Neurofibromin Deficiency and Extracellular Matrix Cooperate to Increase Transforming Potential through FAK-Dependent Signaling
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Cell Cultures
2.3. Isolation of SCs from Neurofibromas
2.4. The 3D Culture In Vitro
2.5. Cell Lysates and Western Immunoblot Analysis
2.6. Immunofluorescence Microscopy
2.7. Statistical Analysis
3. Results
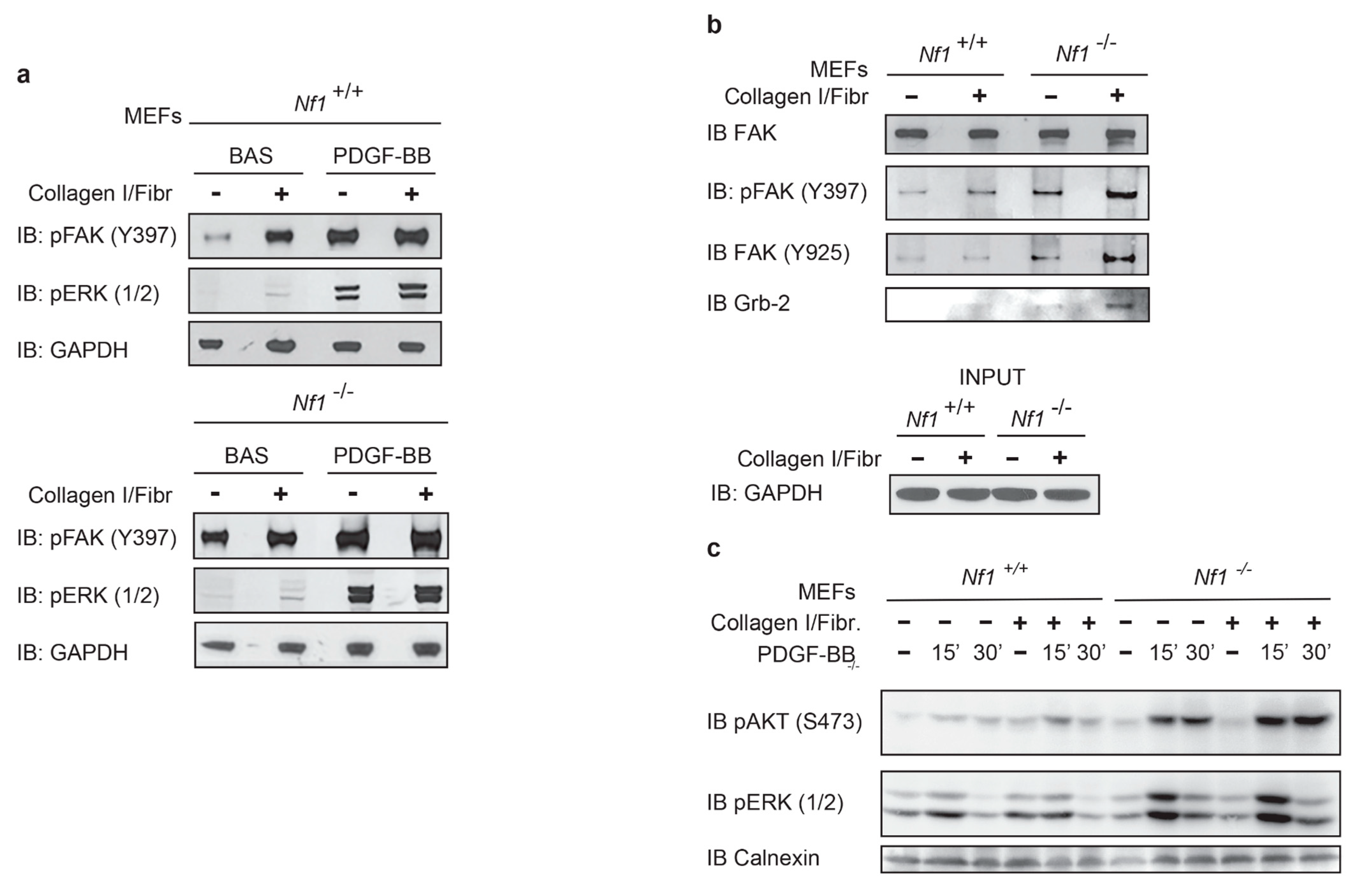
3.1. Neurofibromin Deficiency Affects Phosphorylation Kinetics of ERK and FAK under PDGF-BB Stimulation
3.2. Cell Transformation Induced by Neurofibromin Deficiency Is Increased by PDGF-BB and Further Enhanced by Collagen/Fibronectin
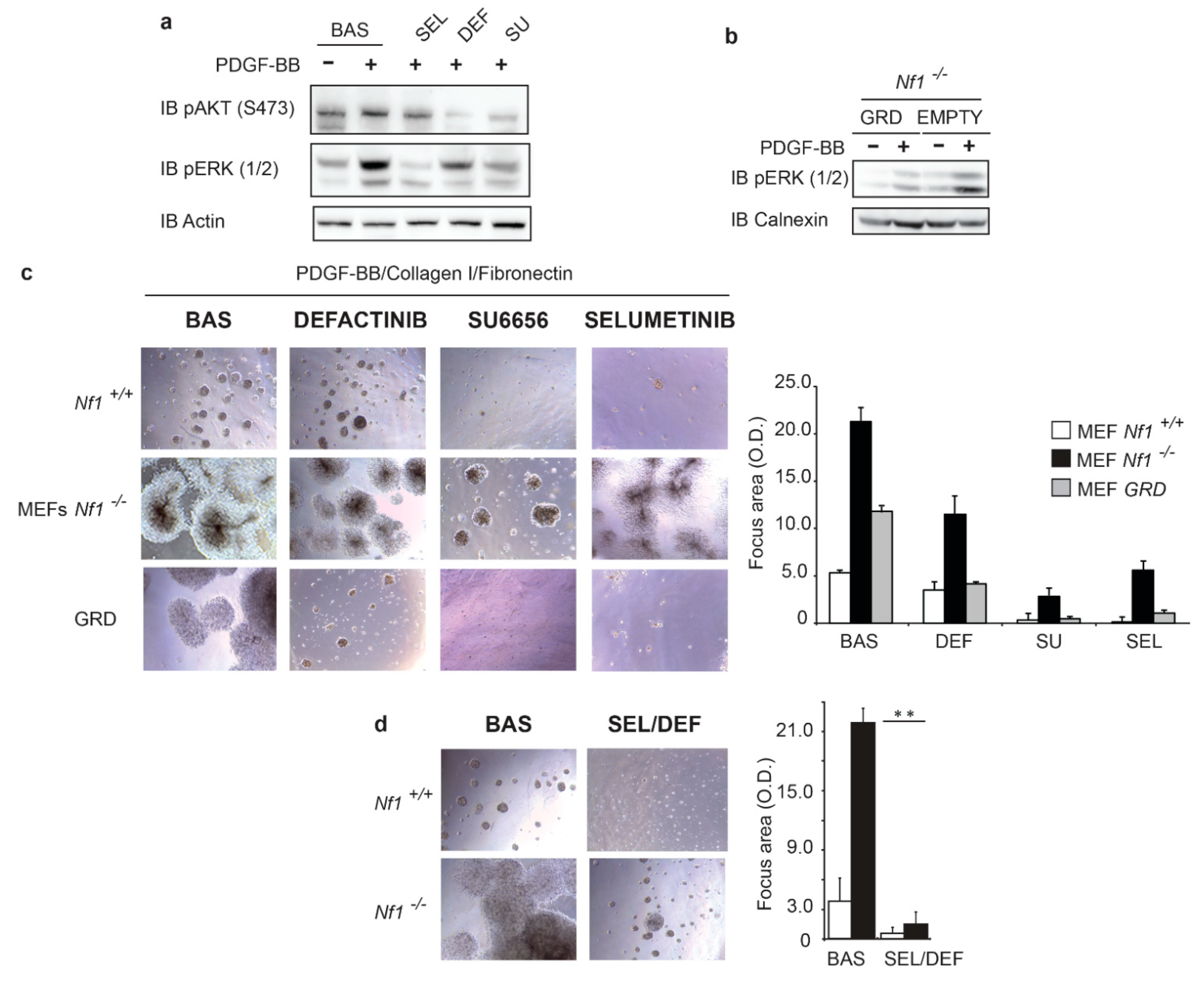
3.3. Oncogenic Signaling Mediated by Both RAS and FAK Signaling Sustains Transformation in Nfn-Deficient Cells
3.4. Neurofibromin Deficiency and FAK Cooperate to Fuel Tumorigenicity of SCs from PNs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottfried, O.N.; Viskochil, D.H.; Couldwell, W.T. Neurofibromatosis Type 1 and tumorigenesis: Molecular mechanisms and therapeutic implications. Neurosurg. Focus 2010, 28, E8. [Google Scholar] [CrossRef]
- Young, E.D.; Ingram, D.; Metcalf-Doetsch, W.; Khan, D.; Al Sannaa, G.; Le Loarer, F.; Lazar, A.J.F.; Slopis, J.; Torres, K.E.; Lev, D.; et al. Clinicopathological variables of sporadic schwannomas of peripheral nerve in 291 patients and expression of biologically relevant markers. J. Neurosurg. 2018, 129, 805–814. [Google Scholar] [CrossRef]
- Tucker, T.; Riccardi, V.M.; Sutcliffe, M.; Vielkind, J.; Wechsler, J.; Wolkenstein, P.; Friedman, J.M. Different Patterns of Mast Cells Distinguish Diffuse from Encapsulated Neurofibromas in Patients with Neurofibromatosis 1. J. Histochem. Cytochem. 2011, 59, 584–590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parrinello, S.; Lloyd, A.C. Neurofibroma development in NF1—Insights into tumour initiation. Trends Cell Biol. 2009, 19, 395–403. [Google Scholar] [CrossRef]
- Cichowski, K.; Jacks, T. NF1 tumor suppressor gene function: Narrowing the GAP. Cell 2001, 104, 593–604. [Google Scholar] [CrossRef]
- Le, L.Q.; Parada, L.F. Tumor microenvironment and neurofibromatosis type I: Connecting the GAPs. Oncogene 2007, 26, 4609–4616. [Google Scholar] [CrossRef]
- Gross, A.M.; Dombi, E.; Widemann, B.C. Current status of MEK inhibitors in the treatment of plexiform neurofibromas. Child’s Nerv. Syst. 2020, 36, 2443–2452. [Google Scholar] [CrossRef]
- Gross, A.M.; Frone, M.; Gripp, K.W.; Gelb, B.D.; Schoyer, L.; Schill, L.; Stronach, B.; Biesecker, L.G.; Esposito, D.; Hernandez, E.R.; et al. Advancing RAS/RASopathy therapies: An NCI-sponsored intramural and extramural collaboration for the study of RASopathies. Am. J. Med. Genet. Part A 2020, 182, 866–876. [Google Scholar] [CrossRef]
- Gross, A.M.; Widemann, B.C. Clinical trial design in neurofibromatosis type 1 as a model for other tumor predisposition syndromes. Neuro-Oncol. Adv. 2020, 2, i134–i140. [Google Scholar] [CrossRef]
- Gross, A.M.; Wolters, P.L.; Dombi, E.; Baldwin, A.; Whitcomb, P.; Fisher, M.J.; Weiss, B.; Kim, A.; Bornhorst, M.; Shah, A.C.; et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N. Engl. J. Med. 2020, 382, 1430–1442. [Google Scholar] [CrossRef]
- Harrisingh, M.C.; Lloyd, A.C. Ras/Raf/ERK signalling and NF1. Cell Cycle 2004, 3, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.J.; Przybyla, L.; Weaver, V.M. Tissue Force Programs Cell Fate and Tumor Aggression. Cancer Discov. 2017, 7, 1224–1237. [Google Scholar] [CrossRef]
- Ratner, N.; Miller, S.J. A RASopathy gene commonly mutated in cancer: The neurofibromatosis type 1 tumour suppressor. Nat. Rev. Cancer 2015, 15, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guan, J.L. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009, 28, 35–49. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.-S.; Zhang, J.; Zhao, W.; Yang, X.-M.; Li, X.; Jiang, T.-S.; Yao, L.-B. Focal adhesion kinase signaling pathway participates in the formation of choroidal neovascularization and regulates the proliferation and migration of choroidal microvascular endothelial cells by acting through HIF-1 and VEGF expression in RPE cells. Exp. Eye Res. 2009, 88, 910–918. [Google Scholar] [CrossRef]
- Hauck, C.R.; Hsia, D.A.; Ilic, D.; Schlaepfer, D.D. v-Src SH3-enhanced interaction with focal adhesion kinase at beta 1 integrin-containing invadopodia promotes cell invasion. J. Biol. Chem. 2002, 277, 12487–12490. [Google Scholar] [CrossRef]
- Hauck, C.R.; Hsia, D.A.; Puente, X.S.; Cheresh, D.A.; Schlaepfer, D.D. FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth. EMBO J. 2002, 21, 6289–6302. [Google Scholar] [CrossRef]
- Hauck, C.R.; Hsia, D.A.; Schlaepfer, D.D. Focal Adhesion Kinase Facilitates Platelet-derived Growth Factor-BB-stimulated ERK2 Activation Required for Chemotaxis Migration of Vascular Smooth Muscle Cells. J. Biol. Chem. 2000, 275, 41092–41099. [Google Scholar] [CrossRef]
- Hauck, C.R.; Hsia, D.A.; Schlaepfer, D.D. The Focal Adhesion Kinase--A Regulator of Cell Migration and Invasion. IUBMB Life 2002, 53, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Hauck, C.R.; Hunter, T.; Schlaepfer, D.D. The v-Src SH3 Domain Facilitates a Cell Adhesion-independent Association with Focal Adhesion Kinase. J. Biol. Chem. 2001, 276, 17653–17662. [Google Scholar] [CrossRef] [PubMed]
- Goode, E.L.; Chenevix-Trench, G.; Song, H.; Ramus, S.J.; Notaridou, M.; Lawrenson, K.; Widschwendter, M.; Vierkant, R.A.; Larson, M.C.; Kjaer, S.K.; et al. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat. Genet. 2010, 42, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Grove, M.; Komiyama, N.H.; Nave, K.-A.; Grant, S.G.; Sherman, D.L.; Brophy, P.J. FAK is required for axonal sorting by Schwann cells. J. Cell Biol. 2007, 176, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jiang, M.N.; Zhang, C.H.; Li, C.; Jia, Y.J. Effects of Ganfukang on expression of connective tissue growth factor and focal adhesion kinase/protein kinase B signal pathway in hepatic fibrosis rats. Chin. J. Integr. Med. 2014, 20, 438–444. [Google Scholar] [CrossRef]
- Chen, J.-S.; Li, H.-S.; Huang, J.-Q.; Dong, S.-H.; Huang, Z.-J.; Yi, W.; Zhan, G.-F.; Feng, J.-T.; Sun, J.-C.; Huang, X.-H. MicroRNA-379-5p inhibits tumor invasion and metastasis by targeting FAK/AKT signaling in hepatocellular carcinoma. Cancer Lett. 2016, 375, 73–83. [Google Scholar] [CrossRef]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef]
- Mohanty, A.; Pharaon, R.R.; Nam, A.; Salgia, S.; Kulkarni, P.; Massarelli, E. FAK-targeted and combination therapies for the treatment of cancer: An overview of phase I and II clinical trials. Expert Opin. Investig. Drugs 2020, 29, 399–409. [Google Scholar] [CrossRef]
- O’Brien, S.; Golubovskaya, V.M.; Conroy, J.; Liu, S.; Wang, D.; Liu, B.; Cance, W.G. FAK inhibition with small molecule inhibitor Y15 decreases viability, clonogenicity, and cell attachment in thyroid cancer cell lines and synergizes with targeted therapeutics. Oncotarget 2014, 5, 7945–7959. [Google Scholar] [CrossRef]
- Marusak, C.; Thakur, V.; Li, Y.; Freitas, J.T.; Zmina, P.M.; Thakur, V.S.; Chang, M.; Gao, M.; Tan, J.; Xiao, M.; et al. Targeting Extracellular Matrix Remodeling Restores BRAF Inhibitor Sensitivity in BRAFi-resistant Melanoma. Clin. Cancer Res. 2020, 26, 6039–6050. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, D.; Wang, Y.; Zhang, W.; Zhang, J.; Fan, J.; Zhan, Q.; Chen, J. Focal adhesion kinase (FAK) inhibitor-defactinib suppresses the malignant progression of human esophageal squamous cell carcinoma (ESCC) cells via effective blockade of PI3K/AKT axis and downstream molecular network. Mol. Carcinog. 2021, 60, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Canel, M.; Serrels, A.; Miller, D.; Timpson, P.; Serrels, B.; Frame, M.C.; Brunton, V.G. Quantitative In vivo Imaging of the Effects of Inhibiting Integrin Signaling via Src and FAK on Cancer Cell Movement: Effects on E-cadherin Dynamics. Cancer Res. 2010, 70, 9413–9422. [Google Scholar] [CrossRef]
- Jiang, H.; Hegde, S.; Knolhoff, B.L.; Zhu, Y.; Herndon, J.M.; Meyer, M.A.; Nywening, T.M.; Hawkins, T.M.N.W.G.; Shapiro, I.M.; Weaver, D.T.; et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 2016, 22, 851–860. [Google Scholar] [CrossRef]
- Gerber, D.E.; Camidge, D.R.; Morgensztern, D.; Cetnar, J.; Kelly, R.J.; Ramalingam, S.S.; Spigel, D.R.; Jeong, W.; Scaglioni, P.P.; Zhang, S.; et al. Phase 2 study of the focal adhesion kinase inhibitor defactinib (VS-6063) in previously treated advanced KRAS mutant non-small cell lung cancer. Lung Cancer 2020, 139, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Tavora, B.; Reynolds, L.E.; Batista, S.; Demircioglu, F.; Fernandez, I.; Lechertier, T.; Lees, D.M.; Wong, P.-P.; Alexopoulou, A.; Elia, G.; et al. Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy. Nature 2014, 514, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, L.-J.; Neubauer, D.R.; Muir, D.F.; Wallace, M.R. Immortalization of human normal and NF1 neurofibroma Schwann cells. Lab. Investig. 2016, 96, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.D.; Barkan, B.; Friedman, E.; Kloog, Y.; Stein, R.E. The tumor suppressor neurofibromin confers sensitivity to apoptosis by Ras-dependent and Ras-independent pathways. Cell Death Differ. 2006, 14, 895–906. [Google Scholar] [CrossRef]
- Chiara, F.; Bishayee, S.; Heldin, C.H.; Demoulin, J.B. Autoinhibition of the platelet-derived growth factor beta-receptor tyrosine kinase by its C-terminal tail. J. Biol. Chem. 2004, 279, 19732–19738. [Google Scholar] [CrossRef]
- Chiara, F.; Goumans, M.J.; Forsberg, H.; Ahgren, A.; Rasola, A.; Aspenstrom, P.; Wernstedt, C.; Hellberg, C.; Heldin, C.H.; Heuchel, R. A gain of function mutation in the activation loop of platelet-derived growth factor beta-receptor deregulates its kinase activity. J. Biol. Chem. 2004, 279, 42516–42527. [Google Scholar] [CrossRef]
- Dixon, R.D.; Chen, Y.; Ding, F.; Khare, S.D.; Prutzman, K.C.; Schaller, M.D.; Campbell, S.L.; Dokholyan, N.V. New Insights into FAK Signaling and Localization Based on Detection of a FAT Domain Folding Intermediate. Structure 2004, 12, 2161–2171. [Google Scholar] [CrossRef]
- Golubovskaya, V.; Curtin, L.; Groman, A.; Sexton, S.; Cance, W.G. In vivo toxicity, metabolism and pharmacokinetic properties of FAK inhibitor 14 or Y15 (1, 2, 4, 5-benzenetetramine tetrahydrochloride). Arch. Toxicol. 2014, 89, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.W.; Kaplan, S.; Lowenstein, E.J.; Schlessinger, J.; Bar-Sagi, D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature 1993, 363, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, D.D.; Hauck, C.R.; Sieg, D.J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999, 71, 435–478. [Google Scholar] [CrossRef]
- Guarino, M. Plexiform schwannoma. Immunohistochemistry of Schwann cell markers, intermediate filaments and extracellular matrix components. Pathol. Res. Pract. 1993, 189, 913–920. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Momeni, S.A.; Zarandi, P.K.; Chalbatani, G.M.; Dana, H.; Mirzaei, H.R.; Akbari, M.E.; Miri, S.R. The Role and Function of Ras-association domain family in Cancer: A Review. Genes Dis. 2019, 6, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Masgras, I.; Ciscato, F.; Brunati, A.M.; Tibaldi, E.; Indraccolo, S.; Curtarello, M.; Chiara, F.; Cannino, G.; Papaleo, E.; Lambrughi, M.; et al. Absence of Neurofibromin Induces an Oncogenic Metabolic Switch via Mitochondrial ERK-Mediated Phosphorylation of the Chaperone TRAP1. Cell Rep. 2017, 18, 659–672. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Errico, A.; Stocco, A.; Riccardi, V.M.; Gambalunga, A.; Bassetto, F.; Grigatti, M.; Ferlosio, A.; Tadini, G.; Garozzo, D.; Ferraresi, S.; et al. Neurofibromin Deficiency and Extracellular Matrix Cooperate to Increase Transforming Potential through FAK-Dependent Signaling. Cancers 2021, 13, 2329. https://doi.org/10.3390/cancers13102329
Errico A, Stocco A, Riccardi VM, Gambalunga A, Bassetto F, Grigatti M, Ferlosio A, Tadini G, Garozzo D, Ferraresi S, et al. Neurofibromin Deficiency and Extracellular Matrix Cooperate to Increase Transforming Potential through FAK-Dependent Signaling. Cancers. 2021; 13(10):2329. https://doi.org/10.3390/cancers13102329
Chicago/Turabian StyleErrico, Andrea, Anna Stocco, Vincent M. Riccardi, Alberto Gambalunga, Franco Bassetto, Martina Grigatti, Amedeo Ferlosio, Gianluca Tadini, Debora Garozzo, Stefano Ferraresi, and et al. 2021. "Neurofibromin Deficiency and Extracellular Matrix Cooperate to Increase Transforming Potential through FAK-Dependent Signaling" Cancers 13, no. 10: 2329. https://doi.org/10.3390/cancers13102329
APA StyleErrico, A., Stocco, A., Riccardi, V. M., Gambalunga, A., Bassetto, F., Grigatti, M., Ferlosio, A., Tadini, G., Garozzo, D., Ferraresi, S., Trevisan, A., Giustini, S., Rasola, A., & Chiara, F. (2021). Neurofibromin Deficiency and Extracellular Matrix Cooperate to Increase Transforming Potential through FAK-Dependent Signaling. Cancers, 13(10), 2329. https://doi.org/10.3390/cancers13102329









