Physical Activity as an Imperative Support in Breast Cancer Management
Abstract
Simple Summary
Abstract
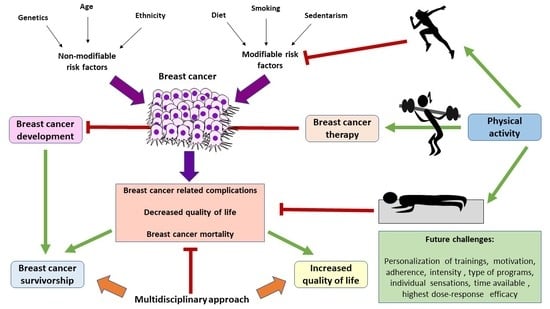
1. Introduction
2. PA in the Prevention of BC
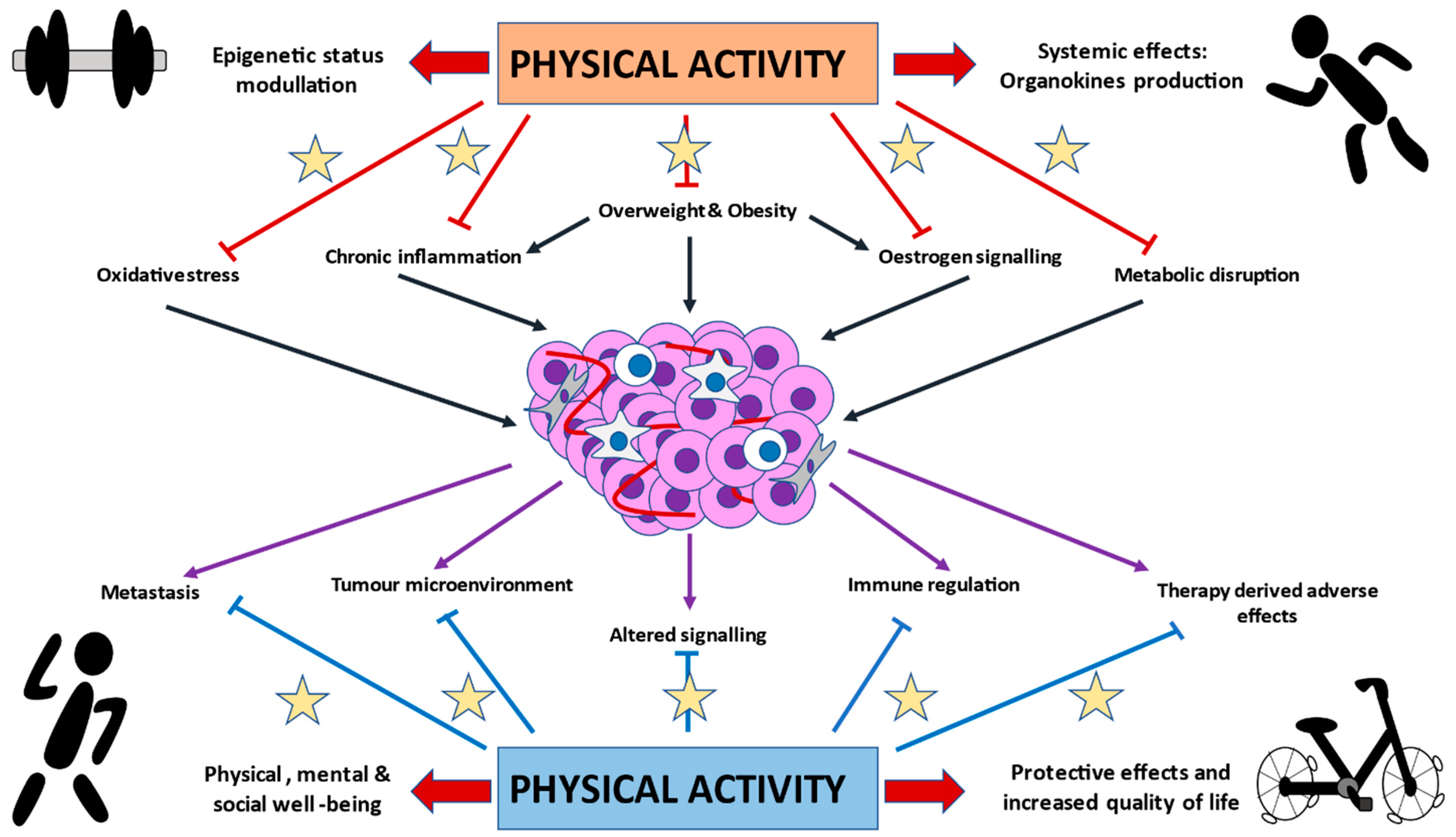
3. Exploring the Biological and Molecular Basis of PA in BC Development and Progression
3.1. PA and Sexual Hormones
3.2. Immunomodulatory Effects of PA
3.3. Metabolic Effects of PA
3.4. PA and Oxidative Stress
3.5. PA Direct Actions in BC Tumors
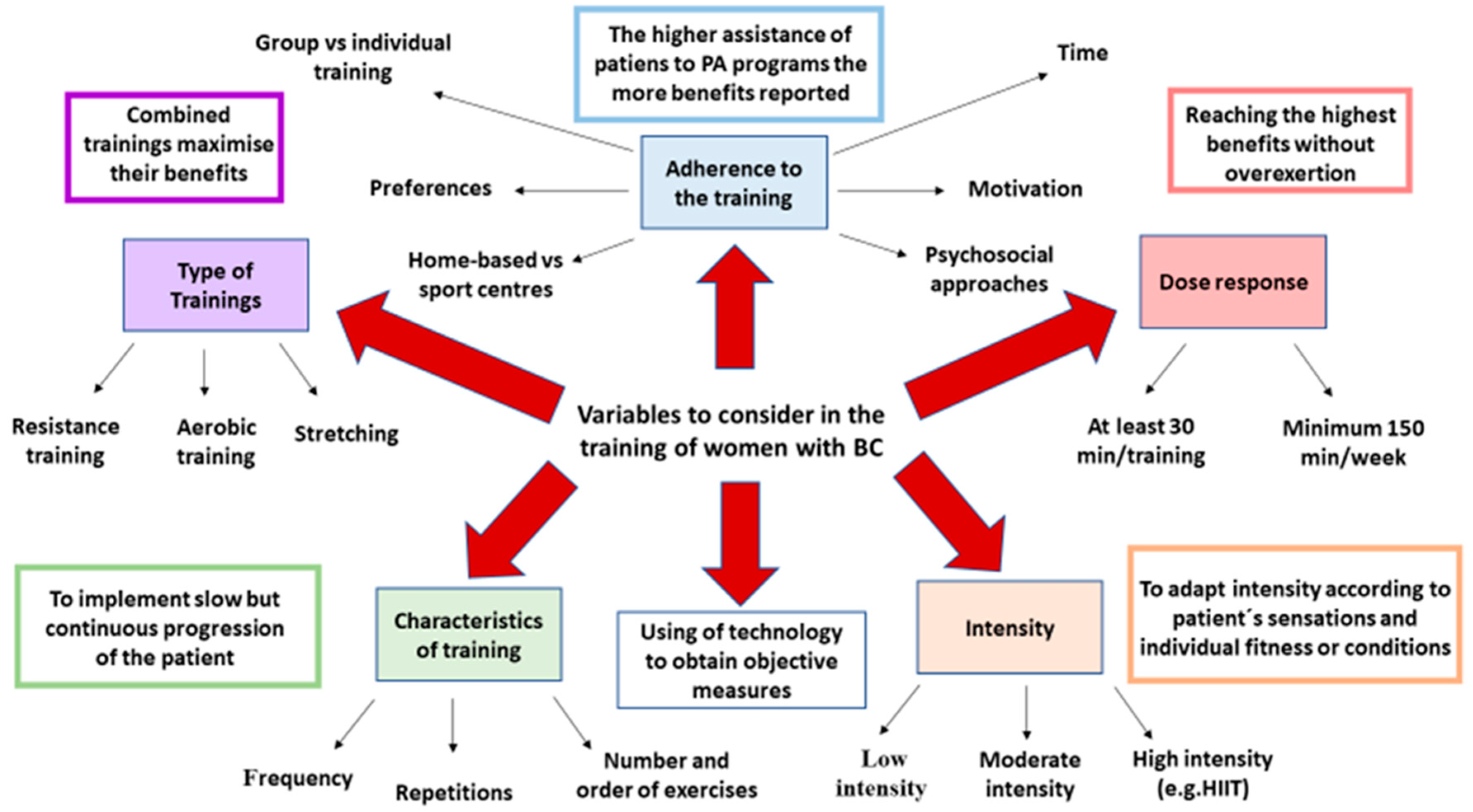
4. Specific Considerations of Training Women with BC
5. The Association of PA with BC Mortality and Survival
6. PA in BC Management
6.1. PA and Cardiovascular Disease in BC
6.2. PA and Lymphedema
6.3. PA and Body Composition in BC
6.4. PA and Peripheral Neuropathy
6.5. BC-Related Cognitive Dysfunction and PA
7. Physical Activity in the QOL of BC Patients
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| PA | Physical activity |
| BC | Breast cancer |
| TNBC | Triple-negative breast cancer |
| RT | Resistance/strength training |
| AT | Aerobic/endurance training |
| HIIT | High-intensity interval training |
| QOL | Quality of life |
| CVD | Cardiovascular disease |
| BCRL | Breast cancer-related lymphedema |
| PN | Peripheral neuropathy |
References
- Fahad Ullah, M. Breast Cancer: Current Perspectives on the Disease Status. Adv. Exp. Med. Biol. 2019, 51–64. [Google Scholar] [CrossRef]
- Rojas, K.; Stuckey, A. Breast Cancer Epidemiology and Risk Factors. Clin. Obstet. Gynecol. 2016, 59, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Wörmann, B. Breast Cancer: Basics, Screening, Diagnostics and Treatment. Med. Monatsschr. Pharm. 2017, 40, 55–64. [Google Scholar] [PubMed]
- Varghese, F.; Wong, J. Breast Cancer in the Elderly. Surg. Clin. N. Am. 2018, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Radecka, B.; Litwiniuk, M. Breast Cancer in Young Women. Ginekol. Pol. 2016, 659–663. [Google Scholar] [CrossRef]
- Liu, N.; Johnson, K.J.; Ma, C.X. Male Breast Cancer: An Updated Surveillance, Epidemiology, and End Results Data Analysis. Clin. Breast Cancer 2018, 18, e997–e1002. [Google Scholar] [CrossRef]
- Cao, A.; Huang, L.; Shao, Z. The Preventive Intervention of Hereditary Breast Cancer. Adv. Exp. Med. Biol. 2017, 1026, 41–57. [Google Scholar] [CrossRef]
- Abdelwahab Yousef, A.J. Male Breast Cancer: Epidemiology and Risk Factors. Semin. Oncol. 2017, 267–272. [Google Scholar] [CrossRef]
- Kapil, U.; Bhadoria, A.S.; Sareen, N.; Singh, P.; Dwivedi, S.N. Reproductive Factors and Risk of Breast Cancer: A Review. Indian J. Cancer 2014, 51, 571–576. [Google Scholar] [CrossRef]
- Guerrero, V.G.; Baez, A.F.; González, C.G.C.; González, C.G.M. Monitoring Modifiable Risk Factors for Breast Cancer: An Obligation for Health Professionals. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2017, 41. [Google Scholar] [CrossRef]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and Current Knowledge of Breast Cancer. Biol. Res. 2017, 33. [Google Scholar] [CrossRef] [PubMed]
- Taherian-Fard, A.; Srihari, S.; Ragan, M.A. Breast Cancer Classification: Linking Molecular Mechanisms to Disease Prognosis. Brief. Bioinform. 2014, 16, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Z.; Su, K.; Zeng, J. Clinicopathological Classification and Traditional Prognostic Indicators of Breast Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 8500–8505. [Google Scholar] [PubMed]
- Maughan, K.L.; Lutterbie, M.A.; Ham, P. Treatment of Breast Cancer. Am. Fam. Phys. 2010, 81, 1339–1346. [Google Scholar]
- Shien, T.; Iwata, H. Adjuvant and Neoadjuvant Therapy for Breast Cancer. Jpn. J. Clin. Oncol. 2020, 225–229. [Google Scholar] [CrossRef]
- Nagini, S. Breast Cancer: Current Molecular Therapeutic Targets and New Players. Anticancer Agents Med. Chem. 2017, 17, 152–163. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Guijarro, L.G.; Casanova, C.; Coca, S.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N.; Asúnsolo, Á. The Regulatory Role of Mitochondrial MicroRNAs (MitomiRs) in Breast Cancer: Translational Implications Present and Future. Cancers 2020, 12, 2443. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Buján, J.; García-Honduvilla, N.; Coca, S. Signal Transduction Pathways in Breast Cancer: The Important Role of PI3K/Akt/MTOR. J. Oncol. 2020, 2020. [Google Scholar] [CrossRef]
- Bergin, A.R.T.; Loi, S. Triple-Negative Breast Cancer: Recent Treatment Advances. F1000Research 2019, F1000. [Google Scholar] [CrossRef]
- Carioli, G.; Malvezzi, M.; Rodriguez, T.; Bertuccio, P.; Negri, E.; La Vecchia, C. Trends and Predictions to 2020 in Breast Cancer Mortality in Europe. Breast 2017, 36, 89–95. [Google Scholar] [CrossRef]
- Villar, R.R.; Fernández, S.P.; Garea, C.C.; Pillado, M.T.S.; Barreiro, V.B.; Martín, C.G. Quality of Life and Anxiety in Women with Breast Cancer before and after Treatment. Rev. Lat. Am. Enfermagem 2017, 25, e2958. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.V.; Bergerot, C.D.; Barbosa, L.R.; de Carvalho Tavares Calux, N.M.; Elias, S.; Ashing, K.T.; de Domenico, E.B.L. Impact of Breast Cancer and Quality of Life of Women Survivors. Rev. Bras. Enferm. 2018, 71, 2916–2921. [Google Scholar] [CrossRef] [PubMed]
- Tsaras, K.; Papathanasiou, I.V.; Mitsi, D.; Veneti, A.; Kelesi, M.; Zyga, S.; Fradelos, E.C. Assessment of Depression and Anxiety in Breast Cancer Patients: Prevalence and Associated Factors. Asian Pac. J. Cancer Prev. 2018, 19, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Gudenkauf, L.M.; Ehlers, S.L. Psychosocial Interventions in Breast Cancer Survivorship Care. Breast 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Winters-Stone, K.; Lee, A.; Schmitz, K.H. Cancer, Physical Activity, and Exercise. Compr. Physiol. 2012, 2775–2809. [Google Scholar] [CrossRef] [PubMed]
- Koçer, S.; Beuchat-Mamie, S.; Sperisen, N.; Molnar, P. [Physical Activity and Cancer]. Swiss Sports Exerc. Med. 2018, 42–47. [Google Scholar] [CrossRef]
- Kolak, A.; Kamińska, M.; Sygit, K.; Budny, A.; Surdyka, D.; Kukiełka-Budny, B.; Burdan, F. Primary and Secondary Prevention of Breast Cancer. Ann. Agric. Environ. Med. 2017, 24, 549–553. [Google Scholar] [CrossRef]
- Boyne, D.J.; O’Sullivan, D.E.; Olij, B.F.; King, W.D.; Friedenreich, C.M.; Brenner, D.R. Physical Activity, Global DNA Methylation, and Breast Cancer Risk: A Systematic Literature Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2018, 1320–1331. [Google Scholar] [CrossRef]
- Castelló, A.; Martín, M.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Sánchez, P.; Ramos, M.; Antón, A.; et al. Lower Breast Cancer Risk among Women Following the World Cancer Research Fund and American Institute for Cancer Research Lifestyle Recommendations: Epigeicam Case-Control Study. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Harvie, M.; Howell, A.; Evans, D.G. Can Diet and Lifestyle Prevent Breast Cancer: What Is the Evidence? Am. Soc. Clin. Oncol. Educ. B 2015, e66–e73. [Google Scholar] [CrossRef]
- Friedenreich, C.M. Physical Activity and Breast Cancer: Review of the Epidemiologic Evidence and Biologic Mechanisms. Recent Results Cancer Res. 2011, 125–139. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Zhang, Y.; Xie, Q.; Tan, X. Physical Activity and Risk of Breast Cancer: A Meta-Analysis of 38 Cohort Studies in 45 Study Reports. Value Health 2019, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Lope, V.; Martín, M.; Castelló, A.; Casla, S.; Ruiz, A.; Baena-Cañada, J.M.; Casas, A.M.; Calvo, L.; Bermejo, B.; Muñoz, M.; et al. Physical Activity and Breast Cancer Risk by Pathological Subtype. Gynecol. Oncol. 2017, 144, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Niehoff, N.M.; Nichols, H.B.; Zhao, S.; White, A.J.; Sandler, D.P. Adult Physical Activity and Breast Cancer Risk in Women with a Family History of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 51–58. [Google Scholar] [CrossRef]
- Romieu, I.I.; Amadou, A.; Chajes, V. The Role of Diet, Physical Activity, Body Fatness, and Breastfeeding in Breast Cancer in Young Women: Epidemiological Evidence. Rev. Investig. Clin. 2017, 69, 193–203. [Google Scholar] [CrossRef]
- Kudela, E.; Samec, M.; Kubatka, P.; Nachajova, M.; Laucekova, Z.; Liskova, A.; Dokus, K.; Biringer, K.; Simova, D.; Gabonova, E.; et al. Breast Cancer in Young Women: Status Quo and Advanced Disease Management by a Predictive, Preventive, and Personalized Approach. Cancers 2019, 11, 1791. [Google Scholar] [CrossRef]
- Niehoff, N.M.; White, A.J.; Sandler, D.P. Childhood and Teenage Physical Activity and Breast Cancer Risk. Breast Cancer Res. Treat. 2017, 164, 697–705. [Google Scholar] [CrossRef]
- Lammert, J.; Lubinski, J.; Gronwald, J.; Huzarski, T.; Armel, S.; Eisen, A.; Meschino, W.S.; Lynch, H.T.; Snyder, C.; Eng, C.; et al. Physical activity during adolescence and young adulthood and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2018, 169, 561–571. [Google Scholar] [CrossRef]
- Liu, Y.; Tobias, D.K.; Sturgeon, K.M.; Rosner, B.; Malik, V.; Cespedes, E.; Joshi, A.D.; Eliassen, A.H.; Colditz, G.A. Physical Activity from Menarche to First Pregnancy and Risk of Breast Cancer. Int. J. Cancer 2016, 139, 1223–1230. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, D.; Kang, S. Physical Activity and Risk of Breast Cancer: A Meta-Analysis of Prospective Studies. Breast Cancer Res. Treat. 2013, 137, 869–882. [Google Scholar] [CrossRef]
- Kyu, H.H.; Bachman, V.F.; Alexander, L.T.; Mumford, J.E.; Afshin, A.; Estep, K.; Veerman, J.L.; Delwiche, K.; Iannarone, M.L.; Moyer, M.L.; et al. Physical Activity and Risk of Breast Cancer, Colon Cancer, Diabetes, Ischemic Heart Disease, and Ischemic Stroke Events: Systematic Review and Dose-Response Meta-Analysis for the Global Burden of Disease Study 2013. BMJ 2016, 354, i3857. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Abar, L.; Cariolou, M.; Nanu, N.; Greenwood, D.C.; Bandera, E.V.; McTiernan, A.; Norat, T. World Cancer Research Fund International: Continuous Update Project—Systematic Literature Review and Meta-Analysis of Observational Cohort Studies on Physical Activity, Sedentary Behavior, Adiposity, and Weight Change and Breast Cancer Risk. Cancer Causes Control 2019, 1183–1200. [Google Scholar] [CrossRef] [PubMed]
- de Boer, M.C.; Wörner, E.A.; Verlaan, D.; van Leeuwen, P.A.M. The Mechanisms and Effects of Physical Activity on Breast Cancer. Clin. Breast Cancer 2017, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Neilson, H.K.; Farris, M.S.; Courneya, K.S. Physical Activity and Cancer Outcomes: A Precision Medicine Approach. Clin. Cancer Res. 2016, 4766–4775. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Kurzer, M.S. Estrogen Metabolism and Breast Cancer. Cancer Lett. 2015, 28, 231–243. [Google Scholar] [CrossRef]
- Neilson, H.K.; Friedenreich, C.M.; Brockton, N.T.; Millikan, R.C. Physical Activity and Postmenopausal Breast Cancer: Proposed Biologic Mechanisms and Areas for Future Research. Cancer Epidemiol. Biomark. Prev. 2009, 11–27. [Google Scholar] [CrossRef]
- Coyle, Y.M. Physical Activity as a Negative Modulator of Estrogen-Induced Breast Cancer. Cancer Causes Control 2008, 1021–1029. [Google Scholar] [CrossRef]
- Pizot, C.; Boniol, M.; Mullie, P.; Koechlin, A.; Boniol, M.; Boyle, P.; Autier, P. Physical Activity, Hormone Replacement Therapy and Breast Cancer Risk: A Meta-Analysis of Prospective Studies. Eur. J. Cancer 2016, 52, 138–154. [Google Scholar] [CrossRef]
- Lipovka, Y.; Konhilas, J.P. The Complex Nature of Oestrogen Signalling in Breast Cancer: Enemy or Ally? Biosci. Rep. 2016. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Shaw, E.; Neilson, H.K.; Brenner, D.R. Epidemiology and Biology of Physical Activity and Cancer Recurrence. J. Mol. Med. 2017, 1029–1041. [Google Scholar] [CrossRef]
- Argolo, D.F.; Hudis, C.A.; Iyengar, N.M. The Impact of Obesity on Breast Cancer. Curr. Oncol. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Brown, K.A. Obesity and Breast Cancer—Role of Estrogens and the Molecular Underpinnings of Aromatase Regulation in Breast Adipose Tissue. Mol. Cell. Endocrinol. 2018, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Karpe, F.; Lafontan, M.; Frayn, K. Physical Activity and Exercise in the Regulation of Human Adipose Tissue Physiology. Physiol. Rev. 2012, 92, 157–191. [Google Scholar] [CrossRef] [PubMed]
- Zimta, A.A.; Tigu, A.B.; Muntean, M.; Cenariu, D.; Slaby, O.; Berindan-Neagoe, I. Molecular Links between Central Obesity and Breast Cancer. Int. J. Mol. Sci. 2019, 20, 5364. [Google Scholar] [CrossRef] [PubMed]
- Andò, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, Leptin and Breast Cancer: Epidemiological Evidence and Proposed Mechanisms. Cancers 2019, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Cespedes Feliciano, E.M.; Chen, W.Y.; Bradshaw, P.T.; Prado, C.M.; Alexeeff, S.; Albers, K.B.; Castillo, A.L.; Caan, B.J. Adipose Tissue Distribution and Cardiovascular Disease Risk among Breast Cancer Survivors. J. Clin. Oncol. 2019, 37, 2528–2536. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-Inflammatory Effects of Exercise: Role in Diabetes and Cardiovascular Disease. Eur. J. Clin. Investig. 2017, 600–611. [Google Scholar] [CrossRef]
- Batatinha, H.A.P.; Biondo, L.A.; Lira, F.S.; Castell, L.M.; Rosa-Neto, J.C. Nutrients, Immune System, and Exercise: Where Will It Take Us? Nutrition 2019, 151–156. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 646–674. [Google Scholar] [CrossRef]
- Jiang, X.; Shapiro, D.J. The Immune System and Inflammation in Breast Cancer. Mol. Cell. Endocrinol. 2014, 382, 673–682. [Google Scholar] [CrossRef]
- Goldberg, J.E.; Schwertfeger, K.L. Proinflammatory Cytokines in Breast Cancer: Mechanisms of Action and Potential Targets for Therapeutics. Curr. Drug Targets 2010, 11, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Van Mackelenbergh, M.; Wesch, D.; Mundhenke, C. Physical Activity Influences the Immune System of Breast Cancer Patients. J. Cancer Res. Ther. 2017, 392–398. [Google Scholar] [CrossRef]
- Hagar, A.; Wang, Z.; Koyama, S.; Serrano, J.A.; Melo, L.; Vargas, S.; Carpenter, R.; Foley, J. Endurance Training Slows Breast Tumor Growth in Mice by Suppressing Treg Cells Recruitment to Tumors. BMC Cancer 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, M.; Jones, L.W.; Wilcock, A. Immunological and Hormonal Effects of Exercise: Implications for Cancer Cachexia. Curr. Opin. Support. Palliat. Care 2013, 7, 376–382. [Google Scholar] [CrossRef]
- Neilson, H.K.; Conroy, S.M.; Friedenreich, C.M. The Influence of Energetic Factors on Biomarkers of Postmenopausal Breast Cancer Risk. Curr. Nutr. Rep. 2014, 3, 22–34. [Google Scholar] [CrossRef]
- Hackney, A.C.; Lane, A.R. Exercise and the Regulation of Endocrine Hormones. Prog. Mol. Biol. Transl. Sci. 2015, 135, 293–311. [Google Scholar] [CrossRef]
- Moghetti, P.; Bacchi, E.; Brangani, C.; Donà, S.; Negri, C. Metabolic Effects of Exercise. Front. Horm. Res. 2016, 47, 44–57. [Google Scholar] [CrossRef]
- Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; King, J.A.; Miyashita, M.; Thackray, A.E.; Batterham, R.L.; et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef]
- Balkau, B.; Mhamdi, L.; Oppert, J.M.; Nolan, J.; Golay, A.; Porcellati, F.; Laakso, M.; Ferrannini, E. Physical Activity and Insulin Sensitivity the RISC Study. Diabetes 2008, 57, 2613–2618. [Google Scholar] [CrossRef]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The Endocrine Coupling of Skeletal Muscle and Bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical Activity and Muscle–Brain Crosstalk. Nat. Rev. Endocrinol. Nat. 2019, 15, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef] [PubMed]
- Peres Valgas da Silva, C.; Hernández-Saavedra, D.; White, J.D.; Stanford, K.I. Cold and Exercise: Therapeutic Tools to Activate Brown Adipose Tissue and Combat Obesity. Biology 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Gallè, F.; Valeriani, F.; Cattaruzza, M.S.; Ubaldi, F.; Romano Spica, V.; Liguori, G.; Calimeri, S.; Bono, R.; Privitera, G.; Fabiani, L.; et al. Exploring the Association between Physical Activity and Gut Microbiota Composition: A Review of Current Evidence. Ann. Ig. 2019, 31, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Plaza-DÍaz, J.; Álvarez-Mercado, A.I.; Ruiz-Marín, C.M.; Reina-Pérez, I.; Pérez-Alonso, A.J.; Sánchez-Andujar, M.B.; Torné, P.; Gallart-Aragón, T.; Sánchez-Barrón, M.T.; Reyes Lartategui, S.; et al. Association of Breast and Gut Microbiota Dysbiosis and the Risk of Breast Cancer: A Case-Control Clinical Study. BMC Cancer 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Jezierska-Drutel, A.; Rosenzweig, S.A.; Neumann, C.A. Role of Oxidative Stress and the Microenvironment in Breast Cancer Development and Progression. Adv. Cancer Res. 2013, 119, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Sadati Zarrini, A.; Moslemi, D.; Parsian, H.; Vessal, M.; Mosapour, A.; Shirkhani Kelagari, Z. The Status of Antioxidants, Malondialdehyde and Some Trace Elements in Serum of Patients with Breast Cancer. Casp. J. Intern. Med. 2016, 7, 31–36. [Google Scholar]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2019, 24, 4771–4778. [Google Scholar] [CrossRef]
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; Carvalho, D.P.; Fortunato, R.S. The Role of Oxidative Stress on Breast Cancer Development and Therapy. Tumor Biol. 2016, 37, 4281–4291. [Google Scholar] [CrossRef]
- Wen, C.; Wu, L.; Fu, L.; Wang, B.; Zhou, H. Unifying Mechanism in the Initiation of Breast Cancer by Metabolism of Estrogen (Review). Mol. Med. Reps. 2017, 1001–1006. [Google Scholar] [CrossRef]
- Soares, J.P.; Silva, A.M.; Oliveira, M.M.; Peixoto, F.; Gaivão, I.; Mota, M.P. Effects of Combined Physical Exercise Training on DNA Damage and Repair Capacity: Role of Oxidative Stress Changes. Age (Omaha) 2015, 37. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, B.; Malfa, G.A.; Strazzanti, A.; Gangi, S.; Di Giacomo, C.; Basile, F.; Renis, M. Effects of Physical Activity on Systemic Oxidative/DNA Status in Breast Cancer Survivors. Oncol. Lett. 2017, 13, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Pialoux, V.; Wang, Q.; Shaw, E.; Brenner, D.R.; Waltz, X.; Conroy, S.M.; Johnson, R.; Woolcott, C.G.; Poulin, M.J.; et al. Effects of Exercise on Markers of Oxidative Stress: An Ancillary Analysis of the Alberta Physical Activity and Breast Cancer Prevention Trial. BMJ Open Sport Exerc. Med. 2016, 2, e000171. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Milne, G.L.; Sandler, D.P.; Nichols, H.B. Oxidative Stress in Relation to Diet and Physical Activity among Premenopausal Women. Br. J. Nutr. 2016, 116, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Gurer-Orhan, H.; Ince, E.; Konyar, D.; Saso, L.; Suzen, S. The Role of Oxidative Stress Modulators in Breast Cancer. Curr. Med. Chem. 2017, 25, 4084–4101. [Google Scholar] [CrossRef] [PubMed]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of Doxorubicin on Apoptosis and Oxidative Stress in Breast Cancer Cell Lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Gago-Dominguez, M.; Jiang, X.; Esteban Castelao, J. Lipid Peroxidation and the Protective Effect of Physical Exercise on Breast Cancer. Med. Hypotheses 2007, 68, 1138–1143. [Google Scholar] [CrossRef]
- McCullough, L.E.; Santella, R.M.; Cleveland, R.J.; Bradshaw, P.T.; Millikan, R.C.; North, K.E.; Olshan, A.F.; Eng, S.M.; Ambrosone, C.B.; Ahn, J.; et al. Polymorphisms in Oxidative Stress Genes, Physical Activity, and Breast Cancer Risk. Cancer Causes Control 2012, 23, 1949–1958. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Bacabac, R.G.; Bakker, A.D. Mechanical Loading and How It Affects Bone Cells: The Role of the Osteocyte Cytoskeleton in Maintaining Our Skeleton. Eur. Cells Mater. 2012, 24, 278–291. [Google Scholar] [CrossRef]
- Ma, Y.H.V.; Xu, L.; Mei, X.; Middleton, K.; You, L. Mechanically Stimulated Osteocytes Reduce the Bone-Metastatic Potential of Breast Cancer Cells in Vitro by Signaling through Endothelial Cells. J. Cell. Biochem. 2019, 120, 7590–7601. [Google Scholar] [CrossRef]
- Hart, N.H.; Galvão, D.A.; Saunders, C.; Taaffe, D.R.; Feeney, K.T.; Spry, N.A.; Tsoi, D.; Martin, H.; Chee, R.; Clay, T.; et al. Mechanical Suppression of Osteolytic Bone Metastases in Advanced Breast Cancer Patients: A Randomised Controlled Study Protocol Evaluating Safety, Feasibility and Preliminary Efficacy of Exercise as a Targeted Medicine. Trials 2018, 19, 695. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Quail, D.F.; Zhang, X.; White, R.M.; Jones, L.W. Exercise-Dependent Regulation of the Tumour Microenvironment. Nat. Rev. Cancer 2017, 17, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Buss, L.A.; Dachs, G.U. Effects of Exercise on the Tumour Microenvironment. Adv. Exp. Med. Biol. 2020, 1225, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Gholamian, S.; Hosseini, S.R.A.; Rashidlamir, A.; Aghaalinejad, H. The Effects of Interval Aerobic Training on Mesenchymal Biomarker Gene Expression, the Rate of Tumor Volume, and Cachexia in Mice with Breast Cancer. Iran. J. Basic Med. Sci. 2020, 23, 244–250. [Google Scholar] [CrossRef]
- Molanouri Shamsi, M.; Chekachak, S.; Soudi, S.; Quinn, L.S.; Rangbar, K.; Chenari, J.; Yazdi, M.H.; Mahdavi, M. Combined Effect of Aerobic Interval Training and Selenium Nanoparticles on Expression of IL-15 and IL-10/TNF-α Ratio in Skeletal Muscle of 4T1 Breast Cancer Mice with Cachexia. Cytokine 2017, 90, 100–108. [Google Scholar] [CrossRef]
- Jones, L.W.; Viglianti, B.L.; Tashjian, J.A.; Kothadia, S.M.; Keir, S.T.; Freedland, S.J.; Potter, M.Q.; Moon, E.J.; Schroeder, T.; Herndon, J.E.; et al. Effect of Aerobic Exercise on Tumor Physiology in an Animal Model of Human Breast Cancer. J. Appl. Physiol. 2010, 108, 343–348. [Google Scholar] [CrossRef]
- Betof, A.S.; Lascola, C.D.; Weitzel, D.; Landon, C.; Scarbrough, P.M.; Devi, G.R.; Palmer, G.; Jones, L.W.; Dewhirst, M.W. Modulation of Murine Breast Tumor Vascularity, Hypoxia, and Chemotherapeutic Response by Exercise. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Agostini, D.; Natalucci, V.; Baldelli, G.; De Santi, M.; Zeppa, S.D.; Vallorani, L.; Annibalini, G.; Lucertini, F.; Federici, A.; Izzo, R.; et al. New Insights into the Role of Exercise in Inhibiting MTOR Signaling in Triple-Negative Breast Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 5896786. [Google Scholar] [CrossRef]
- Smallbone, K.; Maini, P.K.; Gatenby, R.A. Episodic, Transient Systemic Acidosis Delays Evolution of the Malignant Phenotype: Possible Mechanism for Cancer Prevention by Increased Physical Activity. Biol. Direct 2010, 5. [Google Scholar] [CrossRef]
- Brenner, D.R.; Ruan, Y.; Adams, S.C.; Courneya, K.S.; Friedenreich, C.M. The Impact of Exercise on Growth Factors (VEGF and FGF2): Results from a 12-Month Randomized Intervention Trial. Eur. Rev. Aging Phys. Act. 2019, 16. [Google Scholar] [CrossRef]
- Pudkasam, S.; Polman, R.; Pitcher, M.; Fisher, M.; Chinlumprasert, N.; Stojanovska, L.; Apostolopoulos, V. Physical Activity and Breast Cancer Survivors: Importance of Adherence, Motivational Interviewing and Psychological Health. Maturitas 2018, 116, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Taran, S.; Burke, S.; Sabiston, C.M. A Qualitative Exploration of Barriers and Motivators to Physical Activity Participation in Women Treated for Breast Cancer. Disabil. Rehabil. 2013, 35, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, A.; Arving, C.; Johansson, B.; Igelström, H.; Nordin, K. Perceived Barriers to and Facilitators of Being Physically Active during Adjuvant Cancer Treatment. Patient Educ. Couns. 2016, 99, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Milne, H.M.; Wallman, K.E.; Gullfoyle, A.; Gordon, S.; Courneya, K.S. Self-Determination Theory and Physical Activity among Breast Cancer Survivors. J. Sport Exerc. Psychol. 2008, 30, 23–38. [Google Scholar] [CrossRef]
- Brunet, J.; Burke, S.M.; Sabiston, C.M. The Benefits of Being Self-Determined in Promoting Physical Activity and Affective Well-Being among Women Recently Treated for Breast Cancer. Psychooncology 2013, 22, 2245–2252. [Google Scholar] [CrossRef]
- Short, C.E.; James, E.L.; Plotnikoff, R.C. How Social Cognitive Theory Can Help Oncology-Based Health Professionals Promote Physical Activity among Breast Cancer Survivors. Eur. J. Oncol. Nurs. 2013, 17, 482–489. [Google Scholar] [CrossRef]
- Hartman, S.J.; Rosen, R.K. Breast Cancer Relatives’ Physical Activity Intervention Needs and Preferences: Qualitative Results. BMC Womens Health 2017, 17. [Google Scholar] [CrossRef]
- Delrieu, L.; Vallance, J.K.; Morelle, M.; Fervers, B.; Pialoux, V.; Friedenreich, C.; Dufresne, A.; Bachelot, T.; Heudel, P.E.; Trédan, O.; et al. Physical Activity Preferences before and after Participation in a 6-Month Physical Activity Intervention among Women with Metastatic Breast Cancer. Eur. J. Cancer Care (Engl.) 2020, 29. [Google Scholar] [CrossRef]
- Desbiens, C.; Filion, M.; Brien, M.C.; Hogue, J.C.; Laflamme, C.; Lemieux, J. Impact of Physical Activity in Group versus Individual Physical Activity on Fatigue in Patients with Breast Cancer: A Pilot Study. Breast 2017, 35, 8–13. [Google Scholar] [CrossRef]
- Abdin, S.; Lavallée, J.F.; Faulkner, J.; Husted, M. A Systematic Review of the Effectiveness of Physical Activity Interventions in Adults with Breast Cancer by Physical Activity Type and Mode of Participation. Psycho-Oncology 2019, 28, 1381–1393. [Google Scholar] [CrossRef]
- Pisu, M.; Demark-Wahnefried, W.; Kenzik, K.M.; Oster, R.A.; Lin, C.P.; Manne, S.; Alvarez, R.; Martin, M.Y. A Dance Intervention for Cancer Survivors and Their Partners (RHYTHM). J. Cancer Surviv. 2017, 11, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Boing, L.; Do Bem Fretta, T.; De Carvalho Souza Vieira, M.; Pereira, G.S.; Moratelli, J.; Sperandio, F.F.; Bergmann, A.; Baptista, F.; Dias, M.; De Azevedo Guimarães, A.C. Pilates and Dance to Patients with Breast Cancer Undergoing Treatment: Study Protocol for a Randomized Clinical Trial—MoveMama Study. Trials 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Uth, J.; Fristrup, B.; Sørensen, V.; Helge, E.W.; Christensen, M.K.; Kjærgaard, J.B.; Møller, T.K.; Mohr, M.; Helge, J.W.; Jørgensen, N.R.; et al. Exercise Intensity and Cardiovascular Health Outcomes after 12 months of Football Fitness Training in Women Treated for Stage I-III Breast Cancer: Results from the Football Fitness After Breast Cancer (ABC) Randomized Controlled Trial. Prog. Cardiovasc. Dis. 2020, 63, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Harrington, S.; Padua, D.; Battaglini, C.; Michener, L.A.; Giuliani, C.; Myers, J.; Groff, D. Comparison of Shoulder Flexibility, Strength, and Function between Breast Cancer Survivors and Healthy Participants. J. Cancer Surviv. 2011, 5, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Peel, A.B.; Thomas, S.M.; Dittus, K.; Jones, L.W.; Lakoski, S.G. Cardiorespiratory Fitness in Breast Cancer Patients: A Call for Normative Values. J. Am. Heart Assoc. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harbor Perspect. Med. 2018, 8. [Google Scholar] [CrossRef]
- An, K.Y.; Morielli, A.R.; Kang, D.W.; Friedenreich, C.M.; McKenzie, D.C.; Gelmon, K.; Mackey, J.R.; Reid, R.D.; Courneya, K.S. Effects of Exercise Dose and Type during Breast Cancer Chemotherapy on Longer-Term Patient-Reported Outcomes and Health-Related Fitness: A Randomized Controlled Trial. Int. J. Cancer 2020, 146, 150–160. [Google Scholar] [CrossRef]
- Jones, L.M.; Stoner, L.; Baldi, J.C.; McLaren, B. Circuit Resistance Training and Cardiovascular Health in Breast Cancer Survivors. Eur. J. Cancer Care (Engl.) 2020, 29. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and Resistance Exercise Improves Physical Fitness, Bone Health, and Quality of Life in Overweight and Obese Breast Cancer Survivors: A Randomized Controlled Trial 11 Medical and Health Sciences 1117 Public Health and Health Services. Breast Cancer Res. 2018, 20. [Google Scholar] [CrossRef]
- Hiraoui, M.; Al-Haddabi, B.; Gmada, N.; Doutrellot, P.L.; Mezlini, A.; Ahmaidi, S. Effects of Combined Supervised Intermittent Aerobic, Muscle Strength and Home-Based Walking Training Programs on Cardiorespiratory Responses in Women with Breast Cancer. Bull. Cancer 2019, 106, 527–537. [Google Scholar] [CrossRef]
- Soriano-Maldonado, A.; Carrera-Ruiz, Á.; Díez-Fernández, D.M.; Esteban-Simón, A.; Maldonado-Quesada, M.; Moreno-Poza, N.; García-Martínez, M.D.M.; Alcaraz-García, C.; Vázquez-Sousa, R.; Moreno-Martos, H.; et al. Effects of a 12-Week Resistance and Aerobic Exercise Program on Muscular Strength and Quality of Life in Breast Cancer Survivors: Study Protocol for the EFICAN Randomized Controlled Trial. Medicine 2019, 98, e17625. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; McNeil, J.; O’Reilly, R.; Morielli, A.R.; Friedenreich, C.M. Dose-Response Effects of Aerobic Exercise on Quality of Life in Postmenopausal Women: Results from the Breast Cancer and Exercise Trial in Alberta (BETA). Ann. Behav. Med. 2017, 51, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; McKenzie, D.C.; Gelmon, K.; MacKey, J.R.; Reid, R.D.; Yasui, Y.; Friedenreich, C.M.; Forbes, C.C.; Trinh, L.; Jespersen, D.; et al. A Multicenter Randomized Trial of the Effects of Exercise Dose and Type on Psychosocial Distress in Breast Cancer Patients Undergoing Chemotherapy. Cancer Epidemiol. Biomark. Prev. 2014, 23, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Leach, H.J.; Danyluk, J.M.; Culos-Reed, S.N. Design and Implementation of a Community-Based Exercise Program for Breast Cancer Patients. Curr. Oncol. 2014, 21, 267–271. [Google Scholar] [CrossRef]
- Leach, H.J.; Danyluk, J.M.; Nishimura, K.C.; Culos-Reed, S.N. Benefits of 24 versus 12 Weeks of Exercise and Wellness Programming for Women Undergoing Treatment for Breast Cancer. Support. Care Cancer 2016, 24, 4597–4606. [Google Scholar] [CrossRef]
- Wengström, Y.; Bolam, K.A.; Mijwel, S.; Sundberg, C.J.; Backman, M.; Browall, M.; Norrbom, J.; Rundqvist, H. Optitrain: A Randomised Controlled Exercise Trial for Women with Breast Cancer Undergoing Chemotherapy. BMC Cancer 2017, 17. [Google Scholar] [CrossRef]
- Ross, L.M.; Porter, R.R.; Durstine, J.L. High-Intensity Interval Training (HIIT) for Patients with Chronic Diseases. J. Sport Health Sci. 2016, 139–144. [Google Scholar] [CrossRef]
- Scharhag-Rosenberger, F.; Kuehl, R.; Klassen, O.; Schommer, K.; Schmidt, M.E.; Ulrich, C.M.; Wiskemann, J.; Steindorf, K. Exercise Training Intensity Prescription in Breast Cancer Survivors: Validity of Current Practice and Specific Recommendations. J. Cancer Surviv. 2015, 9, 612–619. [Google Scholar] [CrossRef]
- Lopez, P.; Galvão, D.A.; Taaffe, D.R.; Newton, R.U.; Souza, G.; Trajano, G.S.; Pinto, R.S. Resistance Training in Breast Cancer Patients Undergoing Primary Treatment: A Systematic Review and Meta-Regression of Exercise Dosage. Breast Cancer 2020, 1–9. [Google Scholar] [CrossRef]
- Bloomquist, K.; Adamsen, L.; Hayes, S.C.; Lillelund, C.; Andersen, C.; Christensen, K.B.; Oturai, P.; Ejlertsen, B.; Tuxen, M.K.; Møller, T. Heavy-Load Resistance Exercise during Chemotherapy in Physically Inactive Breast Cancer Survivors at Risk for Lymphedema: A Randomized Trial. Acta Oncol. (Madr.) 2019, 58, 1667–1675. [Google Scholar] [CrossRef]
- Mijwel, S.; Backman, M.; Bolam, K.A.; Jervaeus, A.; Sundberg, C.J.; Margolin, S.; Browall, M.; Rundqvist, H.; Wengström, Y. Adding High-Intensity Interval Training to Conventional Training Modalities: Optimizing Health-Related Outcomes during Chemotherapy for Breast Cancer: The OptiTrain Randomized Controlled Trial. Breast Cancer Res. Treat. 2018, 168, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Mijwel, S.; Jervaeus, A.; Bolam, K.A.; Norrbom, J.; Bergh, J.; Rundqvist, H.; Wengström, Y. High-Intensity Exercise during Chemotherapy Induces Beneficial Effects 12 Months into Breast Cancer Survivorship. J. Cancer Surviv. 2019, 13, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Mijwel, S.; Backman, M.; Bolam, K.A.; Olofsson, E.; Norrbom, J.; Bergh, J.; Sundberg, C.J.; Wengström, Y.; Rundqvist, H. Highly Favorable Physiological Responses to Concurrent Resistance and High-Intensity Interval Training during Chemotherapy: The OptiTrain Breast Cancer Trial. Breast Cancer Res. Treat. 2018, 169, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Mijwel, S.; Bolam, K.A.; Gerrevall, J.; Foukakis, T.; Wengström, Y.; Rundqvist, H. Effects of Exercise on Chemotherapy Completion and Hospitalization Rates: The OptiTrain Breast Cancer Trial. Oncologist 2020, 25, 23–32. [Google Scholar] [CrossRef] [PubMed]
- (McNeil, J.; Brenner, D.R.; Stone, C.R.; O’Reilly, R.; Ruan, Y.; Vallance, J.K.; Courneya, K.S.; Thorpe, K.E.; Klein, D.J.; Friedenreich, C.M. Activity Tracker to Prescribe Various Exercise Intensities in Breast Cancer Survivors. Med. Sci. Sports Exerc. 2019, 51, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, B.D.; Ahmed, R.; Amireault, S.; Sabiston, C.M. Changes in Light-, Moderate-, and Vigorous-Intensity Physical Activity and Changes in Depressive Symptoms in Breast Cancer Survivors: A Prospective Observational Study. Support. Care Cancer 2017, 25, 3305–3312. [Google Scholar] [CrossRef]
- Kokts-Porietis, R.L.; Stone, C.R.; Friedenreich, C.M.; Froese, A.; McDonough, M.; McNeil, J. Breast Cancer Survivors’ Perspectives on a Home-Based Physical Activity Intervention Utilizing Wearable Technology. Support. Care Cancer 2019, 27, 2885–2892. [Google Scholar] [CrossRef]
- Wagoner, C.W.; Choi, S.K.; Deal, A.M.; Lee, J.T.; Wood, W.A.; Muss, H.B.; Nyrop, K.A. Establishing Physical Activity in Breast Cancer: Self-Report versus Activity Tracker. Breast Cancer Res. Treat. 2019, 176, 395–400. [Google Scholar] [CrossRef]
- Johnsson, A.; Broberg, P.; Krüger, U.; Johnsson, A.; Tornberg, Å.B.; Olsson, H. Physical Activity and Survival Following Breast Cancer. Eur. J. Cancer Care (Engl.) 2019, 28. [Google Scholar] [CrossRef]
- Mctiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Jung, A.Y.; Behrens, S.; Schmidt, M.; Thoene, K.; Obi, N.; Hüsing, A.; Benner, A.; Steindorf, K.; Chang-Claude, J. Pre- To Postdiagnosis Leisure-Time Physical Activity and Prognosis in Postmenopausal Breast Cancer Survivors. Breast Cancer Res. 2019, 21. [Google Scholar] [CrossRef] [PubMed]
- Palesh, O.; Kamen, C.; Sharp, S.; Golden, A.; Neri, E.; Spiegel, D.; Koopman, C. Physical Activity and Survival in Women with Advanced Breast Cancer. Cancer Nurs. 2018, 41, E31–E38. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. A Meta-Analysis of the Association between Physical Activity and Breast Cancer Mortality. Cancer Nurs. 2019, 42, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Lemanne, D.; Cassileth, B.; Gubili, J. The Role of Physical Activity in Cancer Prevention, Treatment, Recovery, and Survivorship. Oncology 2013, 27, 580–585. [Google Scholar]
- Cannioto, R.A.; Hutson, A.; Dighe, S.; McCann, W.; McCann, S.E.; Zirpoli, G.R.; Barlow, W.; Kelly, K.M.; DeNysschen, C.A.; Hershman, D.L.; et al. Physical Activity before, during and after Chemotherapy for High-Risk Breast Cancer: Relationships with Survival. JNCI J. Natl. Cancer Inst. 2020, djaa046. [Google Scholar] [CrossRef]
- Versteeg, K.S.; Blauwhoff-Buskermolen, S.; Buffart, L.M.; de van der Schueren, M.A.E.; Langius, J.A.E.; Verheul, H.M.W.; Maier, A.B.; Konings, I.R. Higher Muscle Strength Is Associated with Prolonged Survival in Older Patients with Advanced Cancer. Oncologist 2018, 23, 580–585. [Google Scholar] [CrossRef]
- Ording, A.G.; Garne, J.P.; Nyström, P.M.W.; Frøslev, T.; Sørensen, H.T.; Lash, T.L. Comorbid Diseases Interact with Breast Cancer to Affect Mortality in the First Year after Diagnosis-A Danish Nationwide Matched Cohort Study. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Vardar-Yagli, N.; Sener, G.; Saglam, M.; Calik-Kutukcu, E.; Arikan, H.; Inal-Ince, D.; Savci, S.; Altundag, K.; Kutluk, T.; Ozisik, Y.; et al. Associations among Physical Activity, Comorbidity, Functional Capacity, Peripheral Muscle Strength and Depression in Breast Cancer Survivors. Asian Pac. J. Cancer Prev. 2015, 16, 585–589. [Google Scholar] [CrossRef]
- McTiernan, A. Weight, Physical Activity and Breast Cancer Survival. Proc. Nutr. Soc. 2018, 77, 403–411. [Google Scholar] [CrossRef]
- Da Mata Tiezzi, M.F.B.; De Andrade, J.M.; Romao, A.P.M.S.; Tiezzi, D.G.; Lerri, M.R.; Carrara, H.A.H.; Lara, L.A.S. Quality of Life in Women with Breast Cancer Treated with or without Chemotherapy. Cancer Nurs. 2017, 40, 108–116. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Cardinal, B.J. Effects of Physical Activity on Common Side Effects of Breast Cancer Treatment. Breast Cancer 2012, 19, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Palesh, O.; Scheiber, C.; Kesler, S.; Mustian, K.; Koopman, C.; Schapira, L. Management of Side Effects during and Post-Treatment in Breast Cancer Survivors. Breast J. 2018, 24, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Gernaat, S.A.M.; Ho, P.J.; Rijnberg, N.; Emaus, M.J.; Baak, L.M.; Hartman, M.; Grobbee, D.E.; Verkooijen, H.M. Risk of Death from Cardiovascular Disease Following Breast Cancer: A Systematic Review. Breast Cancer Res. Treat. 2017, 164, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Mulvagh, S.L. The Connection between the Breast and Heart in a Woman: Breast Cancer and Cardiovascular Disease. Clin. Cardiol. 2018, 41, 253–257. [Google Scholar] [CrossRef]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; Dent, S.; Kondapalli, L.; Ky, B.; Okwuosa, T.; et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef]
- Bowles, E.J.A.; Wellman, R.; Feigelson, H.S.; Onitilo, A.A.; Freedman, A.N.; Delate, T.; Allen, L.A.; Nekhlyudov, L.; Goddard, K.A.B.; Davis, R.L.; et al. Risk of Heart Failure in Breast Cancer Patients after Anthracycline and Trastuzumab Treatment: A Retrospective Cohort Study. J. Natl. Cancer Inst. 2012, 104, 1293–1305. [Google Scholar] [CrossRef]
- Antunes, P.; Esteves, D.; Nunes, C.; Amarelo, A.; Fonseca-Moutinho, J.; Afreixo, V.; Costa, H.; Alves, A.; Joaquim, A. Effects of Physical Exercise on Outcomes of Cardiac (Dys)Function in Women with Breast Cancer Undergoing Anthracycline or Trastuzumab Treatment: Study Protocol for a Systematic Review. Syst. Revs. 2019, 8. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Davis, M.K. Exercise Prevention of Cardiovascular Disease in Breast Cancer Survivors. J. Oncol. 2015, 2015, 917606. [Google Scholar] [CrossRef]
- Ginzac, A.; Passildas, J.; Gadéa, E.; Abrial, C.; Molnar, I.; Trésorier, R.; Duclos, M.; Thivat, E.; Durando, X. Treatment-Induced Cardiotoxicity in Breast Cancer: A Review of the Interest of Practicing a Physical Activity. Oncology 2019, 96, 223–234. [Google Scholar] [CrossRef]
- Pituskin, E.; Haykowsky, M.; McNeely, M.; Mackey, J.; Chua, N.; Paterson, I. Rationale and Design of the Multidisciplinary Team IntervenTion in CArdio-ONcology Study (TITAN). BMC Cancer 2016, 16. [Google Scholar] [CrossRef]
- Antunes, P.; Esteves, D.; Nunes, C.; Sampaio, F.; Ascensão, A.; Vilela, E.; Teixeira, M.; Amarelo, A.L.; Joaquim, A. Impact of Exercise Training on Cardiotoxicity and Cardiac Health Outcomes in Women with Breast Cancer Anthracycline Chemotherapy: A Study Protocol for a Randomized Controlled Trial. Trials 2019, 20. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Shave, R.E.; Bland, K.A.; Bovard, J.M.; Eves, N.D.; Gelmon, K.A.; McKenzie, D.C.; Virani, S.A.; Stöhr, E.J.; Warburton, D.E.R.; et al. Protective Effects of Acute Exercise Prior to Doxorubicin on Cardiac Function of Breast Cancer Patients: A Proof-of-Concept RCT. Int. J. Cardiol. 2017, 245, 263–270. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Mackey, J.R.; Thompson, R.B.; Jones, L.W.; Paterson, D.I. Adjuvant Trastuzumab Induces Ventricular Remodeling despite Aerobic Exercise Training. Clin. Cancer Res. 2009, 15, 4963–4967. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, K.M.; Ky, B.; Libonati, J.R.; Schmitz, K.H. The Effects of Exercise on Cardiovascular Outcomes before, during, and after Treatment for Breast Cancer. Breast Cancer Res. Treat. 2014, 219–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacquinot, Q.; Meneveau, N.; Chatot, M.; Bonnetain, F.; Degano, B.; Bouhaddi, M.; Dumoulin, G.; Vernerey, D.; Pivot, X.; Mougin, F. A Phase 2 Randomized Trial to Evaluate the Impact of a Supervised Exercise Program on Cardiotoxicity at 3 Months in Patients with HER2 Overexpressing Breast Cancer Undergoing Adjuvant Treatment by Trastuzumab: Design of the CARDAPAC Study. BMC Cancer 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.; Stanway, S.; Farmer, R.E.; Strongman, H.; Thomas, S.; Lyon, A.R.; Smeeth, L.; Bhaskaran, K. Long Term Adjuvant Endocrine Therapy and Risk of Cardiovascular Disease in Female Breast Cancer Survivors: Systematic Review. BMJ (Online) 2018, 363, k3845. [Google Scholar] [CrossRef] [PubMed]
- Saghatchian, M.; Lesur, A. Management of Side Effects Related to Adjuvant Hormone Therapy in Young Women with Breast Cancer. Bull. Cancer 2019, 106, S37–S42. [Google Scholar] [CrossRef]
- Hojan, K.; Molińska-Glura, M.; Milecki, P. Physical Activity and Body Composition, Body Physique, and Quality of Life in Premenopausal Breast Cancer Patients during Endocrine Therapy- A Feasibility Study. Acta Oncol. 2013, 52, 319–326. [Google Scholar] [CrossRef]
- De Glas, N.A.; Fontein, D.B.Y.; Bastiaannet, E.; Pijpe, A.; De Craen, A.J.M.; Liefers, G.J.; Nortier, H.J.R.; De Haes, H.J.C.J.M.; Van De Velde, C.J.H.; Van Leeuwen, F.E. Physical Activity and Survival of Postmenopausal, Hormone Receptor-Positive Breast Cancer Patients: Results of the Tamoxifen Exemestane Adjuvant Multicenter Lifestyle Study. Cancer 2014, 120, 2847–2854. [Google Scholar] [CrossRef]
- Suesada, M.M.; de Andrade Carvalho, H.; de Albuquerque, A.L.P.; Salge, J.M.; Stuart, S.R.; Takagaki, T.Y. Impact of Thoracic Radiotherapy on Respiratory Function and Exercise Capacity in Patients with Breast Cancer. J. Bras. Pneumol. 2018, 44, 469–476. [Google Scholar] [CrossRef]
- Lipsett, A.; Barrett, S.; Haruna, F.; Mustian, K.; O’Donovan, A. The Impact of Exercise during Adjuvant Radiotherapy for Breast Cancer on Fatigue and Quality of Life: A Systematic Review and Meta-Analysis. Breast 2017, 144–155. [Google Scholar] [CrossRef]
- Hwang, J.H.; Chang, H.J.; Shim, Y.H.; Park, W.H.; Park, W.; Huh, S.J.; Yang, J.H. Effects of Supervised Exercise Therapy in Patients Receiving Radiotherapy for Breast Cancer. Yonsei Med. J. 2008, 49, 443–450. [Google Scholar] [CrossRef]
- Zimmer, P.; Schmidt, M.E.; Prentzell, M.T.; Berdel, B.; Wiskemann, J.; Kellner, K.H.; Debus, J.; Ulrich, C.; Opitz, C.A.; Steindorf, K. Resistance Exercise Reduces Kynurenine Pathway Metabolites in Breast Cancer Patients Undergoing Radiotherapy. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Yang, H. Impact of Post-Radiotherapy Exercise on Women with Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Rehabil. Med. 2020, 52. [Google Scholar] [CrossRef] [PubMed]
- Piraux, E.; Caty, G.; Aboubakar Nana, F.; Reychler, G. Effects of Exercise Therapy in Cancer Patients Undergoing Radiotherapy Treatment: A Narrative Review. SAGE Open Med. 2020, 8, 205031212092265. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast Cancer-Related Lymphedema: Risk Factors, Precautionary Measures, and Treatments. Gland Surg. 2018, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Kilbreath, S.L.; Refshauge, K.M.; Beith, J.M.; Ward, L.C.; Ung, O.A.; Dylke, E.S.; French, J.R.; Yee, J.; Koelmeyer, L.; Gaitatzis, K. Risk Factors for Lymphoedema in Women with Breast Cancer: A Large Prospective Cohort. Breast 2016, 28, 29–36. [Google Scholar] [CrossRef]
- Miller, C.L.; Colwell, A.S.; Horick, N.; Skolny, M.N.; Jammallo, L.S.; O’Toole, J.A.; Shenouda, M.N.; Sadek, B.T.; Swaroop, M.N.; Ferguson, C.M.; et al. Immediate Implant Reconstruction Is Associated with a Reduced Risk of Lymphedema Compared to Mastectomy Alone a Prospective Cohort Study. Ann. Surg. 2016, 263, 399–405. [Google Scholar] [CrossRef]
- De Vrieze, T.; Gebruers, N.; Nevelsteen, I.; Tjalma, W.A.A.; Thomis, S.; De Groef, A.; Dams, L.; Van der Gucht, E.; Devoogdt, N. Physical Activity Level and Age Contribute to Functioning Problems in Patients with Breast Cancer-Related Lymphedema: A Multicentre Cross-Sectional Study. Support. Care Cancer 2020, 28, 5717–5731. [Google Scholar] [CrossRef]
- Baumann, F.T.; Reike, A.; Reimer, V.; Schumann, M.; Hallek, M.; Taaffe, D.R.; Newton, R.U.; Galvao, D.A. Effects of Physical Exercise on Breast Cancer-Related Secondary Lymphedema: A Systematic Review. Breast Cancer Res. Treat. 2018, 170, 1–13. [Google Scholar] [CrossRef]
- Rogan, S.; Taeymans, J.; Luginbuehl, H.; Aebi, M.; Mahnig, S.; Gebruers, N. Therapy Modalities to Reduce Lymphoedema in Female Breast Cancer Patients: A Systematic Review and Meta-Analysis. Breast Cancer Res. Treat. 2016, 159, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.; Mortimer, P.; Judd, P.A. A Randomized Controlled Trial of Weight Reduction as a Treatment for Breast Cancer-Related Lymphedema. Cancer 2007, 110, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Winkels, R.M.; Sturgeon, K.M.; Kallan, M.J.; Dean, L.T.; Zhang, Z.; Evangelisti, M.; Brown, J.C.; Sarwer, D.B.; Troxel, A.B.; Denlinger, C.; et al. The Women in Steady Exercise Research (WISER) Survivor Trial: The Innovative Transdisciplinary Design of a Randomized Controlled Trial of Exercise and Weight-Loss Interventions among Breast Cancer Survivors with Lymphedema. Contemp. Clin. Trials 2017, 61, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Troxel, A.B.; Dean, L.T.; Demichele, A.; Brown, J.C.; Sturgeon, K.; Zhang, Z.; Evangelisti, M.; Spinelli, B.; Kallan, M.J.; et al. Effect of Home-Based Exercise and Weight Loss Programs on Breast Cancer-Related Lymphedema Outcomes among Overweight Breast Cancer Survivors: The WISER Survivor Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Letellier, M.E.; Towers, A.; Shimony, A.; Tidhar, D. Breast Cancer-Related Lymphedema: A Randomized Controlled Pilot and Feasibility Study. Am. J. Phys. Med. Rehabil. 2014, 93. [Google Scholar] [CrossRef] [PubMed]
- Dönmez, A.A.; Kapucu, S. The Effectiveness of a Clinical and Home-Based Physical Activity Program and Simple Lymphatic Drainage in the Prevention of Breast Cancer-Related Lymphedema: A Prospective Randomized Controlled Study. Eur. J. Oncol. Nurs. 2017, 31, 12–21. [Google Scholar] [CrossRef]
- Sander, A.P.; Wilson, J.; Izzo, N.; Mountford, S.A.; Hayes, K.W. Factors That Affect Decisions about Physical Activity and Exercise in Survivors of Breast Cancer: A Qualitative Study. Phys. Ther. 2012, 92, 525–536. [Google Scholar] [CrossRef]
- Cormie, P.; Pumpa, K.; Galvão, D.A.; Turner, E.; Spry, N.; Saunders, C.; Zissiadis, Y.; Newton, R.U. Is It Safe and Efficacious for Women with Lymphedema Secondary to Breast Cancer to Lift Heavy Weights during Exercise: A Randomised Controlled Trial. J. Cancer Surviv. 2013, 7, 413–424. [Google Scholar] [CrossRef]
- Cormie, P.; Singh, B.; Hayes, S.; Peake, J.M.; Galvão, D.A.; Taaffe, D.R.; Spry, N.; Nosaka, K.; Cornish, B.; Schmitz, K.H.; et al. Acute Inflammatory Response to Low-, Moderate-, and High-Load Resistance Exercise in Women with Breast Cancer-Related Lymphedema. Integr. Cancer Ther. 2016, 15, 308–317. [Google Scholar] [CrossRef]
- Klassen, O.; Schmidt, M.E.; Ulrich, C.M.; Schneeweiss, A.; Potthoff, K.; Steindorf, K.; Wiskemann, J. Muscle Strength in Breast Cancer Patients Receiving Different Treatment Regimes. J. Cachexia. Sarcopenia Muscle 2017, 8, 305–316. [Google Scholar] [CrossRef]
- Jung, G.H.; Kim, J.H.; Chung, M.S. Changes in Weight, Body Composition, and Physical Activity among Patients with Breast Cancer under Adjuvant Chemotherapy. Eur. J. Oncol. Nurs. 2020, 44. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.M.G.A.; Winkels, R.M.; Kruif, J.T.C.M.; Laarhoven, H.W.M.; Visser, M.; Vries, J.H.M.; Vries, Y.C.; Kampman, E. Weight Change during Chemotherapy in Breast Cancer Patients: A Meta-Analysis. BMC Cancer 2017, 17. [Google Scholar] [CrossRef]
- Tonosaki, A.; Ishikawa, M. Physical Activity Intensity and Health Status Perception of Breast Cancer Patients Undergoing Adjuvant Chemotherapy. Eur. J. Oncol. Nurs. 2014, 18, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.L.; Crumley, D.; McTiernan, A.; Bernstein, L.; Baumgartner, R.; Gilliland, F.D.; Kriska, A.; Ballard-Barbash, R. Physical Activity Levels before and after a Diagnosis of Breast Carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) Study. Cancer 2003, 97, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, J.; McLaughlin, S.L.; Hazard-Jenkins, H.; Infante, A.M.; Montgomery, C.; Davis, M.; Pistilli, E.E. Dysregulation of Metabolic-Associated Pathways in Muscle of Breast Cancer Patients: Preclinical Evaluation of Interleukin-15 Targeting Fatigue. J. Cachexia. Sarcopenia Muscle 2018, 9, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Elsea, C.R.; Kneiss, J.A.; Wood, L.J. Induction of IL-6 by Cytotoxic Chemotherapy Is Associated with Loss of Lean Body and Fat Mass in Tumor-Free Female Mice. Biol. Res. Nurs. 2015, 17, 549–557. [Google Scholar] [CrossRef]
- Marques, V.A.; Ferreira-Junior, J.B.; Lemos, T.V.; Moraes, R.F.; Junior, J.R.S.; Alves, R.R.; Silva, M.S.; de Freitas-Junior, R.; Vieira, C.A. Effects of Chemotherapy Treatment on Muscle Strength, Quality of Life, Fatigue, and Anxiety in Women with Breast Cancer. Int. J. Environ. Res. Public Health 2020, 17, 7289. [Google Scholar] [CrossRef]
- Stene, G.B.; Helbostad, J.L.; Balstad, T.R.; Riphagen, I.I.; Kaasa, S.; Oldervoll, L.M. Effect of Physical Exercise on Muscle Mass and Strength in Cancer Patients during Treatment-A Systematic Review. Crit. Rev. Oncol. Hematol. 2013, 573–593. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Kaufman, S.A. Should Resistance Exercise Be Recommended during Breast Cancer Treatment? Med. Hypotheses 2010, 75, 192–195. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; Van Loon, L.J.C. Satellite Cells in Human Skeletal Muscle; From Birth to Old Age. Age 2014, 36, 545–557. [Google Scholar] [CrossRef]
- Serra, M.C.; Ryan, A.S.; Ortmeyer, H.K.; Addison, O.; Goldberg, A.P. Resistance Training Reduces Inflammation and Fatigue and Improves Physical Function in Older Breast Cancer Survivors. Menopause 2018, 25, 211–216. [Google Scholar] [CrossRef]
- Bolam, K.A.; Mijwel, S.; Rundqvist, H.; Wengström, Y. Two-Year Follow-up of the OptiTrain Randomised Controlled Exercise Trial. Breast Cancer Res. Treat. 2019, 175. [Google Scholar] [CrossRef]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Ramírez-Vélez, R. Effects of Supervised Multimodal Exercise Interventions on Cancer-Related Fatigue: Systematic Review and Meta-Analysis of Randomized Controlled Trials. BioMed Res. Int. 2015, 328636. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, W.D.N.; Gentil, P.; de Moraes, R.F.; Ferreira Júnior, J.B.; Campos, M.H.; de Lira, C.A.B.; Freitas Júnior, R.; Bottaro, M.; Vieira, C.A. Chronic Effects of Resistance Training in Breast Cancer Survivors. BioMed Res. Int. 2017, 8367803. [Google Scholar] [CrossRef] [PubMed]
- De Kruif, J.T.C.M.; Visser, M.; Van Den Berg, M.M.G.A.; Derks, M.J.M.; De Boer, M.R.; Van Laarhoven, H.W.M.; De Vries, J.H.M.; De Vries, Y.C.; Kampman, E.; Winkels, R.W.; et al. Longitudinal Mixed Methods Study on Changes in Body Weight, Body Composition, and Lifestyle in Breast Cancer Patients during Chemotherapy and in a Comparison Group of Women without Cancer: Study Protocol. BMC Cancer 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- McNeely, M.L.; Campbell, K.; Ospina, M.; Rowe, B.H.; Dabbs, K.; Klassen, T.P.; Mackey, J.; Courneya, K. Exercise Interventions for Upper-Limb Dysfunction Due to Breast Cancer Treatment. Cochrane Database Syst. Rev. 2010, 6, CD005211. [Google Scholar] [CrossRef]
- Yang, A.; Sokolof, J.; Gulati, A. The Effect of Preoperative Exercise on Upper Extremity Recovery Following Breast Cancer Surgery: A Systematic Review. Int. J. Rehabil. Res. 2018, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Hamood, R.; Hamood, H.; Merhasin, I.; Keinan-Boker, L. Hormone Therapy and Osteoporosis in Breast Cancer Survivors: Assessment of Risk and Adherence to Screening Recommendations. Osteoporos. Int. 2019, 30, 187–200. [Google Scholar] [CrossRef]
- Hadji, P.; Aapro, M.S.; Body, J.J.; Gnant, M.; Brandi, M.L.; Reginster, J.Y.; Zillikens, M.C.; Glüer, C.C.; de Villiers, T.; Baber, R.; et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in Postmenopausal Women with Hormone Sensitive Breast Cancer: Joint Position Statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J. Bone Oncol. 2017, 1–12. [Google Scholar] [CrossRef]
- Brown, J.C.; Mao, J.J.; Stricker, C.; Hwang, W.T.; Tan, K.S.; Schmitz, K.H. Aromatase Inhibitor Associated Musculoskeletal Symptoms Are Associated with Reduced Physical Activity among Breast Cancer Survivors. Breast J. 2014, 20, 22–28. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Dobek, J.; Nail, L.; Bennett, J.A.; Leo, M.C.; Naik, A.; Schwartz, A. Strength Training Stops Bone Loss and Builds Muscle in Postmenopausal Breast Cancer Survivors: A Randomized, Controlled Trial. Breast Cancer Res. Treat. 2011, 127, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, L.S.; Bloom, J.; Stewart, S.; Sellmeyer, D.E. A Randomized Controlled Trial of Exercise to Prevent Bone Loss in Premenopausal Women with Breast Cancer. J. Women’s Health 2019, 28, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.E.; Jung, M.S.; Sohn, E.H.; Kim, M.; Yoo, H.S.; Bae, K.; Kim, J.R.; Lee, J.S. The Evaluation of Changes in Peripheral Neuropathy and Quality-of-Life Using Low-Frequency Electrostimulation in Patients Treated with Chemotherapy for Breast Cancer: A Study Protocol 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. Trials 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, M.K.; Ruterbusch, J.J.; Beebe-Dimmer, J.L.; Simon, M.S.; Albrecht, T.L.; Schwartz, A.G. Risk of Incident Claims for Chemotherapy-Induced Peripheral Neuropathy among Women with Breast Cancer in a Medicare Population. Cancer 2019, 125, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Carozzi, V.A.; Canta, A.; Chiorazzi, A. Chemotherapy-Induced Peripheral Neuropathy: What Do We Know about Mechanisms? Neurosci. Lett. 2015, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Lefaix, J.L.; Pradat, P.F. Radiation-Induced Neuropathy in Cancer Survivors. Radiother. Oncol. R 2012, 273–282. [Google Scholar] [CrossRef]
- Kleckner, I.R.; Kamen, C.; Gewandter, J.S.; Mohile, N.A.; Heckler, C.E.; Culakova, E.; Fung, C.; Janelsins, M.C.; Asare, M.; Lin, P.J.; et al. Effects of Exercise during Chemotherapy on Chemotherapy-Induced Peripheral Neuropathy: A Multicenter, Randomized Controlled Trial. Support. Care Cancer 2018, 26, 1019–1028. [Google Scholar] [CrossRef]
- Dobson, J.L.; McMillan, J.; Li, L. Benefits of Exercise Intervention in Reducing Neuropathic Pain. Front. Cell. Neurosci. 2014, 8, 102. [Google Scholar] [CrossRef]
- Wonders, K.Y. The Effect of Supervised Exercise Training on Symptoms of Chemotherapy-Induced Peripheral Neuropathy. Clin. Breast Cancer 2014, 19, 411–422. [Google Scholar] [CrossRef]
- Wonders, K.Y.; Whisler, G.; Loy, H.; Holt, B.; Bohachek, K.; Wise, R. Ten Weeks of Home-Based Exercise Attenuates Symptoms of Chemotherapy-Induced Peripheral Neuropathy in Breast Cancer Patients. Health Psychol. Res. 2013, 1, 28. [Google Scholar] [CrossRef]
- Kneis, S.; Wehrle, A.; Freyler, K.; Lehmann, K.; Rudolphi, B.; Hildenbrand, B.; Bartsch, H.H.; Bertz, H.; Gollhofer, A.; Ritzmann, R. Balance Impairments and Neuromuscular Changes in Breast Cancer Patients with Chemotherapy-Induced Peripheral Neuropathy. Clin. Neurophysiol. 2016, 127, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.L.; de Heer, H.D.; Bea, J.W. Initiating Exercise Interventions to Promote Wellness in Cancer Patients and Survivors. Oncology 2017, 31, 711–717. [Google Scholar] [PubMed]
- Vollmers, P.L.; Mundhenke, C.; Maass, N.; Bauerschlag, D.; Kratzenstein, S.; Röcken, C.; Schmidt, T. Evaluation of the Effects of Sensorimotor Exercise on Physical and Psychological Parameters in Breast Cancer Patients Undergoing Neurotoxic Chemotherapy. J. Cancer Res. Clin. Oncol. 2018, 144, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Deprez, S.; Kesler, S.R.; Saykin, A.J.; Silverman, D.H.S.; De Ruiter, M.B.; McDonald, B.C. International Cognition and Cancer Task Force Recommendations for Neuroimaging Methods in the Study of Cognitive Impairment in Non-CNS Cancer Patients. J. Natl. Cancer Inst. 2018, 223–231. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Beckjord, E.; Bovbjerg, D.H.; Low, C.A.; Posluszny, D.M.; Lowery, A.E.; Dew, M.A.; Nutt, S.; Arvey, S.R.; Rechis, R. Prevalence of Perceived Cognitive Dysfunction in Survivors of a Wide Range of Cancers: Results from the 2010 LIVESTRONG Survey. J. Cancer Surviv. 2016, 10, 302–311. [Google Scholar] [CrossRef]
- Yang, Y.; Hendrix, C. Cancer-Related Cognitive Impairment in Breast Cancer Patients: Influences of Psychological Variables. Asia-Pac. J. Oncol. Nurs. 2018, 5, 296–306. [Google Scholar] [CrossRef]
- Hermelink, K. Chemotherapy and Cognitive Function in Breast Cancer Patients: The so-Called Chemo Brain. J. Natl. Cancer Inst. Monogr. 2015, 2015, 67–69. [Google Scholar] [CrossRef]
- Hermelink, K.; Bühner, M.; Sckopke, P.; Neufeld, F.; Kaste, J.; Voigt, V.; Münzel, K.; Wuerstlein, R.; Ditsch, N.; Hellerhoff, K.; et al. Chemotherapy and Post-Traumatic Stress in the Causation of Cognitive Dysfunction in Breast Cancer Patients. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-Related Cognitive Impairment: An Update on State of the Art, Detection, and Management Strategies in Cancer Survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 1194. [Google Scholar] [CrossRef]
- Chang, L.; Weiner, L.S.; Hartman, S.J.; Horvath, S.; Jeste, D.; Mischel, P.S.; Kado, D.M. Breast Cancer Treatment and Its Effects on Aging. J. Geriatr. Oncol. E 2019, 10, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.A.; Erickson, K.I.; Bender, C.M.; Conley, Y.P. The Influence of Physical Activity and Epigenomics On Cognitive Function and Brain Health in Breast Cancer. Front. Aging Neurosci. 2020, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Furmaniak, A.C.; Menig, M.; Markes, M.H. Exercise for Women Receiving Adjuvant Therapy for Breast Cancer. Cochrane Database Syst. Rev. 2016, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Kam, J.W.Y.; Neil-Sztramko, S.E.; Liu Ambrose, T.; Handy, T.C.; Lim, H.J.; Hayden, S.; Hsu, L.; Kirkham, A.A.; Gotay, C.C.; et al. Effect of Aerobic Exercise on Cancer-Associated Cognitive Impairment: A Proof-of-Concept RCT. Psychooncology 2018, 27, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Salerno, E.A.; Rowland, K.; Kramer, A.F.; McAuley, E. Acute Aerobic Exercise Effects on Cognitive Function in Breast Cancer Survivors: A Randomized Crossover Trial. BMC Cancer 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Derry, H.M.; Jaremka, L.M.; Bennett, J.M.; Peng, J.; Andridge, R.; Shapiro, C.; Malarkey, W.B.; Emery, C.F.; Layman, R.; Mrozek, E.; et al. Yoga and Self-Reported Cognitive Problems in Breast Cancer Survivors: A Randomized Controlled Trial. Psychooncology 2015, 24, 958–966. [Google Scholar] [CrossRef]
- Myers, J.S.; Mitchell, M.; Krigel, S.; Steinhoff, A.; Boyce-White, A.; Van Goethem, K.; Valla, M.; Dai, J.; He, J.; Liu, W.; et al. Qigong Intervention for Breast Cancer Survivors with Complaints of Decreased Cognitive Function. Support. Care Cancer 2019, 27, 1395–1403. [Google Scholar] [CrossRef]
- Cifu, G.; Power, M.C.; Shomstein, S.; Arem, H. Mindfulness-Based Interventions and Cognitive Function among Breast Cancer Survivors: A Systematic Review 17 Psychology and Cognitive Sciences 1701 Psychology 11 Medical and Health Sciences 1117 Public Health and Health Services. BMC Cancer 2018, 18. [Google Scholar] [CrossRef]
- Northey, J.M.; Pumpa, K.L.; Quinlan, C.; Ikin, A.; Toohey, K.; Smee, D.J.; Rattray, B. Cognition in Breast Cancer Survivors: A Pilot Study of Interval and Continuous Exercise. J. Sci. Med. Sport 2019, 22, 580–585. [Google Scholar] [CrossRef]
- Oberste, M.; Schaffrath, N.; Schmidt, K.; Bloch, W.; Jäger, E.; Steindorf, K.; Hartig, P.; Joisten, N.; Zimmer, P. Protocol for the “Chemobrain in Motion—Study” (CIM—Study): A Randomized Placebo-Controlled Trial of the Impact of a High-Intensity Interval Endurance Training on Cancer Related Cognitive Impairments in Women with Breast Cancer Receiving First-Line Chemotherapy. BMC Cancer 2018, 18. [Google Scholar] [CrossRef]
- Carayol, M.; Ninot, G.; Senesse, P.; Bleuse, J.P.; Gourgou, S.; Sancho-Garnier, H.; Sari, C.; Romieu, I.; Romieu, G.; Jacot, W. Short- and Long-Term Impact of Adapted Physical Activity and Diet Counseling during Adjuvant Breast Cancer Therapy: The “APAD1” Randomized Controlled Trial. BMC Cancer 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Frequently Asked Questions. Available online: https://www.who.int/about/who-we-are/frequently-asked-questions (accessed on 2 November 2020).
- Post, M.W.M. Definitions of Quality of Life: What Has Happened and How to Move On. Top. Spinal Cord Inj. Rehabil. 2014, 20, 167–180. [Google Scholar] [CrossRef]
- Spitzer, W.O.; Dobson, A.J.; Hall, J.; Chesterman, E.; Levi, J.; Shepherd, R.; Battista, R.N.; Catchlove, B.R. Measuring the Quality of Life of Cancer Patients. A Concise QL-Index for Use by Physicians. J. Chronic Dis. 1981, 34, 585–597. [Google Scholar] [CrossRef]
- Nguyen, J.; Popovic, M.; Chow, E.; Cella, D.; Beaumont, J.L.; Chu, D.; Digiovanni, J.; Lam, H.; Pulenzas, N.; Bottomley, A. EORTC QLQ-BR23 and FACT-B for the Assessment of Quality of Life in Patients with Breast Cancer: A Literature Review. J. Comp. Effect. Res. 2015, 4, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jung, Y.S.; Kim, J.Y.; Jo, Y.; Bae, S.H. Trajectories of Health-Related Quality of Life in Breast Cancer Patients. Support. Care Cancer 2020, 28, 3381–3389. [Google Scholar] [CrossRef] [PubMed]
- Shafaee, F.S.; Mirghafourvand, M.; Harischi, S.; Esfahani, A.; Amirzehni, J. Self-Confidence and Quality of Life in Women Undergoing Treatment for Breast Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 733–740. [Google Scholar] [CrossRef]
- Gavric, Z.; Vukovic-Kostic, Z. Assessment of Quality of Life of Women with Breast Cancer. Glob. J. Health Sci. 2015, 8, 1. [Google Scholar] [CrossRef]
- Williams, R.; Müller, M.; Harewood, R.; Stanway, S.; Bhaskaran, K.; Carreira, H. Associations between Breast Cancer Survivorship and Adverse Mental Health Outcomes: A Systematic Review. J. Natl. Cancer Inst. 2018, 110, 1311–1327. [Google Scholar] [CrossRef]
- Ganz, P.A.; Goodwin, P.J. Breast Cancer Survivorship: Where Are We Today? Adv. Exp. Med. Biol. 2015, 862, 1–8. [Google Scholar] [CrossRef]
- Backman, M.; Browall, M.; Sundberg, C.J.; Wengström, Y. Experiencing Health—Physical Activity during Adjuvant Chemotherapy Treatment for Women with Breast Cancer. Eur. J. Oncol. Nurs. 2016, 21, 160–167. [Google Scholar] [CrossRef]
- Penttinen, H.; Utriainen, M.; Kellokumpu-Lehtinen, P.L.; Raitanen, J.; Sievänen, H.; Nikander, R.; Blomqvist, C.; Huovinen, R.; Vehmanen, L.; Saarto, T. Effectiveness of a 12-Month Exercise Intervention on Physical Activity and Quality of Life of Breast Cancer Survivors; Five-Year Results of the BREX-Study. In Vivo (Brooklyn) 2019, 33, 881–888. [Google Scholar] [CrossRef]
- Courneya, K.S.; Vallance, J.K.; Culos-Reed, S.N.; McNeely, M.L.; Bell, G.J.; Mackey, J.R.; Yasui, Y.; Yuan, Y.; Matthews, C.E.; Lau, D.C.W.; et al. The Alberta Moving beyond Breast Cancer (AMBER) Cohort Study: A Prospective Study of Physical Activity and Health-Related Fitness in Breast Cancer Survivors. BMC Cancer 2012, 12. [Google Scholar] [CrossRef]
- Courneya, K.S.; McNeely, M.L.; Culos-Reed, S.N.; Vallance, J.K.; Bell, G.J.; Mackey, J.R.; Matthews, C.E.; Morielli, A.R.; Cook, D.; MacLaughlin, S.; et al. The Alberta Moving beyond Breast Cancer (AMBER) Cohort Study: Recruitment, Baseline Assessment, and Description of the First 500 Participants. BMC Cancer 2016, 16. [Google Scholar] [CrossRef]
- Juvet, L.K.; Thune, I.; Elvsaas, I.K.Ø.; Fors, E.A.; Lundgren, S.; Bertheussen, G.; Leivseth, G.; Oldervoll, L.M. The Effect of Exercise on Fatigue and Physical Functioning in Breast Cancer Patients during and after Treatment and at 6 Months Follow-up: A Meta-Analysis. Breast 2017, 166–177. [Google Scholar] [CrossRef]
- Fleming, L.; Randell, K.; Stewart, E.; Espie, C.A.; Morrison, D.S.; Lawless, C.; Paul, J. Insomnia in Breast Cancer: A Prospective Observational Study. Sleep 2019, 42. [Google Scholar] [CrossRef]
- Chennaoui, M.; Arnal, P.J.; Sauvet, F.; Léger, D. Sleep and Exercise: A Reciprocal Issue? Sleep Med. Rev. 2015, 59–72. [Google Scholar] [CrossRef]
- Baron, K.G.; Reid, K.J.; Zee, P.C. Exercise to Improve Sleep in Insomnia: Exploration of the Bidirectional Effects. J. Clin. Sleep Med. 2013, 9, 819–824. [Google Scholar] [CrossRef]
- Bernard, P.; Ivers, H.; Savard, M.H.; Savard, J. Temporal Relationships between Sleep and Physical Activity among Breast Cancer Patients with Insomnia. Heal. Psychol. 2016, 35. [Google Scholar] [CrossRef]
- Kwak, A.; Jacobs, J.; Haggett, D.; Jimenez, R.; Peppercorn, J. Evaluation and Management of Insomnia in Women with Breast Cancer. Breast Cancer Res. Treat. 2020, 269–277. [Google Scholar] [CrossRef]
- Chen, W.Y.; Giobbie-Hurder, A.; Gantman, K.; Savoie, J.; Scheib, R.; Parker, L.M.; Schernhammer, E.S. A Randomized, Placebo-Controlled Trial of Melatonin on Breast Cancer Survivors: Impact on Sleep, Mood, and Hot Flashes. Breast Cancer Res. Treat. 2014, 145, 381–388. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Courneya, K.S.; Oster, R.A.; Anton, P.M.; Robbs, R.S.; Forero, A.; McAuley, E. Physical Activity and Sleep Quality in Breast Cancer Survivors: A Randomized Trial. Med. Sci. Sports Exerc. 2017, 49, 2009–2015. [Google Scholar] [CrossRef]
- Salibasic, M.; Delibegovic, S. The Quality of Life and Degree of Depression of Patients Suffering from Breast Cancer. Med. Arch. 2018, 72, 202–205. [Google Scholar] [CrossRef]
- Yi, J.C.; Syrjala, K.L. Anxiety and Depression in Cancer Survivors. Med. Clin. N. Am. 2017, 1099–1113. [Google Scholar] [CrossRef]
- Cvetković, J.; Nenadović, M. Depression in Breast Cancer Patients. Psychiatry Res. 2016, 240, 343–347. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, X.; Wang, Y.; Xiong, H.; Zhao, Y.; Sun, F. Effects of Exercise Intervention in Breast Cancer Survivors: A Meta-Analysis of 33 Randomized Controlled Trails. Onco Targets. Ther. 2016, 9, 2153–2168. [Google Scholar] [CrossRef]
- Padin, A.C.; Wilson, S.J.; Bailey, B.E.; Malarkey, W.B.; Lustberg, M.B.; Farrar, W.B.; Povoski, S.P.; Agnese, D.M.; Reinbolt, R.E.; Wesolowski, R.; et al. Physical Activity After Breast Cancer Surgery: Does Depression Make Exercise Feel More Effortful than It Actually Is? Int. J. Behav. Med. 2019, 26, 237–246. [Google Scholar] [CrossRef]
- Ribeiro, F.E.; Palma, M.R.; Silva, D.T.C.; Tebar, W.R.; Vanderlei, L.C.M.; Fregonesi, C.E.P.T.; Christofaro, D.G.D. Relationship of Anxiety and Depression Symptoms with the Different Domains of Physical Activity in Breast Cancer Survivors. J. Affect. Disord. 2020, 273, 210–214. [Google Scholar] [CrossRef]
| Complications | Etiological Care Agent | Favorable Actions Mediated by PA | Interventions | Main Objectives | References |
|---|---|---|---|---|---|
| Cardiovascular diseases (CVD) Approximately 2–10% of BC mortality | Shared risk factors including age, sex, smoking, diet… | Substantial biological benefits from PA (Hormone-levels, antiaging, organokines, etc.) | TITAN study | Multidisciplinary approaches to reduce risk of CVD including from cardiology, clinical nutrition, psychology, PA and physical therapy | NCT01621659 |
| Chemotherapeutic agents mainly trastuzumab, anthracyclines or ET | Cardioprotective effects (Counteracting the oxidative stress derived from chemotherapy in the cardiovascular system) | Effects of physical exercise on cardiac health in women with breast cancer | To assess the impact of 3 days per week with progressive volume and intensity during the period of anthracycline treatment | ISRCTN32617901 | |
| Radiotherapy | Promotion of cardiovascular health | EMBRACE study | To evaluate the potential role of PA in the prevention of chemotherapy-derived cardiotoxicity. | NCT04467411 | |
| Breast Cancer-Related Lymphedema (BCRL) 1 in 5 women will develop this condition | Axillary surgery, lack of breast reconstruction or some adjuvants like taxane-based chemotherapy | PA reported both objective and subjective improvements in patients with BCRL | WISER study (Women In Steady Exercise Research) | To observe the impact of one-year weight loss and exercise in sedentary overweight or obese breast cancer survivors with breast cancer-related lymphedema. Finally, they concluded that home-based training was ineffective for these women | NCT01515124 |
| Non-treatment-related factors (BMI, cellulitis, subclinical oedema | Even RT is safe and completely recommended for patients with BCRL | PAL trial (Physical activity and lymphedema) | Demonstrated the safety and efficacy of RT in BC patients with lymphedema | NCT00194363 | |
| Body composition Including weight gain, increased adiposity, reduced muscle mass, strength, and flexibility, along with bone mass | Chemotherapy (depending on cycles received or certain types of agents such as cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) | RT and AT combined or RT-HIIT improves muscle strength and mass-reducing adiposity, inflammation, and fatigue | COBRA study | To investigate changes in body composition (adipose and muscle tissue) of women with breast cancer treated with or undergoing chemotherapy. | OND1350462 |
| Surgery | PA to promote range of movement of shoulder | Effects of exercise training at different timelines on shoulder dysfunction after BC-modified Radical mastectomy | To describe the most effective routines to deal with shoulder dysfunction after surgery, including isometric shoulder training with RT | NCT03658265 | |
| ET is strongly associated with bone mass loss | RT may collaborate to maintain bone density in women receiving ET | Breast cancer and Exercise (BREX) | Proved the efficacy of regular exercise in the prevention of bone loss and enhancement QOL | NCT00639210 | |
| Peripheral neuropathy Affecting 30–40% of patients during chemotherapy and, in some cases, after this | Chemotherapy Paclitaxel, docetaxel, cisplatin, carboplatin, bortezomib, or vincristine | Attenuation of peripheric neuropathic symptoms by negatively modulating inflammation, pain, sensory, and motor dysfunction | Pain, psychological, and endocannabinoid responses to yoga in breast cancer survivors with chemotherapy-induced neuropathic pain | Combined yoga plus AT as a strategy in BC survivors to deal with peripheral neuropathy | NCT04075097 |
| Cognitive dysfunction Up to 50% of patients with BC may impair its attention, processing speed, memory, and executive functions | Chemotherapy “chemobrain” plus other factors (age, genetics, etc.) | PA directly influences cognitive processes and memory, presenting analgesic and antidepressant effects, acting as potential inductors of well-being | Physical activity and neuropsychological outcomes in a cancer population | To demonstrate if PA also results beneficial for BC patients suffering cognitive dysfunction | NCT02332876 |
| Chemobrain in Motion | To evaluate the adequacy of HIIT in the cognitive functioning of BC patients | DRKS00011390 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Pekarek, L.; Guijarro, L.G.; Castellanos, A.J.; Sanchez-Trujillo, L.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; et al. Physical Activity as an Imperative Support in Breast Cancer Management. Cancers 2021, 13, 55. https://doi.org/10.3390/cancers13010055
Ortega MA, Fraile-Martínez O, García-Montero C, Pekarek L, Guijarro LG, Castellanos AJ, Sanchez-Trujillo L, García-Honduvilla N, Álvarez-Mon M, Buján J, et al. Physical Activity as an Imperative Support in Breast Cancer Management. Cancers. 2021; 13(1):55. https://doi.org/10.3390/cancers13010055
Chicago/Turabian StyleOrtega, Miguel A., Oscar Fraile-Martínez, Cielo García-Montero, Leonel Pekarek, Luis G. Guijarro, Alejandro J. Castellanos, Lara Sanchez-Trujillo, Natalio García-Honduvilla, Melchor Álvarez-Mon, Julia Buján, and et al. 2021. "Physical Activity as an Imperative Support in Breast Cancer Management" Cancers 13, no. 1: 55. https://doi.org/10.3390/cancers13010055
APA StyleOrtega, M. A., Fraile-Martínez, O., García-Montero, C., Pekarek, L., Guijarro, L. G., Castellanos, A. J., Sanchez-Trujillo, L., García-Honduvilla, N., Álvarez-Mon, M., Buján, J., Zapico, Á., Lahera, G., & Álvarez-Mon, M. A. (2021). Physical Activity as an Imperative Support in Breast Cancer Management. Cancers, 13(1), 55. https://doi.org/10.3390/cancers13010055