR improved from 9.31 to 0.80 °C. ME improved from −4.30 to 0.33 °C | B0 drift |
| Bing [38] | 2019 | Forced breath-hold MR-HIFU | Accuracy and stability from 1.2 to 0.6 °C and from 1.4 to 0.8 °C | B0 changes and image gaps due to motion |
| Odeen [39] | 2019 | Different protocols for PRFS MRT for LITT | Factor 2 improvement in the temperature SD | Comparison |
| Tan [40] | 2019 | Motion compensation using principal component analysis and projection onto dipole fields | Reduces temperature SD from 3.02 to 0.86 °C | B0 changes and image gaps due to motion |
| Wu [41] | 2019 | Novel fast spin echo method | TNR efficiency improved by 25% | Feasibility |
| Zhu [42] | 2019 | Feasibility/safety of MRgHIFU | N/A | Feasibility |
| Jonathan [43] | 2018 | Proposed and validated a hybrid radial-EPI temperature mapping pulse sequence | Provides whole brain coverage, temperature SD was 48% higher than standard | Feasibility |
| Kothapalli [44] | 2018 | MRT performance at different anatomical sites | N/A | Comparison |
| Chu [45] | 2016 | Feasibility (safety + performance) MRgHIFU for rectal cancer | Precision and stability of temperature improved from 7.8 and 2.3 °C to 0.3 and 0.6 °C | Feasibility |
| Svedin [46] | 2016 | Correction of respiration artifact in 3D MRT using phase navigators | Temperature measurement improved by a factor of 2.1 | B0 changes and image gaps due to motion |
| Tillander [47] | 2016 | Hyperthermia for deep-seated heating volumes using HIFU | N/A | Feasibility |
| Boulant [48] | 2015 | FID navigator to correct for B0 field and variations induced by breathing | Reduces the temperature SD of the data over the first 8 min from 0.2 to 0.05 °C | B0 drift and B0 changes due to motion |
| De Senneville [49] | 2015 | Approach for motion estimation of abdominal organs | Temperature SD improvement of 0.4 °C and reduction of artefacts by up to 3 °C | B0 changes and image gaps due to motion |
| Gaur [50] | 2015 | Reconstruction method to accelerate MRT | Achieves same temperature error at up to 32× acceleration factors | Acceleration |
| Marx [51] | 2015 | MASTER sequence | Temperature SD improvement from 1.21 to 0.82 °C | Feasibility |
| Mei [52] | 2015 | Different methods for B0 inhomogeneity correction | None | B0 drift |
| Pichardo [53] | 2014 | Multi-baseline MR-based thermometry | Reduced temperature SD from 25.2 to 2.4 °C | B0 changes due to motion |
| Shi [54] | 2014 | partial separability (PS) model and referenceless thermometry introduced | N/A | Feasibility |
| Minalga [55] | 2013 | Integrated multi-channel RF receive coil with MR-HIFU | 163% SNR improvement averaged over all positions investigated | Feasibility |
| Ramsay [56] | 2013 | Segmented GRE-EPI technique | N/A | Feasibility |
| Kickhefel [57] | 2010 | Comparison of fast sequences | Stability improvement from 1.07 to 0.21 °C | Comparison |
| Wyatt [58] | 2010 | Correction of breathing-induced errors using multi-echo fitting methods | Temperature SD from 2.18 to 0.61 °C and bias from 3.17 to −1.26 °C | B0 changes and image gaps due to motion |
| Roujol [59] | 2009 | Reconstruction pipeline for adaptive TSENSE | Image latencies below 90 ms at frame rates up to 40 images/s | Acceleration |
| Wyatt [60] | 2009 | Different stabilization strategies | Improved error by up to 0.5 °C | B0 drift |
| Silcox [61] | 2005 | Ultrasonic heating to control transgene expression spatially using a minimally invasive approach | N/A | Feasibility |
| Sun [62] | 2005 | Adaptive controllers with MRT | N/A | Feasibility |
| Peller [28] | 2002 | Characterize T1 for thermometry | N/A | Feasibility |
| Il’yasov [63] | 1998 | RARE sequence for diffusion MRT | N/A | Feasibility |
| Corbett [64] | 1997 | 1H MR spectroscopy to measure absolute brain temperature | N/A | Feasibility |
| MacFall [65] | 1996 | Chemical shift of water for MRT | N/A | Feasibility |
| De Poorter [66] | 1995 | PRF thermometry in vivo | N/A | Feasibility |
| MacFall [67] | 1995 | Rapid diffusion weighted EPI, being less sensitive to motion | Temperature SD from 1.5 to 0.9 °C | B0 changes due to motion |
| Young [68] | 1994 | Initial investigation of T1 dependence, D and perfusion | N/A | Feasibility |
| Hall [69] | 1990 | Investigation which MR parameter would be best for MRT in vivo | N/A | Comparison |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feddersen, T.V.; Hernandez-Tamames, J.A.; Franckena, M.; van Rhoon, G.C.; Paulides, M.M. Clinical Performance and Future Potential of Magnetic Resonance Thermometry in Hyperthermia. Cancers 2021, 13, 31. https://doi.org/10.3390/cancers13010031
Feddersen TV, Hernandez-Tamames JA, Franckena M, van Rhoon GC, Paulides MM. Clinical Performance and Future Potential of Magnetic Resonance Thermometry in Hyperthermia. Cancers. 2021; 13(1):31. https://doi.org/10.3390/cancers13010031
Chicago/Turabian StyleFeddersen, Theresa V., Juan A. Hernandez-Tamames, Martine Franckena, Gerard C. van Rhoon, and Margarethus M. Paulides. 2021. "Clinical Performance and Future Potential of Magnetic Resonance Thermometry in Hyperthermia" Cancers 13, no. 1: 31. https://doi.org/10.3390/cancers13010031
APA StyleFeddersen, T. V., Hernandez-Tamames, J. A., Franckena, M., van Rhoon, G. C., & Paulides, M. M. (2021). Clinical Performance and Future Potential of Magnetic Resonance Thermometry in Hyperthermia. Cancers, 13(1), 31. https://doi.org/10.3390/cancers13010031








