The Predictive Role of Prostate-Specific Antigen Changes Following Transurethral Resection of the Prostate for Patients with Localized Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
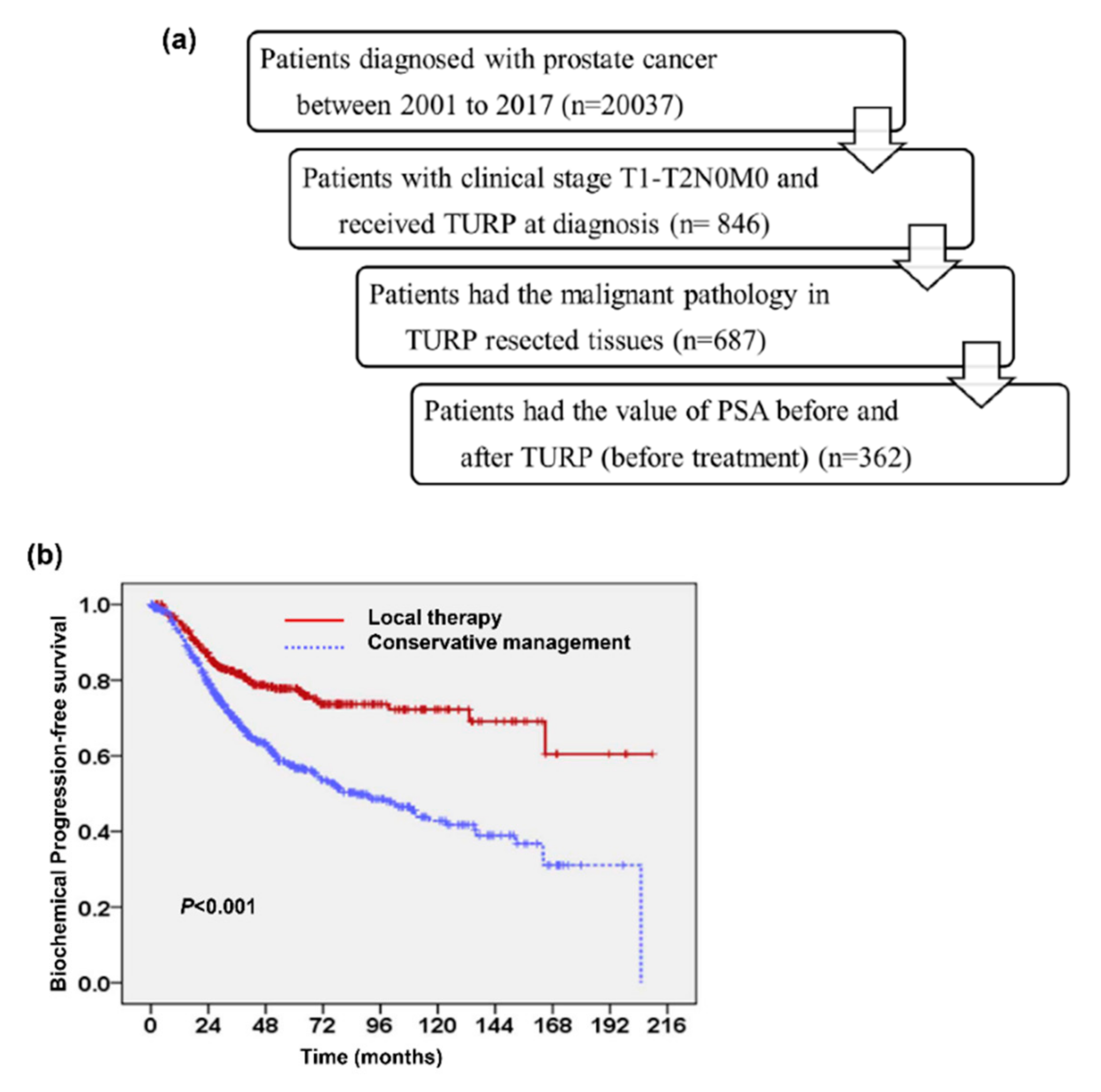
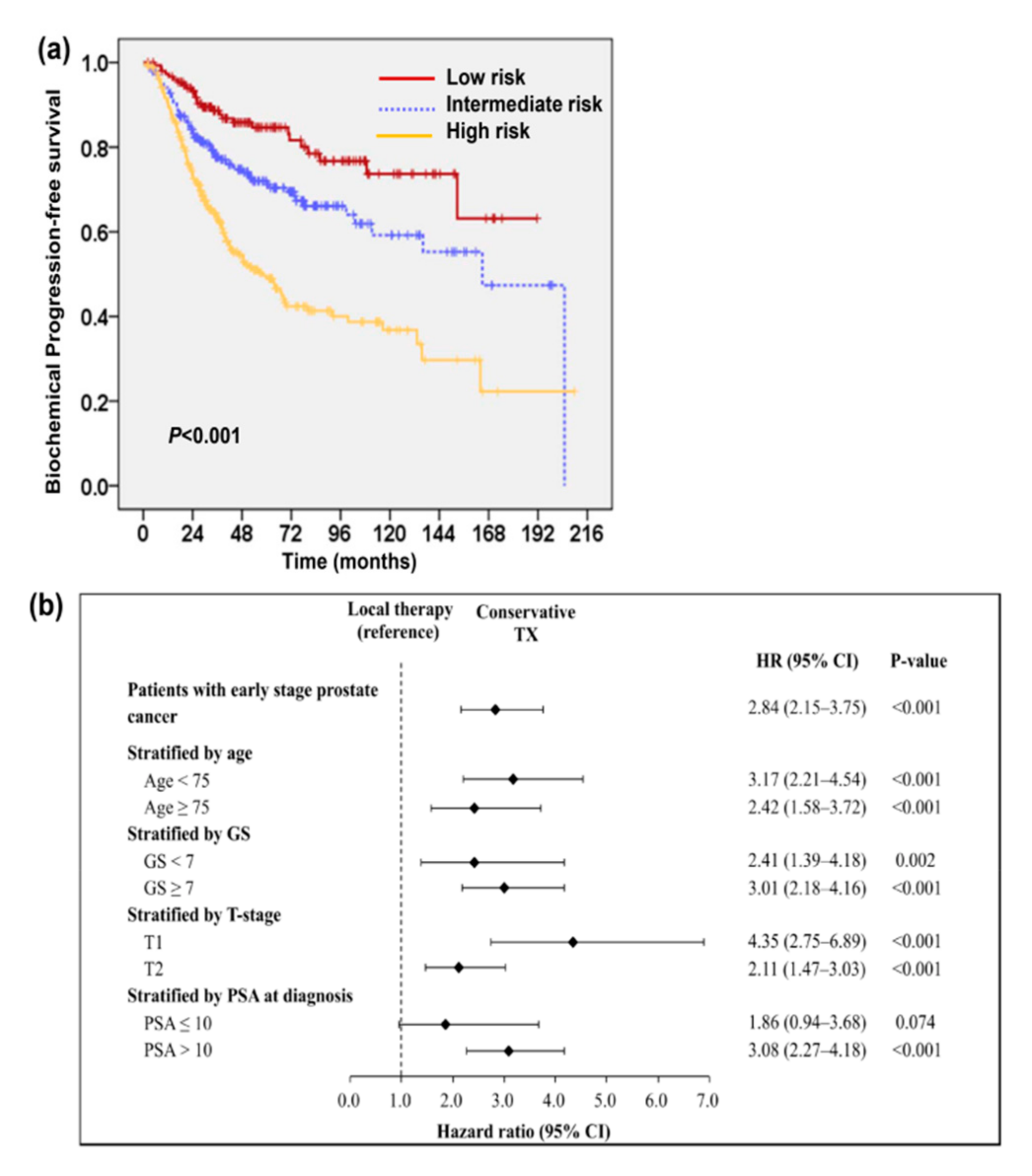
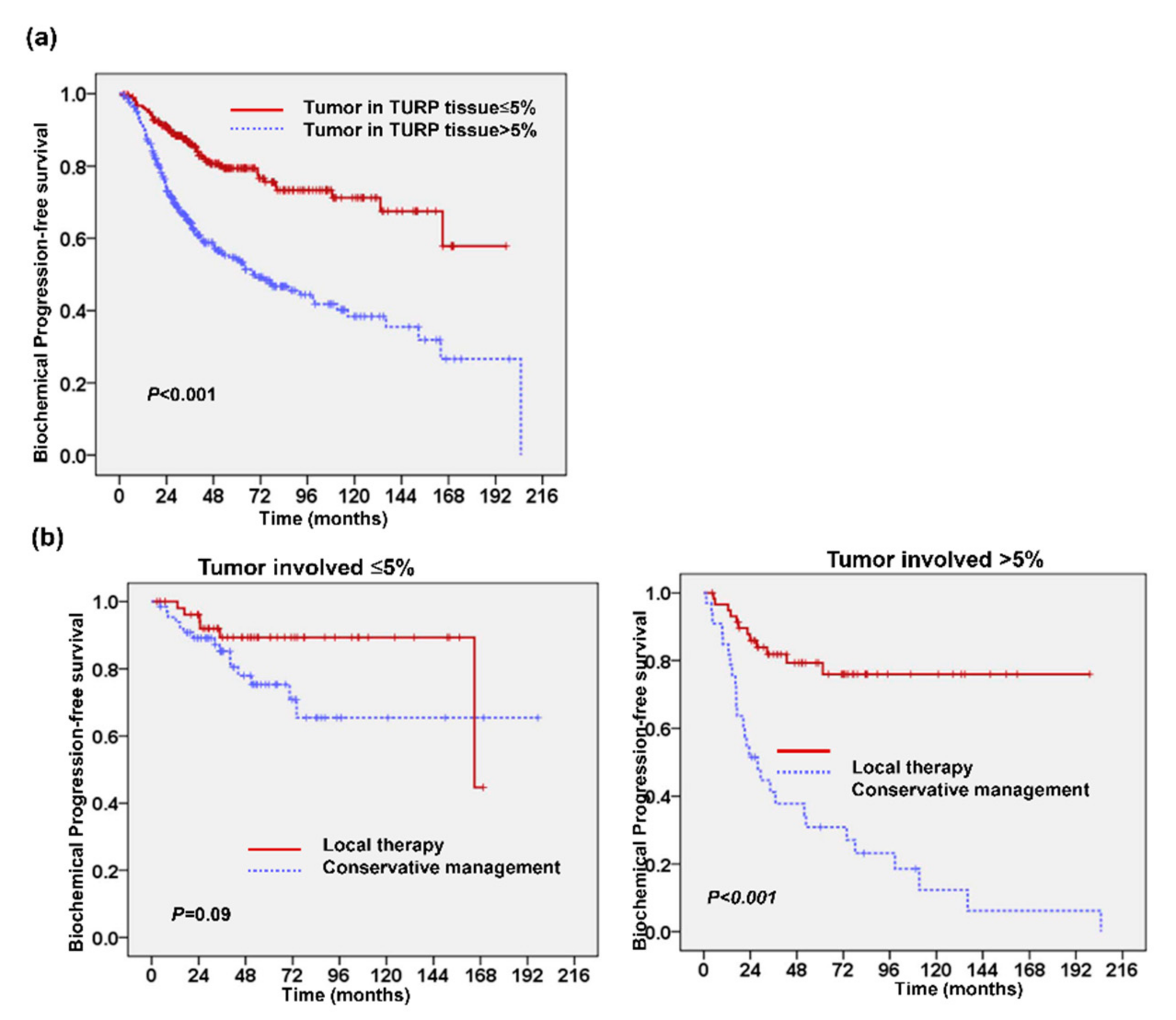
2.1. Predictive Value of Tumor Involvement in Transurethral Resection of the Prostate (TURP)
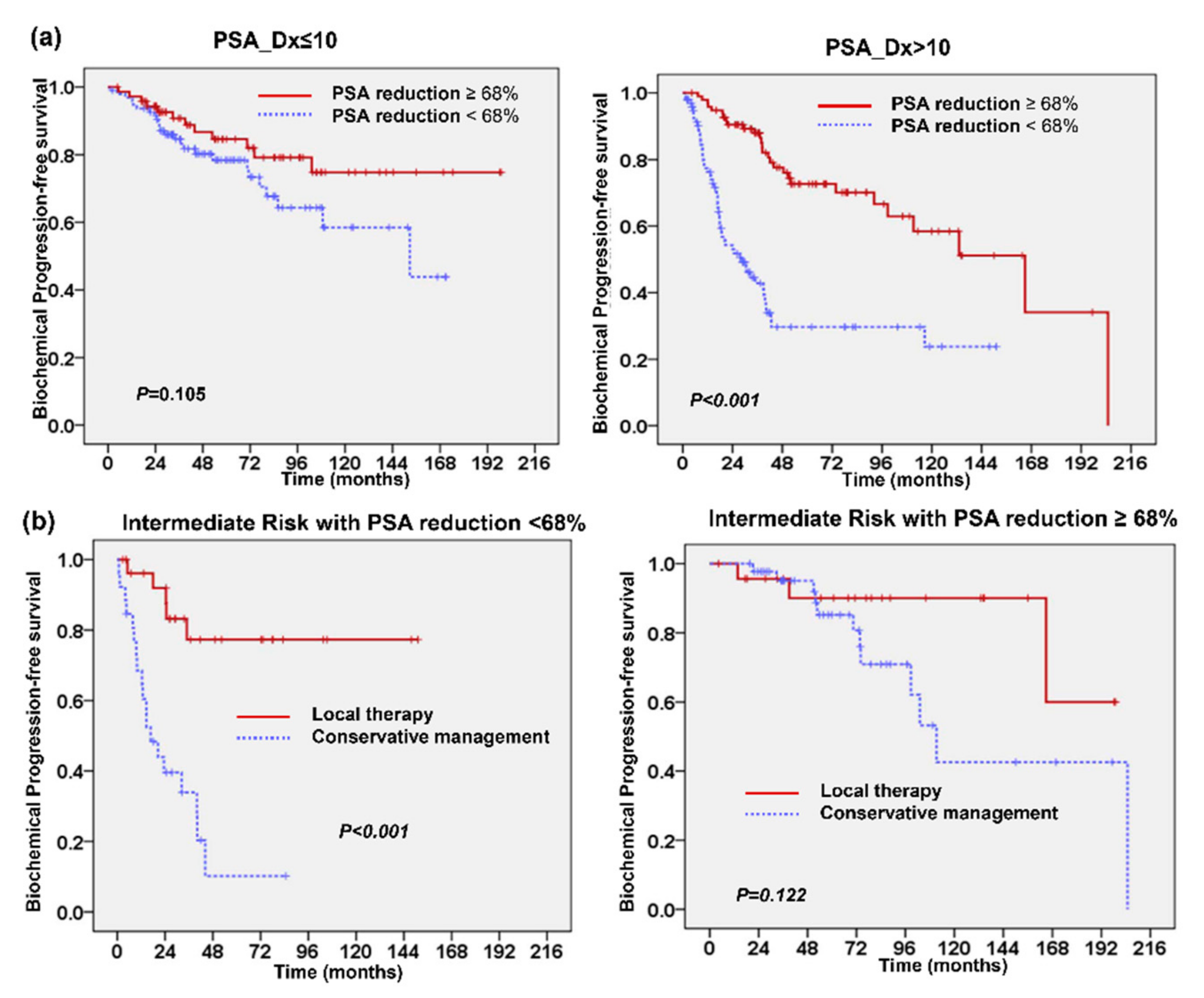
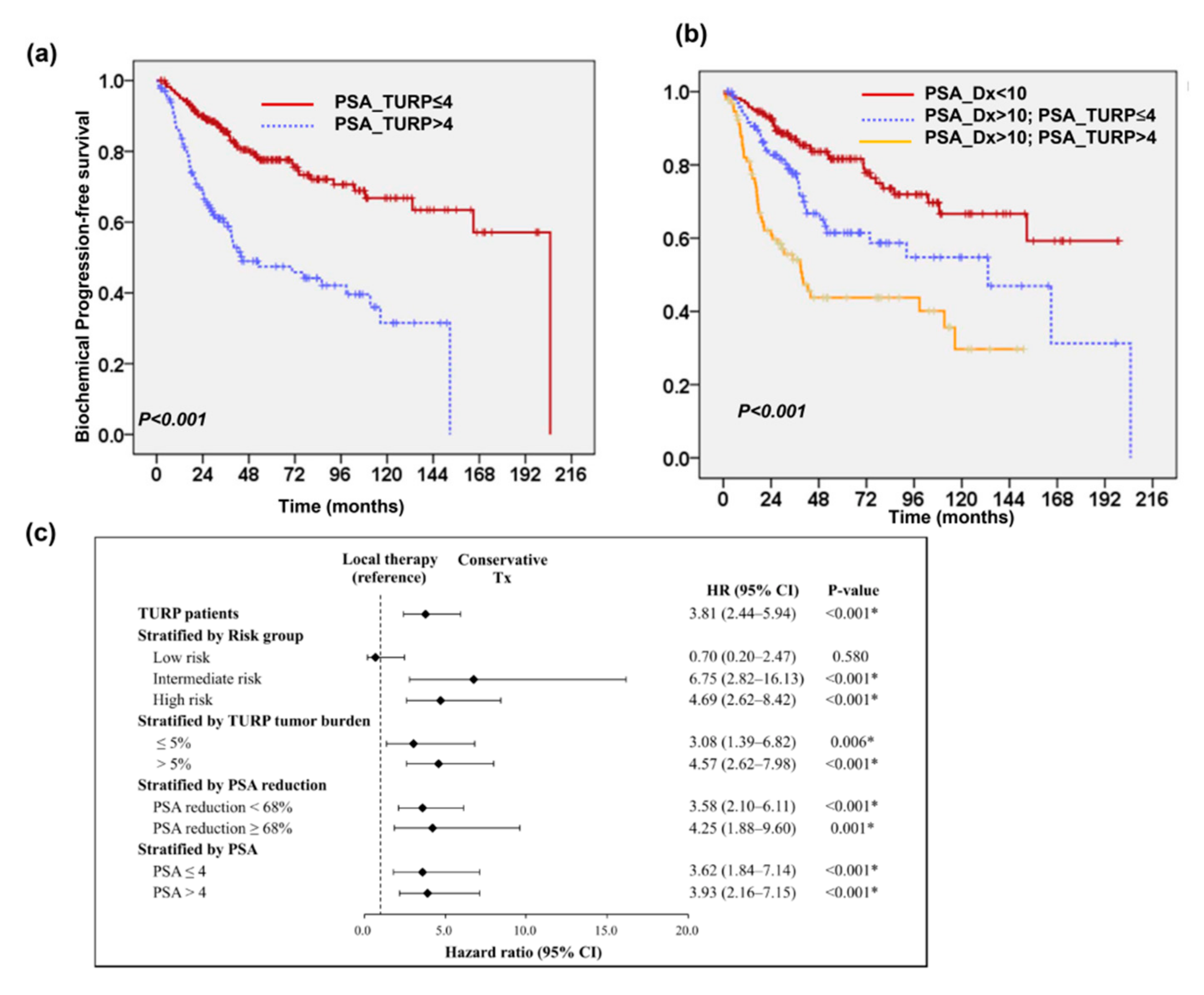
2.2. Changes in Prostate-Specific Antigen (PSA) Following TURP
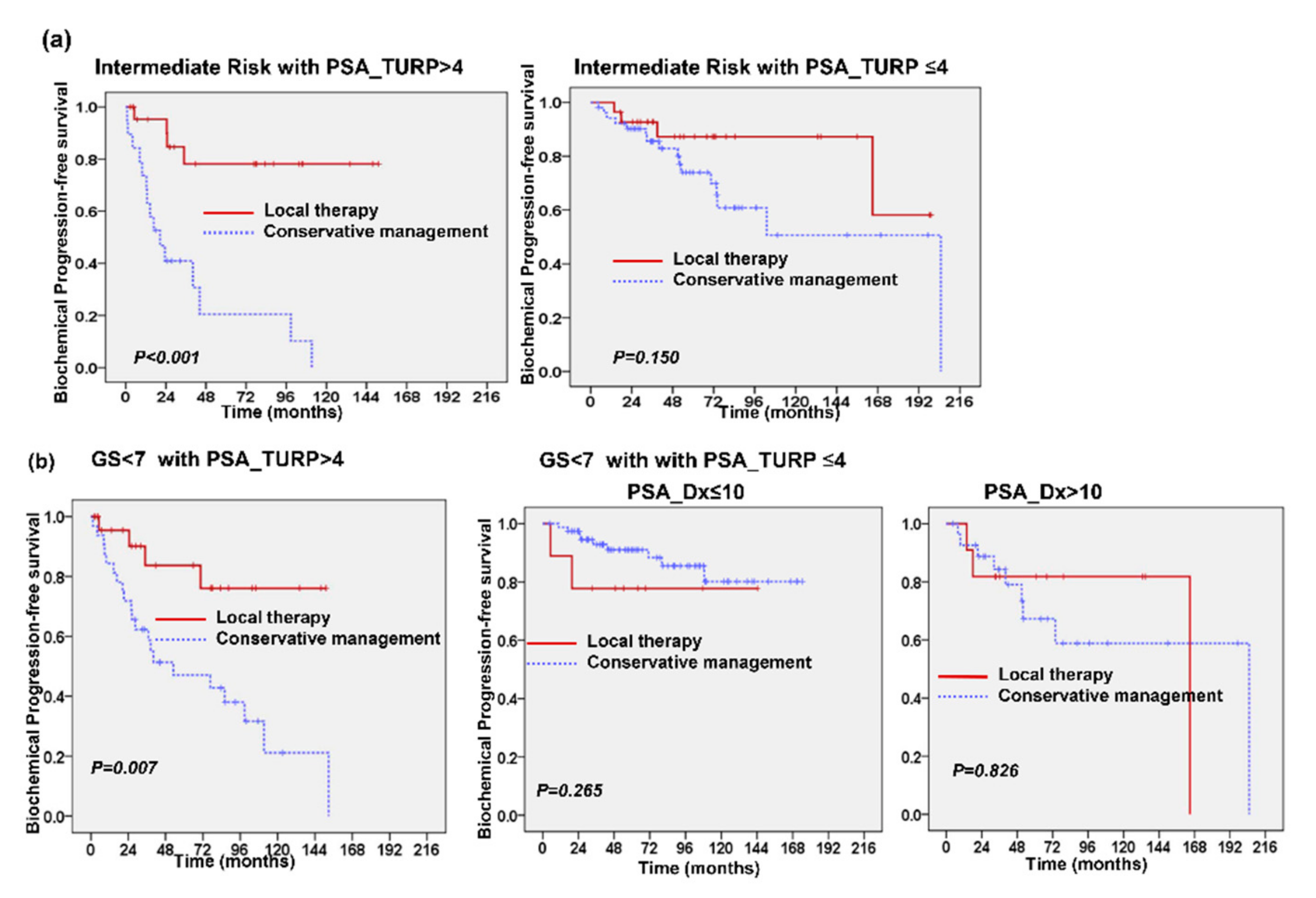
2.3. Predictive Role of PSA Level after TURP
3. Discussion
4. Materials and Methods
4.1. Study Population and Study Design
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, M.C.; Goggins, W.B.; Wang, H.H.; Fung, F.D.; Leung, C.; Wong, S.Y.; Ng, C.F.; Sung, J.J.Y. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur. Urol. 2016, 70, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, N.; Helleman, J.; Bruinsma, S.; Carlsson, S.; Cahill, D.; Brown, C.; Van Hemelrijck, M. Active surveillance for prostate cancer: A systematic review of contemporary worldwide practices. Transl. Androl. Urol. 2018, 7, 83–97. [Google Scholar] [CrossRef]
- Klotz, L.; Vesprini, D.; Sethukavalan, P.; Jethava, V.; Zhang, L.; Jain, S.; Yamamoto, T.; Mamedov, A.; Loblaw, A. Long-Term Follow-Up of a Large Active Surveillance Cohort of Patients with Prostate Cancer. J. Clin. Oncol. 2015, 33, 272–277. [Google Scholar] [CrossRef]
- Diaz, M.; Peabody, J.O.; Kapoor, V.; Sammon, J.; Rogers, C.G.; Stricker, H.; Lane, Z.; Gupta, N.S.; Bhandari, M.; Menon, M. Oncologic Outcomes at 10 Years Following Robotic Radical Prostatectomy. Eur. Urol. 2015, 67, 1168–1176. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef]
- D’Amico, A.V. Treatment or Monitoring for Early Prostate Cancer. N. Engl. J. Med. 2016, 375, 1482–1483. [Google Scholar] [CrossRef]
- Klotz, L. Active surveillance for low-risk prostate cancer. Curr. Opin. Urol. 2017, 27, 225–230. [Google Scholar] [CrossRef]
- Mahal, A.R.; Butler, S.; Franco, I.; Muralidhar, V.; Larios, D.; Pike, L.R.G.; Zhao, S.G.; Sanford, N.N.; Dess, R.T.; Feng, F.Y.; et al. Conservative management of low-risk prostate cancer among young versus older men in the United States: Trends and outcomes from a novel national database. Cancer 2019, 125, 3338–3346. [Google Scholar] [CrossRef]
- Hugosson, J.; Carlsson, S. Overdetection in screening for prostate cancer. Curr. Opin. Urol. 2014, 24, 256–263. [Google Scholar] [CrossRef]
- Loeb, S. Guideline of Guidelines: Prostate Cancer Screening. BJU Int. 2014, 114, 323–325. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Carroll, P.R.; Klotz, L. Active Surveillance for Prostate Cancer: Progress and Promise. J. Clin. Oncol. 2011, 29, 3669–3676. [Google Scholar] [CrossRef]
- Ornstein, D.K.; Rayford, W.; Fusaro, V.A.; Conrads, T.P.; Ross, S.J.; Hitt, B.A.; Wiggins, W.W.; Veenstra, T.D.; Liotta, L.A.; Petricoin, E.F. Serum Proteomic Profiling Can Discriminate Prostate Cancer from Benign Prostates in Men with Total Prostate Specific Antigen Levels between 2.5 and 15.0 ng/mL. J. Urol. 2004, 172, 1302–1305. [Google Scholar] [CrossRef]
- Carter, H.B. Differentiation of lethal and non lethal prostate cancer: PSA and PSA isoforms and kinetics. Asian J. Androl. 2012, 14, 355–360. [Google Scholar] [CrossRef]
- Furuya, Y.; Akakura, K.; Tobe, T.; Ichikawa, T.; Igarashi, T.; Ito, H. Changes in serum prostate-specific antigen following prostatectomy in patients with benign prostate hyperplasia. Int. J. Urol. 2000, 7, 447–451. [Google Scholar] [CrossRef][Green Version]
- Koo, K.C.; Park, S.U.; Rha, K.H.; Hong, S.J.; Yang, S.C.; Hong, C.H.; Chung, B.H. Transurethral resection of the prostate for patients with Gleason score 6 prostate cancer and symptomatic prostatic enlargement: A risk-adaptive strategy for the era of active surveillance. Jpn. J. Clin. Oncol. 2015, 45, 785–790. [Google Scholar] [CrossRef]
- Reich, O.; Gratzke, C.; Stief, C.G. Techniques and Long-Term Results of Surgical Procedures for BPH. Eur. Urol. 2006, 49, 970–978; discussion 978. [Google Scholar] [CrossRef]
- Bill-Axelson, A.; Holmberg, L.; Garmo, H.; Rider, J.R.; Taari, K.; Busch, C.; Nordling, S.; Häggman, M.; Andersson, S.-O.; Spångberg, A.; et al. Radical Prostatectomy or Watchful Waiting in Early Prostate Cancer. N. Engl. J. Med. 2014, 370, 932–942. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Carroll, P.R. Trends in Management for Patients with Localized Prostate Cancer, 1990–2013. JAMA 2015, 314, 80–82. [Google Scholar] [CrossRef]
- Sakr, W.A.; Grignon, D.J.; Crissman, J.D.; Heilbrun, L.K.; Cassin, B.J.; Pontes, J.J.; Haas, G.P. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20–69: An autopsy study of 249 cases. Vivo 1994, 8, 439–443. [Google Scholar]
- Van den Bergh, R.C.; Roemeling, S.; Roobol, M.J.; Wolters, T.; Schroder, F.H.; Bangma, C.H. Prostate-specific antigen kinetics in clinical decision-making during active surveillance for early prostate cancer—A review. Eur. Urol. 2008, 54, 505–516. [Google Scholar] [CrossRef]
- Stamey, T.A.; Yang, N.; Hay, A.R.; McNeal, J.E.; Freiha, F.S.; Redwine, E. Prostate-Specific Antigen as a Serum Marker for Adenocarcinoma of the Prostate. N. Engl. J. Med. 1987, 317, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Shin, S.C.; Cho, J.M.; Kang, J.Y.; Yoo, T.K. The role of transurethral resection of the prostate for patients with an elevated prostate-specific antigen. Prostate Int. 2014, 2, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Van Renterghem, K.; Van Koeveringe, G.; Achten, R.; Van Kerrebroeck, P. Long-Term Clinical Outcome of Diagnostic Transurethral Resection of the Prostate in Patients with Elevated Prostate-Specific Antigen Level and Minor Lower Urinary Tract Symptoms. Urol. Int. 2009, 83, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Briganti, A.; Suardi, N.; Gallina, A.; Salonia, A.; Freschi, M.; Rigatti, P.; Montorsi, F. When should we expect no residual tumor (pT0) once we submit incidental T1a-b prostate cancers to radical prostatectomy? Int. J. Urol. 2011, 18, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Descazeaud, A.; Peyromaure, M.; Salin, A.; Amsellem-Ouazana, D.; Flam, T.; Viellefond, A.; Debre, B.; Zerbib, M. Predictive Factors for Progression in Patients with Clinical Stage T1a Prostate Cancer in the PSA Era. Eur. Urol. 2008, 53, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.J.; Ma, C.G.; Ye, D.W.; Yao, X.D.; Zhang, S.L.; Dai, B.; Zhang, H.L.; Shen, Y.J.; Zhu, Y.; Zhu, Y.P.; et al. Tumor cytoreduction results in better response to androgen ablation—A preliminary report of palliative transurethral resection of the prostate in metastatic hormone sensitive prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 145–149. [Google Scholar] [CrossRef]
- Bayne, C.E.; Williams, S.B.; Cooperberg, M.R.; Gleave, M.E.; Graefen, M.; Montorsi, F.; Novara, G.; Smaldone, M.C.; Sooriakumaran, P.; Wiklund, P.N.; et al. Treatment of the Primary Tumor in Metastatic Prostate Cancer: Current Concepts and Future Perspectives. Eur. Urol. 2016, 69, 775–787. [Google Scholar] [CrossRef]
- Jones, J.S.; Follis, H.W.; Johnson, J.R. Probability of finding T1a and T1b (Incidental) prostate cancer during TURP has decreased in the PSA era. Prostate Cancer Prostatic Dis. 2008, 12, 57–60. [Google Scholar] [CrossRef][Green Version]
- Heidenreich, A.; Bellmunt, J.; Bolla, M.; Joniau, S.; Mason, M.; Matveev, V.; Mottet, N.; Schmid, H.P.; Van Der Kwast, T.; Wiegel, T.; et al. EAU Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Treatment of Clinically Localised Disease. Eur. Urol. 2011, 59, 61–71. [Google Scholar] [CrossRef]
- Fonseca, R.C.; Gomes, C.M.; Meireles, E.B.; Freire, G.C.; Srougi, M. Prostate specific antigen levels following transurethral resection of the prostate. Int. Braz. J. Urol. 2008, 34, 41–48. [Google Scholar] [CrossRef]
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Age | |||
| <75 | Ref | ||
| ≥75 | 1.01 | 0.75–1.35 | 0.98 |
| Gleason score (GS) | |||
| <7 | Ref | ||
| ≥7 | 1.78 | 1.27–2.50 | 0.001 * |
| Clinical stage | |||
| T1 | Ref | ||
| T2 | 0.98 | 0.73–1.32 | 0.91 |
| PSA at diagnosis | |||
| ≤10 | Ref | ||
| >10 | 2.18 | 1.66–3.41 | <0.001 * |
| Cancer in TURP | |||
| ≤5% | Ref | ||
| >5% | 2.24 | 1.62–3.11 | <0.001 * |
| Treatment | |||
| Local therapy | Ref | ||
| Conservative Tx | 3.34 | 2.41–4.63 | <0.001 * |
| No. of Patients | |||
|---|---|---|---|
| PSA_TURP ≤ 4 | PSA_TURP > 4 | p Value | |
| Patients | 228 | 134 | |
| Age | 0.064 | ||
| <75 y/o | 120 | 57 | |
| ≥75 y/o | 108 | 77 | |
| PVR (≥100 mL) | 0.007 | ||
| No | 158 | 110 | |
| Yes | 70 | 24 | |
| GS | 0.039 | ||
| <7 | 126 | 59 | |
| ≥7 | 102 | 75 | |
| Cancer in TURP | 0.077 | ||
| ≤5% | 124 | 57 | |
| >5% | 93 | 64 | |
| Unknown | 11 | 13 | |
| PSA at Diagnosis | <0.001 | ||
| ≤10 | 128 | 40 | |
| >10 | 100 | 94 | |
| Treatment | <0.001 | ||
| Local therapy | 79 | 76 | |
| Conservative Tx | 149 | 58 | |
| Biochemical failure | <0.001 | ||
| No | 176 | 70 | |
| Yes | 52 | 64 | |
| Disease failure a | 0.013 | ||
| No | 201 | 105 | |
| Yes | 27 | 29 | |
| Survival status | 0.005 | ||
| Dead | 45 | 44 | |
| Alive | 183 | 90 | |
| Prostate cancer-specific survival | 0.004 | ||
| Dead | 8 | 15 | |
| Alive | 220 | 119 | |
| Characteristics | Number |
|---|---|
| Age | |
| Range | 45~88 (years) |
| Median | 73.12 (years) |
| <75 | 463 (55%) |
| ≥75 | 383 (45%) |
| Gleason score | |
| <7 | 355 (42%) |
| ≥7 | 477 (56%) |
| Unknown | 14 (1.7%) |
| Clinical T stage | |
| T1 | 441 (52%) |
| T2 | 405 (48%) |
| Cancer in TURP specimen | |
| Cancer (−) | 159 (19%) |
| Cancer (+) | 687 (81%) |
| PSA at diagnosis | |
| ≤10 | 338 (40%) |
| >10 | 508 (60%) |
| Treatment | |
| Local therapy | 359 (42%) |
| Conservative Tx | 487 (58%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-T.; Huang, Y.-C.; Chen, W.-C.; Chen, M.-F. The Predictive Role of Prostate-Specific Antigen Changes Following Transurethral Resection of the Prostate for Patients with Localized Prostate Cancer. Cancers 2021, 13, 74. https://doi.org/10.3390/cancers13010074
Wu C-T, Huang Y-C, Chen W-C, Chen M-F. The Predictive Role of Prostate-Specific Antigen Changes Following Transurethral Resection of the Prostate for Patients with Localized Prostate Cancer. Cancers. 2021; 13(1):74. https://doi.org/10.3390/cancers13010074
Chicago/Turabian StyleWu, Chun-Te, Yun-Ching Huang, Wen-Cheng Chen, and Miao-Fen Chen. 2021. "The Predictive Role of Prostate-Specific Antigen Changes Following Transurethral Resection of the Prostate for Patients with Localized Prostate Cancer" Cancers 13, no. 1: 74. https://doi.org/10.3390/cancers13010074
APA StyleWu, C.-T., Huang, Y.-C., Chen, W.-C., & Chen, M.-F. (2021). The Predictive Role of Prostate-Specific Antigen Changes Following Transurethral Resection of the Prostate for Patients with Localized Prostate Cancer. Cancers, 13(1), 74. https://doi.org/10.3390/cancers13010074




