Afucosylated IgG Targets FcγRIV for Enhanced Tumor Therapy in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies
2.2. Isolation of Primary Effector Cells
2.3. Cell Culture
2.4. Flow Cytometry
2.5. Antibody Dependent Cellular Cytotoxicity (ADCC)
2.6. Antibody Dependent Phagocytosis (ADCP)
2.7. B16 Mouse Melanoma Metastasis Model
2.8. Surface Plasmon Resonance
2.9. Statistical Analysis
3. Results
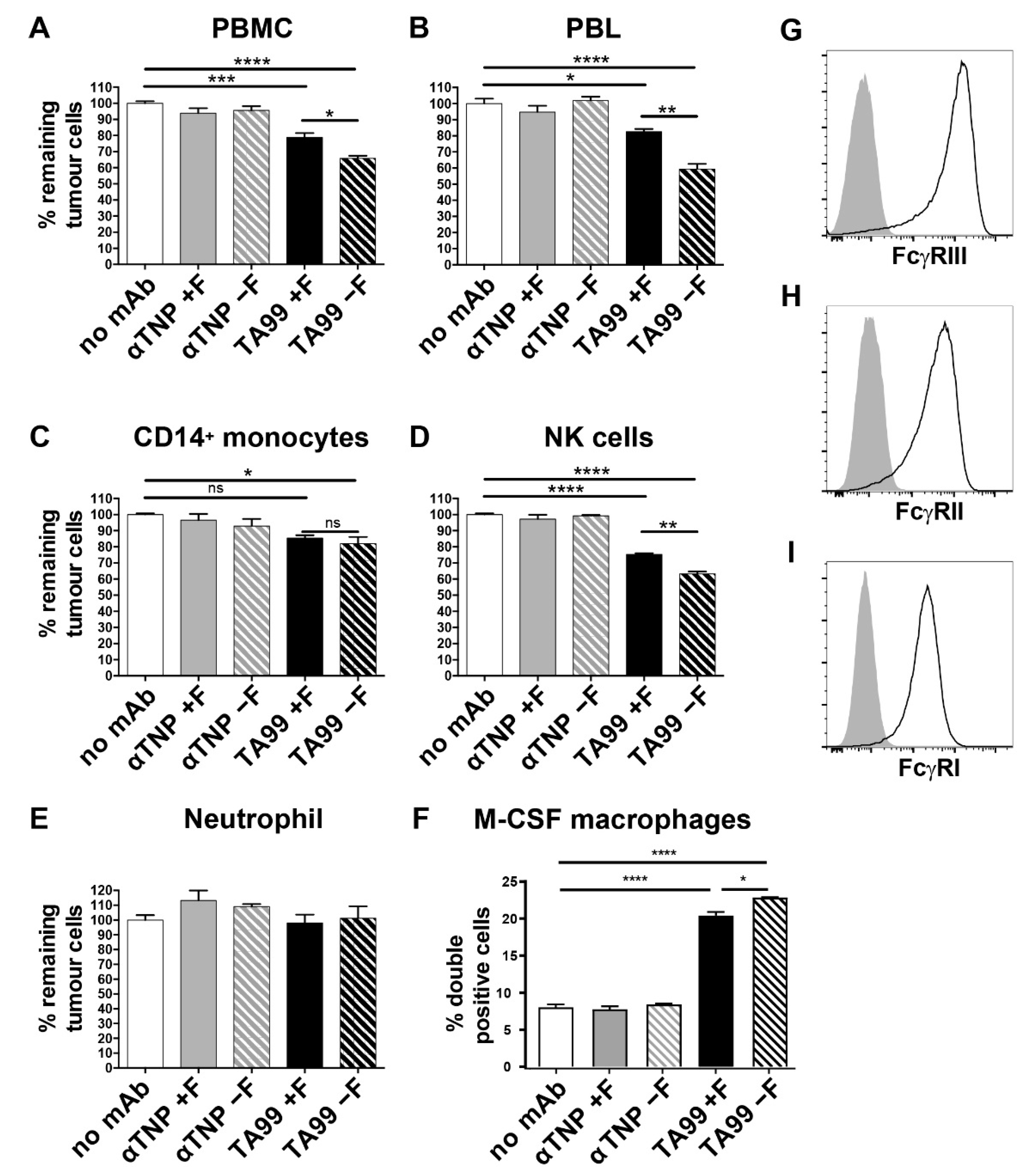
3.1. Lack of Core-Fucose in Human IgG1 Increases the Activation of Human Effector Cells
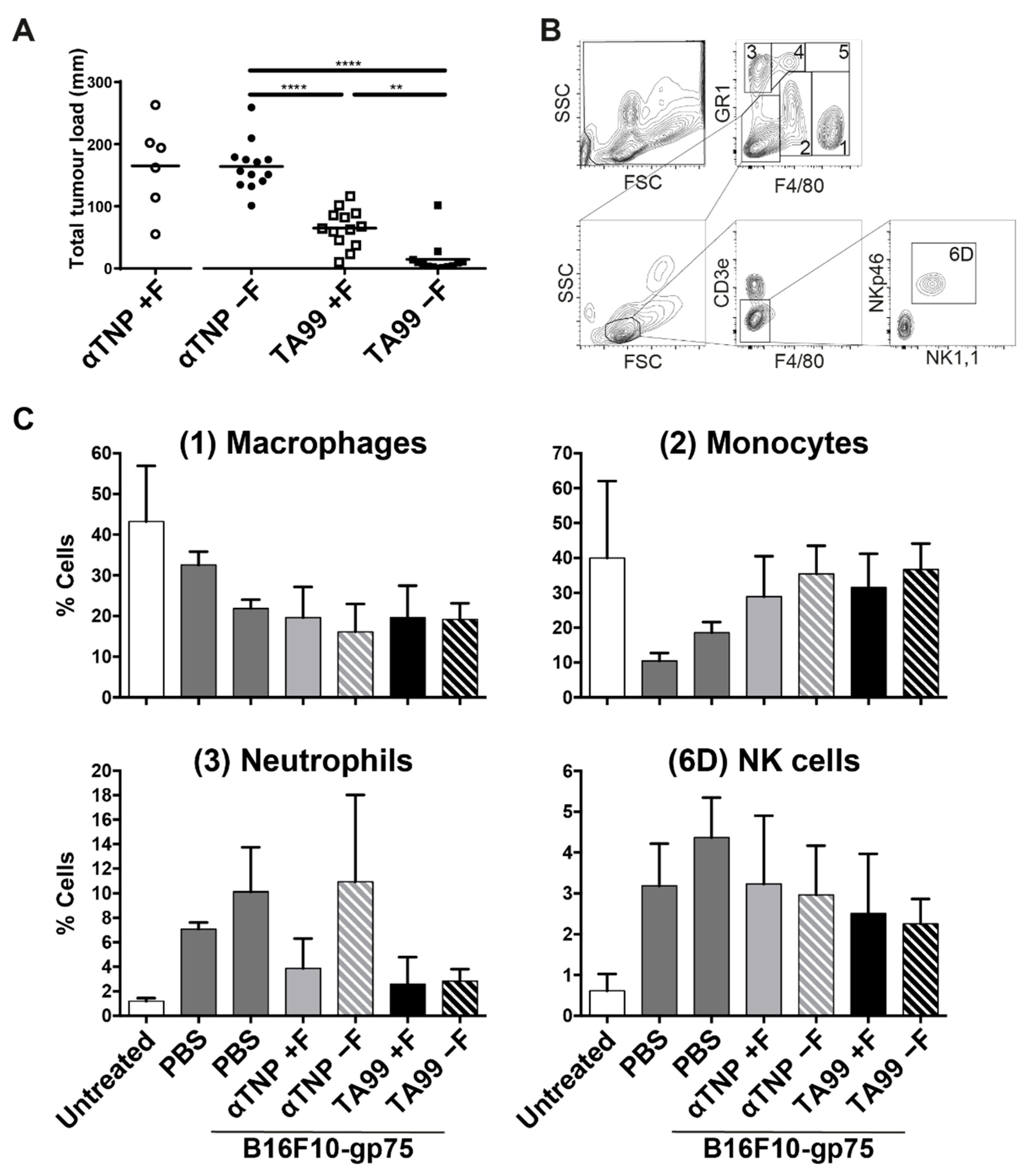
3.2. Mice Treated with Afucosylated hIgG1-TA99 Develop Less Peritoneal Metastasis
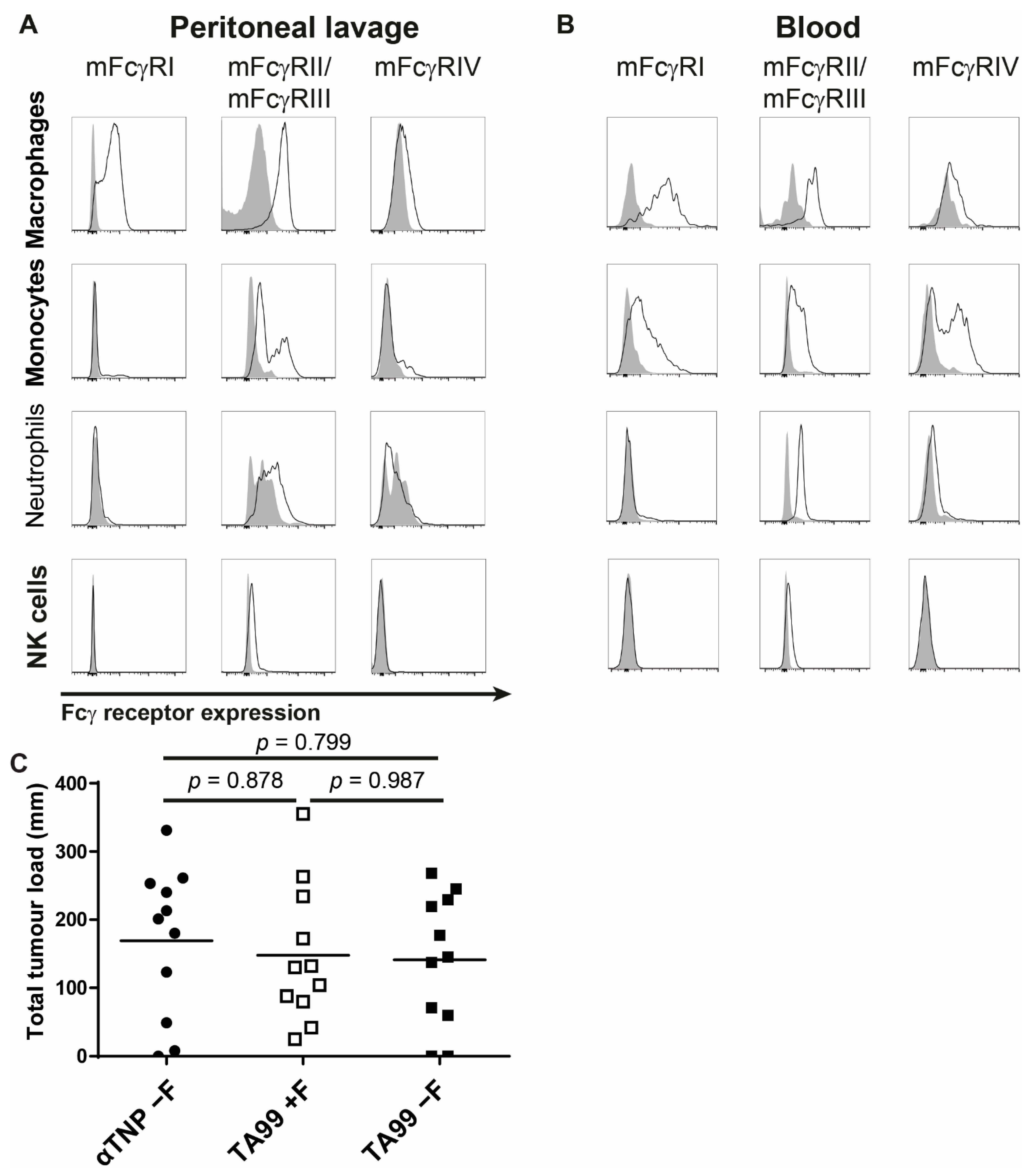
3.3. Afucosylated Human IgG1 Has Increased Affinity for Mouse FcγRIV
3.4. Interaction of Afucosylated Human IgG1 with FcγRIV Is Crucial for Elevated Tumor Clearance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ott, P.A.; Hodi, F.S.; Robert, C. CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin. Cancer Res. 2013, 19, 5300–5309. [Google Scholar] [CrossRef] [Green Version]
- Reichert, J.M.; Dhimolea, E. The future of antibodies as cancer drugs. Drug Discov. Today 2012, 17, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef]
- Liu, R.; Oldham, R.J.; Teal, E.; Beers, S.A.; Cragg, M.S. Fc-Engineering for Modulated Effector Functions—Improving Antibodies for Cancer Treatment. Antibodies 2020, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Saxena, A.; Sidhu, S.S.; Wu, D. Fc Engineering for Developing Therapeutic Bispecific Antibodies and Novel Scaffolds. Front. Immunol. 2017, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimmerjahn, F.; Bruhns, P.; Horiuchi, K.; Ravetch, J.V. FcgammaRIV: A Novel FcR with Distinct IgG Subclass Specificity. Immunity 2005, 23, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Vidarsson, G.; Van De Winkel, J.G.J. Fc receptor and complement receptor-mediated phagocytosis in host defence. Curr. Opin. Infect. Dis. 1998, 11. [Google Scholar] [CrossRef]
- Jefferis, R.; Lund, J. Interaction sites on human IgG-Fc for FcgammaR: Current models. Immunol. Lett. 2002, 82, 57–65. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef]
- Baković, M.P.; Selman, M.H.J.; Hoffmann, M.; Rudan, I.; Campbell, H.; Deelder, A.M.; Lauc, G.; Wuhrer, M. High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. J. Proteome Res. 2013, 12, 821–831. [Google Scholar] [CrossRef] [Green Version]
- Kapur, R.; Kustiawan, I.; Vestrheim, A.; Koeleman, C.A.M.M.; Visser, R.; Einarsdottir, H.K.; Porcelijn, L.; Jackson, D.; Kumpel, B.; Deelder, M.; et al. A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood 2014, 123, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Kapur, R.; Einarsdottir, H.K.; Vidarsson, G. IgG-effector functions: “The Good, The Bad and The Ugly”. Immunol. Lett. 2014, 160, 139–144. [Google Scholar] [CrossRef]
- Larsen, M.D.; de Graaf, E.L.; Sonneveld, M.E.; Plomp, H.R.; Nouta, J.; Hoepel, W.; Chen, H.-J.; Linty, F.; Visser, R.; Brinkhaus, M.; et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science 2020, 371, eabc8378. [Google Scholar] [CrossRef]
- Krištić, J.; Zaytseva, O.O.; Ram, R.; Nguyen, Q.; Novokmet, M.; Vučković, F.; Vilaj, M.; Trbojević-Akmačić, I.; Pezer, M.; Davern, K.M.; et al. Profiling and genetic control of the murine immunoglobulin G glycome. Nat. Chem. Biol. 2018, 14, 516–524. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory actions of intravenous immunoglobulin. Annu. Rev. Immunol. 2008, 26, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.A.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Berkel, P.H.C.; Gerritsen, J.; van Voskuilen, E.; Perdok, G.; Vink, T.; van de Winkel, J.G.J.; Parren, P.W.H.I. Rapid production of recombinant human IgG With improved ADCC effector function in a transient expression system. Biotechnol. Bioeng. 2010, 105, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar] [CrossRef] [Green Version]
- Nimmerjahn, F.; Ravetch, J. V Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005, 310, 1510–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekkers, G.; Bentlage, A.E.H.; Plomp, R.; Visser, R.; Koeleman, C.A.M.; Beentjes, A.; Mok, J.Y.; van Esch, W.J.E.; Wuhrer, M.; Rispens, T.; et al. Conserved FcγR- glycan discriminates between fucosylated and afucosylated IgG in humans and mice. Mol. Immunol. 2018, 94, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Mechetina, L.V.; Najakshin, A.M.; Alabyev, B.Y.; Chikaev, N.A.; Taranin, A.V. Identification of CD16-2, a novel mouse receptor homologous to CD16/Fc gamma RIII. Immunogenetics 2002, 54, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, C.W.; Dekkers, G.; Bentlage, A.E.H.; Treffers, L.W.; Nagelkerke, S.Q.; Lissenberg-Thunnissen, S.; Koeleman, C.A.M.; Wuhrer, M.; van den Berg, T.K.; Rispens, T.; et al. Enhanced Effector Functions Due to Antibody Defucosylation Depend on the Effector Cell Fcγ Receptor Profile. J. Immunol. 2017, 199, 204–211. [Google Scholar] [CrossRef]
- Leabman, M.K.; Meng, Y.G.; Kelley, R.F.; DeForge, L.E.; Cowan, K.J.; Iyer, S. Effects of altered FcγR binding on antibody pharmacokinetics in cynomolgus monkeys. MAbs 2013, 5, 896–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einarsdottir, H.K.; Selman, M.H.J.; Kapur, R.; Scherjon, S.; Koeleman, C.A.M.; Deelder, A.M.; Van Der Schoot, C.E.; Vidarsson, G.; Wuhrer, M. Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconj. J. 2013, 30, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, E.; Niwa, R.; Saji, S.; Muta, M.; Hirose, M.; Iida, S.; Shiotsu, Y.; Satoh, M.; Shitara, K.; Kondo, M.; et al. A nonfucosylated anti-HER2 antibody augments antibody-dependent cellular cytotoxicity in breast cancer patients. Clin. Cancer Res. 2007, 13, 1875–1882. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, C.; Grau, S.; Jäger, C.; Sondermann, P.; Brünker, P.; Waldhauer, I.; Hennig, M.; Ruf, A.; Rufer, A.C.; Stihle, M.; et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. USA 2011, 108, 12669–12674. [Google Scholar] [CrossRef] [Green Version]
- Marcus, R.; Davies, A.; Ando, K.; Klapper, W.; Opat, S.; Owen, C.; Phillips, E.; Sangha, R.; Schlag, R.; Seymour, J.F.; et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N. Engl. J. Med. 2017, 377, 1331–1344. [Google Scholar] [CrossRef]
- Dekkers, G.; Bentlage, A.E.H.; Stegmann, T.C.; Howie, H.L.; Lissenberg-Thunnissen, S.; Zimring, J.; Rispens, T.; Vidarsson, G. Affinity of human IgG subclasses to mouse Fc gamma receptors. MAbs 2017, 9, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Overdijk, M.B.; Verploegen, S.; Ortiz Buijsse, A.; Vink, T.; Leusen, J.H.W.; Bleeker, W.K.; Parren, P.W.H.I. Crosstalk between Human IgG Isotypes and Murine Effector Cells. J. Immunol. 2012, 189, 3430–3438. [Google Scholar] [CrossRef] [PubMed]
- Kruijsen, D.; Einarsdottir, H.K.; Schijf, M.A.; Coenjaerts, F.E.; van der Schoot, E.C.; Vidarsson, G.; van Bleek, G.M. Intranasal administration of antibody-bound respiratory syncytial virus particles efficiently primes virus-specific immune responses in mice. J. Virol. 2013, 87, 7550–7557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayasaradhi, S.; Bouchard, B.; Houghton, A.N. The melanoma antigen gp75 is the human homologue of the mouse b (brown) locus gene product. J. Exp. Med. 1990, 171, 1375–1380. [Google Scholar] [CrossRef]
- Vink, T.; Oudshoorn-Dickmann, M.; Roza, M.; Reitsma, J.-J.J.; de Jong, R.N. A simple, robust and highly efficient transient expression system for producing antibodies. Methods 2014, 65, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Plomp, R.; Hensbergen, P.J.; Rombouts, Y.; Zauner, G.; Dragan, I.; Koeleman, C.A.M.; Deelder, A.M.; Wuhrer, M. Site-Specific N-Glycosylation Analysis of Human Immunoglobulin E. J. Proteome Res. 2014, 13, 536–546. [Google Scholar] [CrossRef]
- Sonneveld, M.E.; Natunen, S.; Sainio, S.; Koeleman, C.A.M.; Holst, S.; Dekkers, G.; Koelewijn, J.; Partanen, J.; van der Schoot, C.E.; Wuhrer, M.; et al. Glycosylation pattern of anti-platelet IgG is stable during pregnancy and predicts clinical outcome in alloimmune thrombocytopenia. Br. J. Haematol. 2016, 174, 310–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekkers, G.; Treffers, L.; Plomp, R.; Bentlage, A.E.H.; de Boer, M.; Koeleman, C.A.M.; Lissenberg-Thunnissen, S.N.; Visser, R.; Brouwer, M.; Mok, J.Y.; et al. Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front. Immunol. 2017, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Reusch, D.; Haberger, M.; Falck, D.; Peter, B.; Maier, B.; Gassner, J.; Hook, M.; Wagner, K.; Bonnington, L.; Bulau, P.; et al. Comparison of methods for the analysis of therapeutic immunoglobulin G Fc-glycosylation profiles-Part 2: Mass spectrometric methods. MAbs 2015, 7. [Google Scholar] [CrossRef]
- Gül, N.; Babes, L.; Siegmund, K.; Korthouwer, R.; Bögels, M.; Braster, R.; Vidarsson, G.; ten Hagen, T.L.M.; Kubes, P.; van Egmond, M. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J. Clin. Invest. 2014, 124, 812–823. [Google Scholar] [CrossRef]
- Braster, R.; O’Toole, T.; van Egmond, M. Myeloid cells as effector cells for monoclonal antibody therapy of cancer. Methods 2014, 65, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Benonisson, H.; Sow, H.S.; Breukel, C.; Claassens, J.W.C.; Brouwers, C.; Linssen, M.M.; Redeker, A.; Fransen, M.F.; van Hall, T.; Ossendorp, F.; et al. FcγRI expression on macrophages is required for antibodymediated tumor protection by cytomegalovirus-based vaccines. Oncotarget 2018, 9, 29392–29402. [Google Scholar] [CrossRef] [PubMed]
- Niwa, R.; Natsume, A.; Uehara, A.; Wakitani, M.; Iida, S.; Uchida, K.; Satoh, M.; Shitara, K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J. Immunol. Methods 2005, 306, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Kubota, T.; Kaneko, E.; Iida, S.; Wakitani, M.; Kobayashi-Natsume, Y.; Kubota, A.; Shitara, K.; Nakamura, K. Enhanced binding affinity for FcgammaRIIIa of fucose-negative antibody is sufficient to induce maximal antibody-dependent cellular cytotoxicity. Mol. Immunol. 2007, 44, 3122–3131. [Google Scholar] [CrossRef]
- Mizushima, T.; Yagi, H.; Takemoto, E.; Shibata-Koyama, M.; Isoda, Y.; Iida, S.; Masuda, K.; Satoh, M.; Kato, K. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells 2011, 16, 1071–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruhns, P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012, 119, 5640–5649. [Google Scholar] [CrossRef]
- Gillis, C.; Gouel-Chéron, A.; Jönsson, F.; Bruhns, P. Contribution of Human FcgammaRs to Disease with Evidence from Human Polymorphisms and Transgenic Animal Studies. Front. Immunol. 2014, 5, 254. [Google Scholar] [CrossRef] [Green Version]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The function of Fcgamma receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Biburger, M.; Lux, A.; Nimmerjahn, F. How immunoglobulin G antibodies kill target cells: Revisiting an old paradigm. Adv. Immunol. 2014, 124, 67–94. [Google Scholar] [CrossRef]
- Niwa, R.; Shoji-Hosaka, E.; Sakurada, M.; Shinkawa, T.; Uchida, K.; Nakamura, K.; Matsushima, K.; Ueda, R.; Hanai, N.; Shitara, K. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004, 64, 2127–2133. [Google Scholar] [CrossRef] [Green Version]
- Junttila, T.T.; Parsons, K.; Olsson, C.; Lu, Y.; Xin, Y.; Theriault, J.; Crocker, L.; Pabonan, O.; Baginski, T.; Meng, G.; et al. Superior In vivo Efficacy of Afucosylated Trastuzumab in the Treatment of HER2-Amplified Breast Cancer. Cancer Res. 2010, 70, 4481–4489. [Google Scholar] [CrossRef] [Green Version]
- Cardarelli, P.M.; Moldovan-Loomis, M.-C.; Preston, B.; Black, A.; Passmore, D.; Chen, T.-H.; Chen, S.; Liu, J.; Kuhne, M.R.; Srinivasan, M.; et al. In vitro and in vivo characterization of MDX-1401 for therapy of malignant lymphoma. Clin. Cancer Res. 2009, 15, 3376–3383. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Liu, L.; Dumitru, C.D.; Cummings, N.R.H.; Cukan, M.; Jiang, Y.; Li, Y.; Li, F.; Mitchell, T.; Mallem, M.R.; et al. Glycoengineered Pichia produced anti-HER2 is comparable to trastuzumab in preclinical study. MAbs 2011, 3, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Ackerman, M.E.; Crispin, M.; Yu, X.; Baruah, K.; Boesch, A.W.; Harvey, D.J.; Dugast, A.-S.S.; Heizen, E.L.; Ercan, A.; Choi, I.; et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Investig. 2013, 123, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Sewatanon, J.; Memoli, M.J.; Wrammert, J.; Bournazos, S.; Bhaumik, S.K.; Pinsky, B.A.; Chokephaibulkit, K.; Onlamoon, N.; Pattanapanyasat, K.; et al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 2017, 355, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Mössner, E.; Brünker, P.; Moser, S.; Püntener, U.; Schmidt, C.; Herter, S.; Grau, R.; Gerdes, C.; Nopora, A.; Van Puijenbroek, E.; et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010, 115, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Niwa, R.; Shitara, K.; Satoh, M. Glyco-engineered Therapeutic Antibodies Therapeutic antibodies as a Second-Generation Antibody Therapy. In Glycoscience: Biology and Medicine; Springer: Tokyo, Japan, 2015; pp. 1501–1508. [Google Scholar]
- Bruhns, P.; Jonsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapur, R.; Della Valle, L.; Sonneveld, M.; Hipgrave Ederveen, A.; Visser, R.; Ligthart, P.; de Haas, M.; Wuhrer, M.; van der Schoot, C.E.; Vidarsson, G. Low anti-RhD IgG-Fc-fucosylation in pregnancy: A new variable predicting severity in haemolytic disease of the fetus and newborn. Br. J. Haematol. 2014, 166, 936–945. [Google Scholar] [CrossRef]
- Peipp, M.; Lammerts van Bueren, J.J.; Schneider-Merck, T.; Bleeker, W.W.K.; Dechant, M.; Beyer, T.; Repp, R.; van Berkel, P.H.C.; Vink, T.; van de Winkel, J.G.J.; et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood 2008, 112, 2390–2399. [Google Scholar] [CrossRef]
- Treffers, L.W.; Van Houdt, M.; Bruggeman, C.W.; Heineke, M.H.; Zhao, X.W.; Van Der Heijden, J.; Nagelkerke, S.Q.; Verkuijlen, P.J.J.H.; Geissler, J.; Lissenberg-Thunnissen, S.; et al. FcγRIIIb restricts antibody-dependent destruction of cancer cells by human neutrophils. Front. Immunol. 2019, 9, 3124. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Gordan, S.; Lux, A. FcgammaR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol. 2015, 36, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Hogarth, P.M.; Pietersz, G.A. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat. Rev. Drug Discov. 2012, 11, 311–331. [Google Scholar] [CrossRef]
- Tang, Y.; Lou, J.; Alpaugh, R.K.; Robinson, M.K.; Marks, J.D.; Weiner, L.M. Regulation of antibody-dependent cellular cytotoxicity by IgG intrinsic and apparent affinity for target antigen. J. Immunol. 2007, 179, 2815–2823. [Google Scholar] [CrossRef]
- Mazor, Y.; Yang, C.; Borrok, M.J.; Ayriss, J.; Aherne, K.; Wu, H.; Dall’Acqua, W.F. Enhancement of Immune Effector Functions by Modulating IgG’s Intrinsic Affinity for Target Antigen. PLoS ONE 2016, 11, e0157788. [Google Scholar] [CrossRef]
- Temming, A.R.; de Taeye, S.W.; de Graaf, E.L.; de Neef, L.A.; Dekkers, G.; Bruggeman, C.W.; Koers, J.; Ligthart, P.; Nagelkerke, S.Q.; Zimring, J.C.; et al. Functional Attributes of Antibodies, Effector Cells, and Target Cells Affecting NK Cell–Mediated Antibody-Dependent Cellular Cytotoxicity. J. Immunol. 2019, 203, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sethuraman, N.; Stadheim, T.A.; Zha, D.; Prinz, B.; Ballew, N.; Bobrowicz, P.; Choi, B.K.; Cook, W.J.; Cukan, M.; et al. Optimization of humanized IgGs in glycoengineeFred Pichia pastoris. Nat. Biotechnol. 2006, 24, 210–215. [Google Scholar] [CrossRef]
- Subedi, G.P.; Barb, A.W. The immunoglobulin G1 N-glycan composition affects binding to each low affinity Fc gamma receptor. MAbs 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Siberil, S.; de Romeuf, C.; Bihoreau, N.; Fernandez, N.; Meterreau, J.L.; Regenman, A.; Nony, E.; Gaucher, C.; Glacet, A.; Jorieux, S.; et al. Selection of a human anti-RhD monoclonal antibody for therapeutic use: Impact of IgG glycosylation on activating and inhibitory Fc gamma R functions. Clin. Immunol. 2006, 118, 170–179. [Google Scholar] [CrossRef]
- Uchida, J.; Hamaguchi, Y.; Oliver, J.A.; Ravetch, J.V.; Poe, J.C.; Haas, K.M.; Tedder, T.F. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J. Exp. Med. 2004, 199, 1659–1669. [Google Scholar] [CrossRef]
- Biburger, M.; Aschermann, S.; Schwab, I.; Lux, A.; Albert, H.; Danzer, H.; Woigk, M.; Dudziak, D.; Nimmerjahn, F. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity 2011, 35, 932–944. [Google Scholar] [CrossRef] [Green Version]
- Montalvao, F.; Garcia, Z.; Celli, S.; Breart, B.; Deguine, J.; Van Rooijen, N.; Bousso, P. The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. J. Clin. Investig. 2013, 123, 5098–5103. [Google Scholar] [CrossRef] [Green Version]
- Albanesi, M.; Mancardi, D.A.; Jönsson, F.; Iannascoli, B.; Fiette, L.; Di Santo, J.P.; Lowell, C.A.; Bruhns, P. Neutrophils mediate antibody-induced antitumor effects in mice. Blood 2013, 122, 3160–3164. [Google Scholar] [CrossRef] [PubMed]
- Boross, P.; Jansen, J.H.M.; van Tetering, G.; Nederend, M.; Brandsma, A.; Meyer, S.; Torfs, E.; van den Ham, H.-J.; Meulenbroek, L.; de Haij, S.; et al. Anti-tumor activity of human IgG1 anti-gp75 TA99 mAb against B16F10 melanoma in human FcgammaRI transgenic mice. Immunol. Lett. 2014, 160, 151–157. [Google Scholar] [CrossRef]
- Leusen, J.H.W. IgA as therapeutic antibody. Mol. Immunol. 2015, 68, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Bakema, J.E.; van Egmond, M. Fc receptor-dependent mechanisms of monoclonal antibody therapy of cancer. Curr. Top. Microbiol. Immunol. 2014, 382, 373–392. [Google Scholar] [CrossRef]
- Aleyd, E.; Heineke, M.H.; van Egmond, M. The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease. Immunol. Rev. 2015, 268, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Van Egmond, M.; Bakema, J.E. Neutrophils as effector cells for antibody-based immunotherapy of cancer. Semin. Cancer Biol. 2012, 23, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Otten, M.A.; Rudolph, E.; Dechant, M.; Tuk, C.W.; Reijmers, R.M.; Beelen, R.H.J.; van de Winkel, J.G.J.; van Egmond, M. Immature Neutrophils Mediate Tumor Cell Killing via IgA but Not IgG Fc Receptors. J. Immunol. 2005, 174, 5472–5480. [Google Scholar] [CrossRef] [Green Version]
- Bevaart, L.; Jansen, M.J.H.; van Vugt, M.J.; Verbeek, J.S.; van de Winkel, J.G.J.; Leusen, J.H.W. The high-affinity IgG receptor, FcgammaRI, plays a central role in antibody therapy of experimental melanoma. Cancer Res. 2006, 66, 1261–1264. [Google Scholar] [CrossRef] [Green Version]
- Otten, M.A.; van der Bij, G.J.; Verbeek, S.J.; Nimmerjahn, F.; Ravetch, J.V.; Beelen, R.H.; van de Winkel, J.G.; van Egmond, M. Experimental antibody therapy of liver metastases reveals functional redundancy between Fc gammaRI and Fc gammaRIV. J. Immunol. 2008, 181, 6829–6836. [Google Scholar] [CrossRef] [Green Version]
- Oosterling, S.J.; van der Bij, G.J.; Meijer, G.A.; Tuk, C.W.; van Garderen, E.; van Rooijen, N.; Meijer, S.; van der Sijp, J.R.; Beelen, R.H.; van Egmond, M. Macrophages direct tumour histology and clinical outcome in a colon cancer model. J. Pathol. 2005, 207, 147–155. [Google Scholar] [CrossRef]
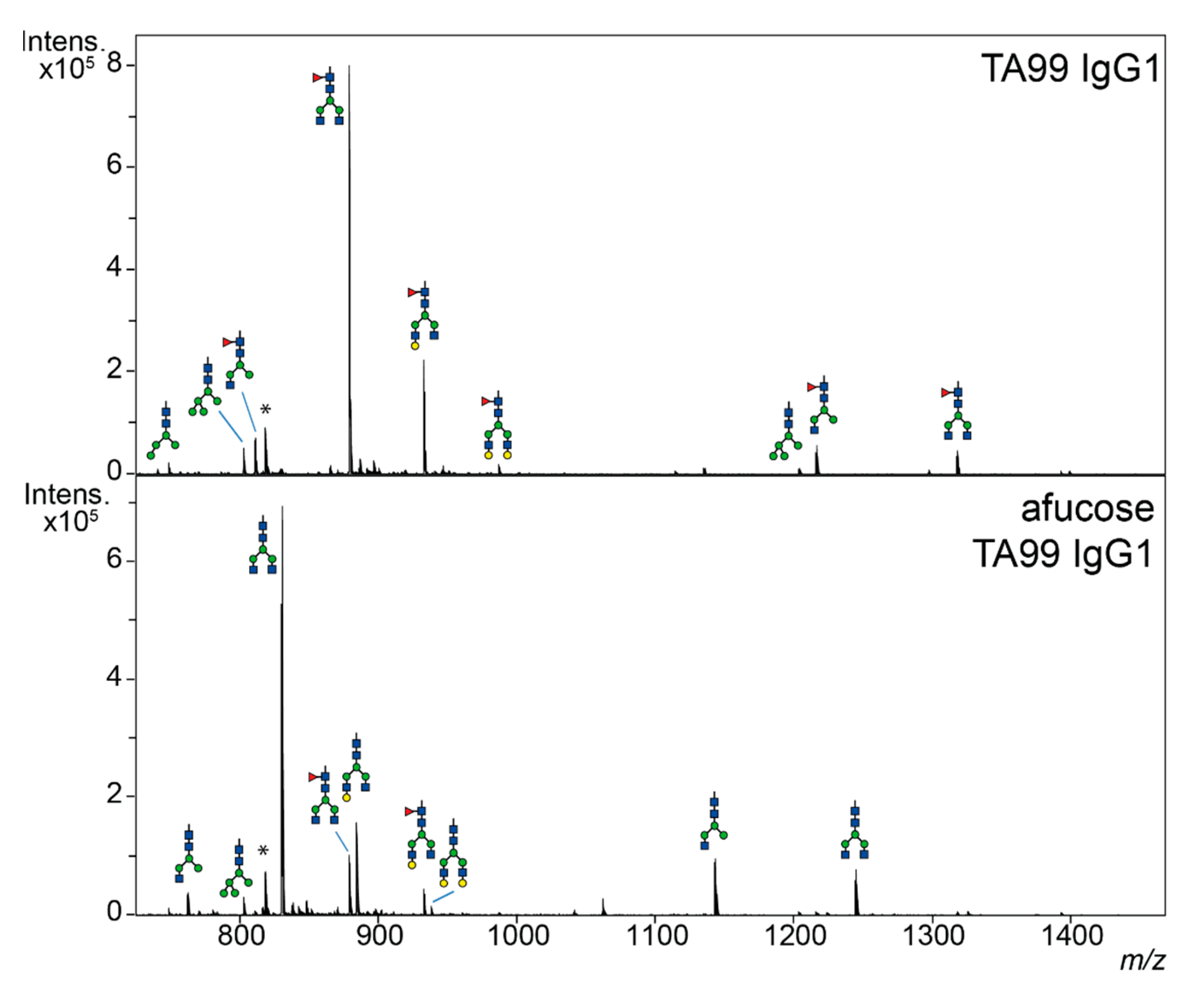


| FcγRI | FcγRII | FcγRIII | FcγRIV | |
|---|---|---|---|---|
| TA99 hIgG1 wt | 1.34 × 10−9 | 0.93 × 10−7 | 2.24 × 10−7 | 8.97 × 10−8 |
| TA99 hIgG1 low fuc | 1.6 × 10−9 | 1.35 × 10−7 | 1.90 × 10−7 | 2.95 × 10−8 |
| Fold change | 0.80 | 0.69 | 1.18 | 3.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braster, R.; Bögels, M.; Benonisson, H.; Wuhrer, M.; Plomp, R.; Bentlage, A.E.H.; Korthouwer, R.; Visser, R.; Verbeek, J.S.; van Egmond, M.; et al. Afucosylated IgG Targets FcγRIV for Enhanced Tumor Therapy in Mice. Cancers 2021, 13, 2372. https://doi.org/10.3390/cancers13102372
Braster R, Bögels M, Benonisson H, Wuhrer M, Plomp R, Bentlage AEH, Korthouwer R, Visser R, Verbeek JS, van Egmond M, et al. Afucosylated IgG Targets FcγRIV for Enhanced Tumor Therapy in Mice. Cancers. 2021; 13(10):2372. https://doi.org/10.3390/cancers13102372
Chicago/Turabian StyleBraster, Rens, Marijn Bögels, Hreinn Benonisson, Manfred Wuhrer, Rosina Plomp, Arthur E. H. Bentlage, Rianne Korthouwer, Remco Visser, J. Sjef Verbeek, Marjolein van Egmond, and et al. 2021. "Afucosylated IgG Targets FcγRIV for Enhanced Tumor Therapy in Mice" Cancers 13, no. 10: 2372. https://doi.org/10.3390/cancers13102372







