Validation of the Combined Biomarker for Prediction of Response to Checkpoint Inhibitor in Patients with Advanced Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Statistical Analysis
2.3. Validation in Another Cohort+
3. Results
3.1. Patient Clinicopathologic Characteristics
3.2. IMAGiC Score/Group and Treatment Outcome
3.3. Association between IMAGiC Score/Group and Other Immunotherapy Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Sosman, J.A.; Atkins, M.B.; Leming, P.D.; et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol. 2019, 5, 1411–1420. [Google Scholar] [CrossRef]
- Shao, C.; Li, G.; Huang, L.; Pruitt, S.; Castellanos, E.; Frampton, G.; Carson, K.R.; Snow, T.; Singal, G.; Fabrizio, D.; et al. Prevalence of High Tumor Mutational Burden and Association With Survival in Patients With Less Common Solid Tumors. JAMA Netw Open 2020, 3, e2025109. [Google Scholar] [CrossRef]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018, 362, eaar3593. [Google Scholar] [CrossRef] [PubMed]
- Auslander, N.; Zhang, G.; Lee, J.S.; Frederick, D.T.; Miao, B.; Moll, T.; Tian, T.; Wei, Z.; Madan, S.; Sullivan, R.J.; et al. Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat. Med. 2018, 24, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.J.; Kang, S.Y.; Kim, S.T.; Kang, W.K.; Lee, J.; Kim, K.-M. Combined biomarker for prediction of response to an immune checkpoint inhibitor in metastatic gastric cancer. Precis. Future Med. 2019, 3, 165–175. [Google Scholar] [CrossRef]
- Jung, H.; Kim, H.S.; Kim, J.Y.; Sun, J.M.; Ahn, J.S.; Ahn, M.J.; Park, K.; Esteller, M.; Lee, S.H.; Choi, J.K. DNA methylation loss promotes immune evasion of tumours with high mutation and copy number load. Nat. Commun. 2019, 10, 4278. [Google Scholar] [CrossRef] [PubMed]
- Conroy, J.M.; Pabla, S.; Nesline, M.K.; Glenn, S.T.; Papanicolau-Sengos, A.; Burgher, B.; Andreas, J.; Giamo, V.; Wang, Y.; Lenzo, F.L.; et al. Next generation sequencing of PD-L1 for predicting response to immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Tan, X.; Xiao, Y.; Wang, X.; Wei, Z.; Wang, J.; Wang, X.; Zhou, H.; Zhang, L.; Shi, Y. Ubiquitin C-terminal hydrolase L1 promotes expression of programmed cell death-ligand 1 in non-small-cell lung cancer cells. Cancer Sci. 2020, 111, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Vielnascher, R.M.; Hainzl, E.; Leitner, N.R.; Rammerstorfer, M.; Popp, D.; Witalisz, A.; Rom, R.; Karaghiosoff, M.; Kolbe, T.; Muller, S.; et al. Conditional ablation of TYK2 in immunity to viral infection and tumor surveillance. Transgenic Res. 2014, 23, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, M.; Du, C.; Zhang, W.; Balaji, K.C. Protein kinase D1: A protein of emerging translational interest. Front. Biosci. 2007, 12, 3757–3767. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhong, H.; Qin, Y.; Wei, W.; Li, Z.; Huang, M.; Luo, X. ARMCX Family Gene Expression Analysis and Potential Prognostic Biomarkers for Prediction of Clinical Outcome in Patients with Gastric Carcinoma. Biomed. Res. Int. 2020, 2020, 3575038. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Schrock, A.B.; Ouyang, C.; Sandhu, J.; Sokol, E.; Jin, D.; Ross, J.S.; Miller, V.A.; Lim, D.; Amanam, I.; Chao, J.; et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann. Oncol. 2019, 30, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Bang, Y.J.; Piha-Paul, S.A.; Razak, A.R.A.; Bennouna, J.; Soria, J.C.; Rugo, H.S.; Cohen, R.B.; O’Neil, B.H.; Mehnert, J.M.; et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J. Clin. Oncol. 2019, 37, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Pender, A.; Titmuss, E.; Pleasance, E.D.; Fan, K.Y.; Pearson, H.; Brown, S.D.; Grisdale, C.J.; Topham, J.T.; Shen, Y.; Bonakdar, M.; et al. Genome and Transcriptome Biomarkers of Response to Immune Checkpoint Inhibitors in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 202–212. [Google Scholar] [CrossRef] [PubMed]




| Clinical Response | CR/PR | SD/PD | Total | p-Value |
|---|---|---|---|---|
| (n = 24) | (n = 49) | (n = 73) | ||
| Age (median & quartile range) | 64.0 (54.0; 71.0) | 59.0 (52.0; 67.0) | 61.0 (52.0; 70.0) | 0.466 |
| Gender | 0.457 | |||
| Female | 10 (41.7%) | 26 (53.1%) | 36 (49.3%) | |
| Male | 14 (58.3%) | 23 (46.9%) | 37 (50.7%) | |
| Cancer type | 0.107 | |||
| Cervix cancer | 0 (0.0%) | 1 (2.0%) | 1 (1.4%) | |
| Cholangiocarcinoma | 3 (12.5%) | 7 (14.3%) | 10 (13.7%) | |
| Colorectal cancer | 4 (16.7%) | 0 (0.0%) | 4 (5.5%) | |
| Gastric cancer | 6 (25.0%) | 13 (26.5%) | 19 (26.0%) | |
| Hepatocellular carcinoma | 0 (0.0%) | 1 (2.0%) | 1 (1.4%) | |
| Melanoma | 9 (37.5%) | 16 (32.7%) | 25 (34.2%) | |
| Sarcoma | 0 (0.0%) | 5 (10.2%) | 5 (6.8%) | |
| Urothelial carcinoma | 2 (8.3%) | 6 (12.2%) | 8 (11.0%) | |
| Treatment line of immunotherapy | 0.948 | |||
| 1 | 8 (33.3%) | 15 (30.6%) | 23 (31.5%) | |
| 2 | 8 (33.3%) | 19 (38.8%) | 27 (37.0%) | |
| ≥3 | 8 (33.3%) | 15 (30.6%) | 23 (31.5%) | |
| Immunotherapy regimen | 0.668 | |||
| Atezolizumab containing | 3 (12.5%) | 5 (10.3%) | 8 (11.0%) | |
| Avelumab containing | 1 (4.2%) | 0 (0.0%) | 1 (1.43) | |
| Durvalumab containing | 6 (25.0%) | 13 (26.4%) | 19 (26.0%) | |
| Nivolumab containing | 4 (16.7%) | 11 (22.6%) | 15 (20.6%) | |
| Pembrolizumab containing | 10 (41.6%) | 20 (40.7%) | 30 (41.1%) | |
| Number of immunotherapy cycle (median & quartile range) | 14.0 (11.0; 19.0) | 7.0 (3.0; 9.0) | 9.0 (5.0; 13.0) | <0.001 |
| Total TMB (median & quartile range) | 7.0 (4.3; 10.2) | 4.7 (3.1; 7.0) | 5.5 (3.1; 7.8) | 0.040 |
| TMB | 0.191 | |||
| High (≥10 mutations per megabase) | 6 (25.0%) | 6 (12.2%) | 12 (16.4%) | |
| Low (<10 mutations per megabase) | 18 (75.0%) | 43 (87.8%) | 61 (83.6%) | |
| MSI status | 0.033 | |||
| MSI-H | 3 (12.5%) | 0 (0.0%) | 3 (4.1%) | |
| MSS | 21 (87.5%) | 49 (100.0%) | 70 (95.9%) | |
| PD-L1 CPS | 4.5 (1.0; 15.5) | 0.0 (0.0; 3.0) | 1.0 (0.0; 5.0) | 0.001 |
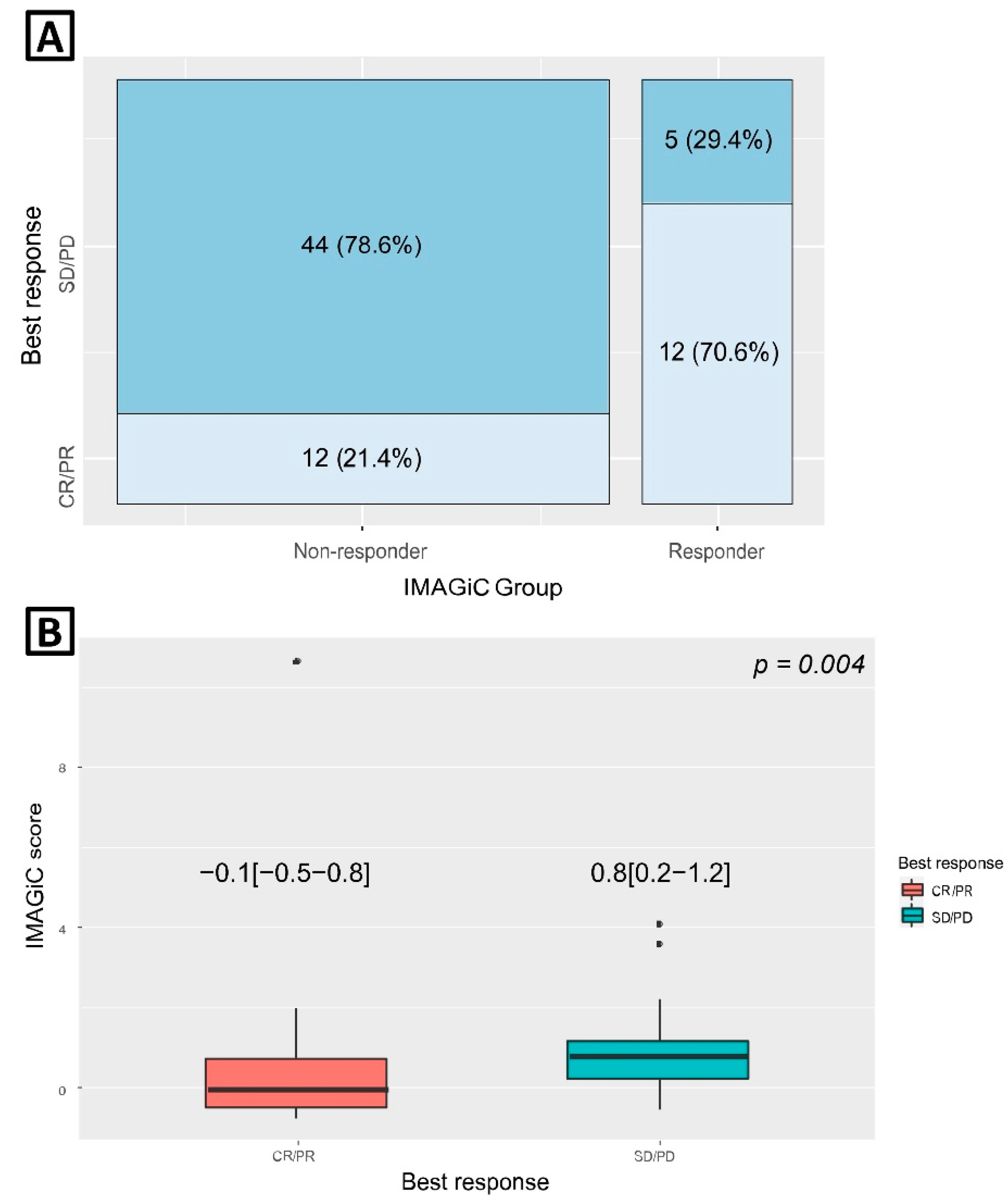
| IMAGiC Group | <0.001 | |||
| Non-responder | 12 (50.0%) | 44 (89.8%) | 56 (76.7%) | |
| Responder | 12 (50.0%) | 5 (10.2%) | 17 (23.3%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-C.; Heo, Y.-J.; Kang, S.-Y.; Lee, J.; Kim, K.-M. Validation of the Combined Biomarker for Prediction of Response to Checkpoint Inhibitor in Patients with Advanced Cancer. Cancers 2021, 13, 2316. https://doi.org/10.3390/cancers13102316
Kim J-C, Heo Y-J, Kang S-Y, Lee J, Kim K-M. Validation of the Combined Biomarker for Prediction of Response to Checkpoint Inhibitor in Patients with Advanced Cancer. Cancers. 2021; 13(10):2316. https://doi.org/10.3390/cancers13102316
Chicago/Turabian StyleKim, Jin-Chul, You-Jeong Heo, So-Young Kang, Jeeyun Lee, and Kyoung-Mee Kim. 2021. "Validation of the Combined Biomarker for Prediction of Response to Checkpoint Inhibitor in Patients with Advanced Cancer" Cancers 13, no. 10: 2316. https://doi.org/10.3390/cancers13102316
APA StyleKim, J.-C., Heo, Y.-J., Kang, S.-Y., Lee, J., & Kim, K.-M. (2021). Validation of the Combined Biomarker for Prediction of Response to Checkpoint Inhibitor in Patients with Advanced Cancer. Cancers, 13(10), 2316. https://doi.org/10.3390/cancers13102316






