Share and Cite
An, H.J.; An, E.; Rabizadeh, S.; Liao, W.-L.; Burrows, J.; Hembrough, T.; Kang, J.H.; Park, C.K.; Kim, T.-J. Quantitative Multiplexed Proteomics Could Assist Therapeutic Decision Making in Non-Small Cell Lung Cancer Patients with Ambiguous ALK Test Results. Cancers 2021, 13, 2337. https://doi.org/10.3390/cancers13102337
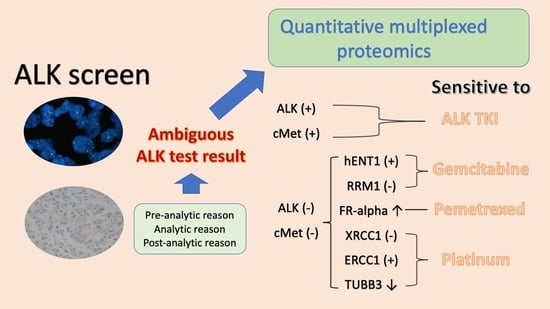
An HJ, An E, Rabizadeh S, Liao W-L, Burrows J, Hembrough T, Kang JH, Park CK, Kim T-J. Quantitative Multiplexed Proteomics Could Assist Therapeutic Decision Making in Non-Small Cell Lung Cancer Patients with Ambiguous ALK Test Results. Cancers. 2021; 13(10):2337. https://doi.org/10.3390/cancers13102337
Chicago/Turabian StyleAn, Ho Jung, Eunkyung An, Shahrooz Rabizadeh, Wei-Li Liao, Jon Burrows, Todd Hembrough, Jin Hyung Kang, Chan Kwon Park, and Tae-Jung Kim. 2021. "Quantitative Multiplexed Proteomics Could Assist Therapeutic Decision Making in Non-Small Cell Lung Cancer Patients with Ambiguous ALK Test Results" Cancers 13, no. 10: 2337. https://doi.org/10.3390/cancers13102337
APA StyleAn, H. J., An, E., Rabizadeh, S., Liao, W.-L., Burrows, J., Hembrough, T., Kang, J. H., Park, C. K., & Kim, T.-J. (2021). Quantitative Multiplexed Proteomics Could Assist Therapeutic Decision Making in Non-Small Cell Lung Cancer Patients with Ambiguous ALK Test Results. Cancers, 13(10), 2337. https://doi.org/10.3390/cancers13102337