Histone Acetyltransferases and Stem Cell Identity
Abstract
:Simple Summary
Abstract
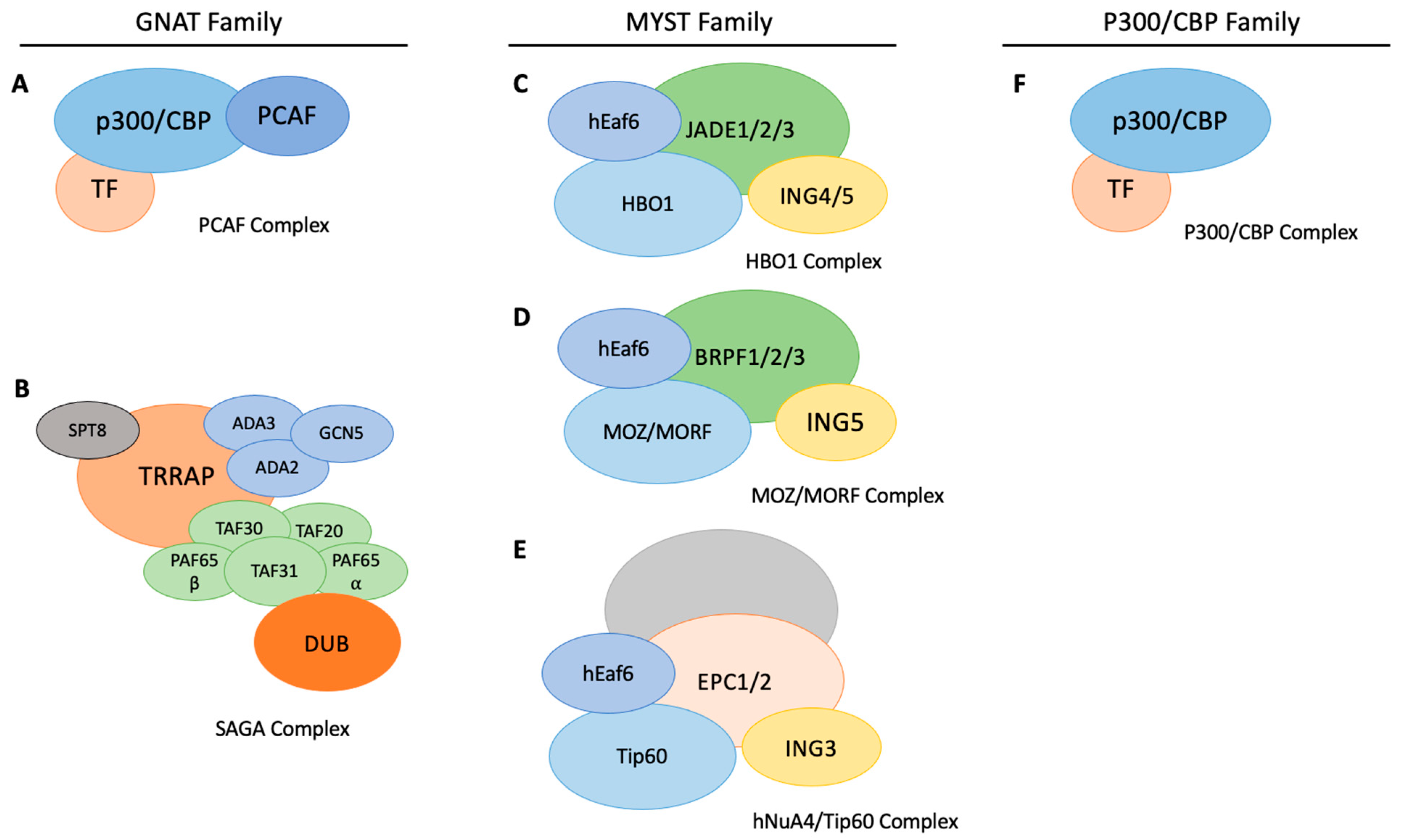
1. Introduction
2. KAT Function in Pluripotent Stem Cells
3. KATs in Hematopoietic Stem Cells
4. KATs in Muscle Satellite Cells
5. KATs in Mesenchymal Stem Cells
6. KATs in Neural Stem Cells
7. KATs in Cancer Stem Cells
8. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gilbert, S.F. Differential Gene Expression. In Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Greenberg, M.V.C.; Bourc’his, D. The Diverse Roles of DNA Methylation in Mammalian Development and Disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cokus, S.J.; Zhang, X.; Chen, P.-Y.; Bostick, M.; Goll, M.G.; Hetzel, J.; Jain, J.; Strauss, S.H.; Halpern, M.E.; et al. Conservation and Divergence of Methylation Patterning in Plants and Animals. Proc. Natl. Acad. Sci. USA 2010, 107, 8689–8694. [Google Scholar] [CrossRef] [Green Version]
- Jansen, A.; Verstrepen, K.J. Nucleosome Positioning in Saccharomyces Cerevisiae. Microbiol. Mol. Biol. Rev. 2011, 75, 301–320. [Google Scholar] [CrossRef] [Green Version]
- Poirier, M.G.; Bussiek, M.; Langowski, J.; Widom, J. Spontaneous Access to DNA Target Sites in Folded Chromatin Fibers. J. Mol. Biol. 2008, 379, 772–786. [Google Scholar] [CrossRef] [Green Version]
- Cabal-Hierro, L.; van Galen, P.; Prado, M.A.; Higby, K.J.; Togami, K.; Mowery, C.T.; Paulo, J.A.; Xie, Y.; Cejas, P.; Furusawa, T.; et al. Chromatin Accessibility Promotes Hematopoietic and Leukemia Stem Cell Activity. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Nalabothula, N.; McVicker, G.; Maiorano, J.; Martin, R.; Pritchard, J.K.; Fondufe-Mittendorf, Y.N. The Chromatin Architectural Proteins HMGD1 and H1 Bind Reciprocally and Have Opposite Effects on Chromatin Structure and Gene Regulation. BMC Genom. 2014, 15, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svaren, J.; Klebanow, E.; Sealy, L.; Chalkley, R. Analysis of the Competition between Nucleosome Formation and Transcription Factor Binding. J. Biol. Chem. 1994, 269, 9335–9344. [Google Scholar] [CrossRef]
- Zaret, K.S.; Carroll, J.S. Pioneer Transcription Factors: Establishing Competence for Gene Expression. Genes Dev. 2011, 25, 2227–2241. [Google Scholar] [CrossRef] [Green Version]
- Bannister, A.J.; Kouzarides, T. Regulation of Chromatin by Histone Modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Allfrey, V.G.; Mirsky, A.E. Structural Modifications of Histones and Their Possible Role in the Regulation of RNA Synthesis. Science 1964, 144, 559. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.; Eisenhaber, F.; O’Carroll, D.; Strahl, B.D.; Sun, Z.W.; Schmid, M.; Opravil, S.; Mechtler, K.; Ponting, C.P.; Allis, C.D.; et al. Regulation of Chromatin Structure by Site-Specific Histone H3 Methyltransferases. Nature 2000, 406, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone Demethylation Mediated by the Nuclear Amine Oxidase Homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, Erasing and Reading Histone Lysine Methylations. Exp. Mol. Med. 2017, 49, e324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, F.S.; Fischl, H.; Murray, S.C.; Mellor, J. Is H3K4me3 Instructive for Transcription Activation? BioEssays 2017, 39, e201600095. [Google Scholar] [CrossRef] [PubMed]
- Wiles, E.T.; Selker, E.U. H3K27 Methylation: A Promiscuous Repressive Chromatin Mark. Curr. Opin. Genet. Dev. 2017, 43, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Gräff, J.; Tsai, L.-H. Histone Acetylation: Molecular Mnemonics on the Chromatin. Nat. Rev. Neurosci. 2013, 14, 97–111. [Google Scholar] [CrossRef]
- Brownell, J.E.; Allis, C.D. Special HATs for Special Occasions: Linking Histone Acetylation to Chromatin Assembly and Gene Activation. Curr. Opin. Genet. Dev. 1996, 6, 176–184. [Google Scholar] [CrossRef]
- Cochran, A.G.; Conery, A.R.; Sims, R.J. Bromodomains: A New Target Class for Drug Development. Nat. Rev. Drug Discov. 2019, 18, 609–628. [Google Scholar] [CrossRef]
- Berndsen, C.E.; Denu, J.M. Catalysis and Substrate Selection by Histone/Protein Lysine Acetyltransferases. Curr. Opin. Struct. Biol. 2008, 18, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The Many Roles of Histone Deacetylases in Development and Physiology: Implications for Disease and Therapy. Nat. Rev. Genet. 2009, 10, 32–42. [Google Scholar] [CrossRef]
- Hodawadekar, S.C.; Marmorstein, R. Chemistry of Acetyl Transfer by Histone Modifying Enzymes: Structure, Mechanism and Implications for Effector Design. Oncogene 2007, 26, 5528–5540. [Google Scholar] [CrossRef] [Green Version]
- Trapp, J.; Jung, M. The Role of NAD+ Dependent Histone Deacetylases (Sirtuins) in Ageing. Curr. Drug Targets 2006, 7, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Barr, E.; Rudnick, D.A. Characterization of the Regulation and Function of Zinc-Dependent Histone Deacetylases During Mouse Liver Regeneration. Hepatology 2013, 57. [Google Scholar] [CrossRef] [Green Version]
- Peleg, S.; Feller, C.; Ladurner, A.G.; Imhof, A. The Metabolic Impact on Histone Acetylation and Transcription in Ageing. Trends Biochem. Sci. 2016, 41, 700–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcea, R.L.; Alberts, B.M. Comparative Studies of Histone Acetylation in Nucleosomes, Nuclei, and Intact Cells. Evidence for Special Factors Which Modify Acetylase Action. J. Biol. Chem. 1980, 255, 11454–11463. [Google Scholar] [CrossRef]
- Marmorstein, R. Structure of Histone Acetyltransferases11Edited by P. W. Wright. J. Mol. Biol. 2001, 311, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, R.; Trievel, R.C. Histone Modifying Enzymes: Structures, Mechanisms, and Specificities. Biochim. Biophys. Acta Gene Regul. Mech. 2009, 1789, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogryzko, V.V. Mammalian Histone Acetyltransferases and Their Complexes. Cell. Mol. Life Sci. 2001, 58, 683–692. [Google Scholar] [CrossRef]
- Sterner, D.E.; Berger, S.L. Acetylation of Histones and Transcription-Related Factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459. [Google Scholar] [CrossRef] [Green Version]
- Shenghui, H.; Nakada, D.; Morrison, S.J. Mechanisms of Stem Cell Self-Renewal. Annu. Rev. Cell Dev. Biol. 2009, 25, 377–406. [Google Scholar] [CrossRef] [Green Version]
- Mitalipov, S.; Wolf, D. Totipotency, Pluripotency and Nuclear Reprogramming. In Engineering of Stem Cells; Martin, U., Ed.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 185–199. ISBN 978-3-540-88806-2. [Google Scholar]
- Ulloa-Montoya, F.; Verfaillie, C.M.; Hu, W.-S. Culture Systems for Pluripotent Stem Cells. J. Biosci. Bioeng. 2005, 100, 12–27. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Tam, W.-L.; Tong, G.Q.; Wu, Q.; Chan, H.-Y.; Soh, B.-S.; Lou, Y.; Yang, J.; Ma, Y.; Chai, L.; et al. Sall4 Modulates Embryonic Stem Cell Pluripotency and Early Embryonic Development by the Transcriptional Regulation of Pou5f1. Nat. Cell Biol. 2006, 8, 1114–1123. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Pandey, R.; Byun, J.S.; Gardner, K.; Qin, Z.; Dou, Y. The Histone Acetyltransferase MOF Is a Key Regulator of the Embryonic Stem Cell Core Transcriptional Network. Cell Stem Cell 2012, 11, 163–178. [Google Scholar] [CrossRef] [Green Version]
- Horne, G.A.; Stewart, H.J.S.; Dickson, J.; Knapp, S.; Ramsahoye, B.; Chevassut, T. Nanog Requires BRD4 to Maintain Murine Embryonic Stem Cell Pluripotency and Is Suppressed by Bromodomain Inhibitor JQ1 Together with Lefty1. Stem Cells Dev. 2015, 24, 879–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardo, M.; Yu, L.; Shen, S.; Tate, P.; Bode, D.; Letney, B.L.; Quelle, D.E.; Skarnes, W.; Choudhary, J.S. Myst2/Kat7 Histone Acetyltransferase Interaction Proteomics Reveals Tumour-Suppressor Niam as a Novel Binding Partner in Embryonic Stem Cells. Sci. Rep. 2017, 7, 8157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Cho, H.I.; Park, S.H.; Kim, J.H.; Chai, Y.G.; Jang, Y.K. The Histone Acetyltransferase Myst2 Regulates Nanog Expression, and Is Involved in Maintaining Pluripotency and Self-Renewal of Embryonic Stem Cells. FEBS Lett. 2015, 589, 941–950. [Google Scholar] [CrossRef]
- Chen, P.B.; Hung, J.-H.; Hickman, T.L.; Coles, A.H.; Carey, J.F.; Weng, Z.; Chu, F.; Fazzio, T.G. Hdac6 Regulates Tip60-P400 Function in Stem Cells. eLife 2013, 2. [Google Scholar] [CrossRef]
- Fazzio, T.G.; Huff, J.T.; Panning, B. Chromatin Regulation Tip(60)s the Balance in Embryonic Stem Cell Self-Renewal. Cell Cycle 2008, 7, 3302–3306. [Google Scholar] [CrossRef] [Green Version]
- Fazzio, T.G.; Huff, J.T.; Panning, B. An RNAi Screen of Chromatin Proteins Identifies Tip60-P400 as a Regulator of Embryonic Stem Cell Identity. Cell 2008, 134, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Moris, N.; Edri, S.; Seyres, D.; Kulkarni, R.; Domingues, A.F.; Balayo, T.; Frontini, M.; Pina, C. Histone Acetyltransferase KAT2A Stabilizes Pluripotency with Control of Transcriptional Heterogeneity. Stem Cells 2018, 36, 1828–1838. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Xu, Y.; Chew, K.-K.; Chen, X.; Ng, H.-H.; Matsudaira, P. Coactivators P300 and CBP Maintain the Identity of Mouse Embryonic Stem Cells by Mediating Long-Range Chromatin Structure. Stem Cells 2014, 32, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Cope, M.; Sidoli, S.; Bhanu, N.V.; Won, K.-J.; Garcia, B.A. Histone H4 Acetylation and the Epigenetic Reader Brd4 Are Critical Regulators of Pluripotency in Embryonic Stem Cells. BMC Genom. 2016, 17, 95. [Google Scholar] [CrossRef]
- Katsumoto, T.; Aikawa, Y.; Iwama, A.; Ueda, S.; Ichikawa, H.; Ochiya, T.; Kitabayashi, I. MOZ Is Essential for Maintenance of Hematopoietic Stem Cells. Genes Dev. 2006, 20, 1321–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, T.; Corcoran, L.M.; Gugasyan, R.; Dixon, M.P.; Brodnicki, T.; Nutt, S.L.; Metcalf, D.; Voss, A.K. Monocytic Leukemia Zinc Finger Protein Is Essential for the Development of Long-Term Reconstituting Hematopoietic Stem Cells. Genes Dev. 2006, 20, 1175–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Campo, F.M.; Costa, G.; Lie-A-Ling, M.; Stifani, S.; Kouskoff, V.; Lacaud, G. MOZ-Mediated Repression of P16(INK) (4) (a) Is Critical for the Self-Renewal of Neural and Hematopoietic Stem Cells. Stem Cells 2014, 32, 1591–1601. [Google Scholar] [CrossRef] [Green Version]
- Khokhar, E.S.; Borikar, S.; Eudy, E.; Stearns, T.; Young, K.; Trowbridge, J.J. Aging-Associated Decrease in the Histone Acetyltransferase KAT6B Is Linked to Altered Hematopoietic Stem Cell Differentiation. Exp. Hematol. 2020, 82, 43–52.e4. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [Green Version]
- Hamed, M.; Khilji, S.; Chen, J.; Li, Q. Stepwise Acetyltransferase Association and Histone Acetylation at the Myod1 Locus during Myogenic Differentiation. Sci. Rep. 2013, 3, 2390. [Google Scholar] [CrossRef] [Green Version]
- Puri, P.L.; Sartorelli, V.; Yang, X.J.; Hamamori, Y.; Ogryzko, V.V.; Howard, B.H.; Kedes, L.; Wang, J.Y.; Graessmann, A.; Nakatani, Y.; et al. Differential Roles of P300 and PCAF Acetyltransferases in Muscle Differentiation. Mol. Cell 1997, 1, 35–45. [Google Scholar] [CrossRef]
- Cao, Y.; Yao, Z.; Sarkar, D.; Lawrence, M.; Sanchez, G.J.; Parker, M.H.; MacQuarrie, K.L.; Davison, J.; Morgan, M.T.; Ruzzo, W.L.; et al. Genome-Wide MyoD Binding in Skeletal Muscle Cells: A Potential for Broad Cellular Reprogramming. Dev. Cell 2010, 18, 662–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Jiao, F.; Wang, J.; Dong, Z.-L.; Wu, M.-J.; Zhao, T.-B.; Li, D.-D.; Wang, X. Human Mesenchymal Stem Cells Derived from Limb Bud Can Differentiate into All Three Embryonic Germ Layers Lineages. Cell. Reprogram. 2012, 14, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Riekstina, U.; Muceniece, R.; Cakstina, I.; Muiznieks, I.; Ancans, J. Characterization of Human Skin-Derived Mesenchymal Stem Cell Proliferation Rate in Different Growth Conditions. Cytotechnology 2008, 58, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W.; et al. Comparative Characteristics of Mesenchymal Stem Cells from Human Bone Marrow, Adipose Tissue, and Umbilical Cord Blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-S.; Hung, S.-C.; Peng, S.-T.; Huang, C.-C.; Wei, H.-M.; Guo, Y.-J.; Fu, Y.-S.; Lai, M.-C.; Chen, C.-C. Mesenchymal Stem Cells in the Wharton’s Jelly of the Human Umbilical Cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Liu, Y.; Jin, C.; Zhang, M.; Tang, F.; Zhou, Y. Histone Acetyltransferase GCN5 Regulates Osteogenic Differentiation of Mesenchymal Stem Cells by Inhibiting NF-ΚB. J. Bone Miner. Res. 2016, 31, 391–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Q.; Xu, H.; Yang, K.; Wang, Y.; Tan, B.; Tian, J.; Zhu, J. Islet-1 Induces the Differentiation of Mesenchymal Stem Cells into Cardiomyocyte-like Cells through the Regulation of Gcn5 and DNMT-1. Mol. Med. Rep. 2017, 15, 2511–2520. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhu, J.; Tian, J. Effects of histone acetylation on differentiation of mesenchymal stem cells. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2008, 25, 159–163. (In Chinese) [Google Scholar] [PubMed]
- Zhang, P.; Liu, Y.; Jin, C.; Zhang, M.; Lv, L.; Zhang, X.; Liu, H.; Zhou, Y. Histone H3K9 Acetyltransferase PCAF Is Essential for Osteogenic Differentiation Through Bone Morphogenetic Protein Signaling and May Be Involved in Osteoporosis. Stem Cells 2016, 34, 2332–2341. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Wang, Y.; Zhang, M.; Lv, L.; Zhang, X.; Zhou, Y. SIRT6 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells through BMP Signaling. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okano, H.; Temple, S. Cell Types to Order: Temporal Specification of CNS Stem Cells. Curr. Opin. Neurobiol. 2009, 19, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Moore, D. Neural Stem Cells: Developmental Mechanisms and Disease Modeling. Cell Tissue Res. 2018, 371, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoof, M.; Launspach, M.; Holdhof, D.; Nguyen, L.; Engel, V.; Filser, S.; Peters, F.; Immenschuh, J.; Hellwig, M.; Niesen, J.; et al. The Transcriptional Coactivator and Histone Acetyltransferase CBP Regulates Neural Precursor Cell Development and Migration. Acta Neuropathol. Commun. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, L.; Yan, K.; Zhou, J.; Zhao, H.; Bertos, N.R.; Park, M.; Wang, E.; Yang, X.-J. The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors. PLoS Genet. 2015, 11. [Google Scholar] [CrossRef] [Green Version]
- Merson, T.D.; Dixon, M.P.; Collin, C.; Rietze, R.L.; Bartlett, P.F.; Thomas, T.; Voss, A.K. The Transcriptional Coactivator Querkopf Controls Adult Neurogenesis. J. Neurosci. 2006, 26, 11359–11370. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Jiao, J. Histone Variant H3.3 Orchestrates Neural Stem Cell Differentiation in the Developing Brain. Cell Death Differ. 2017, 24, 1548–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, B.N.; Dixon, M.P.; Thomas, T.; Voss, A.K. Querkopf Is a Key Marker of Self-Renewal and Multipotency of Adult Neural Stem Cells. J. Cell Sci. 2012, 125, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, M.S.; Oses, C.; Echegaray, C.V.; Solari, C.; Waisman, A.; Álvarez, Y.; Petrone, M.V.; Francia, M.; Schultz, M.; Sevlever, G.; et al. Kat6b Modulates Oct4 and Nanog Binding to Chromatin in Embryonic Stem Cells and Is Required for Efficient Neural Differentiation. J. Mol. Biol. 2019, 431, 1148–1159. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, R.; Yang, X.; Tang, K.; Jing, N. Dual Roles of Histone H3 Lysine 9 Acetylation in Human Embryonic Stem Cell Pluripotency and Neural Differentiation. J. Biol. Chem. 2015, 290, 2508–2520. [Google Scholar] [CrossRef] [Green Version]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A Cell Initiating Human Acute Myeloid Leukaemia after Transplantation into SCID Mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective Identification of Tumorigenic Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-Cell RNA-Seq Highlights Intratumoral Heterogeneity in Primary Glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, X.; Jörg, D.J.; Cavalli, F.M.G.; Richards, L.M.; Nguyen, L.V.; Vanner, R.J.; Guilhamon, P.; Lee, L.; Kushida, M.M.; Pellacani, D.; et al. Fate Mapping of Human Glioblastoma Reveals an Invariant Stem Cell Hierarchy. Nature 2017, 549, 227–232. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, L.; Anokye, J.; Yeung, M.M.; Lam, E.Y.N.; Chan, Y.-C.; Weng, C.-F.; Yeh, P.; Knezevic, K.; Butler, M.S.; Hoegl, A.; et al. HBO1 Is Required for the Maintenance of Leukaemia Stem Cells. Nature 2020, 577, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Ayton, P.M.; Carapeti, M.; Kutok, J.L.; Snyder, C.S.; Williams, I.R.; Cross, N.C.P.; Glass, C.K.; Cleary, M.L.; Gilliland, D.G. MOZ-TIF2-Induced Acute Myeloid Leukemia Requires the MOZ Nucleosome Binding Motif and TIF2-Mediated Recruitment of CBP. Cancer Cell 2003, 3, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Di Martile, M.; Desideri, M.; De Luca, T.; Gabellini, C.; Buglioni, S.; Eramo, A.; Sette, G.; Milella, M.; Rotili, D.; Mai, A.; et al. Histone Acetyltransferase Inhibitor CPTH6 Preferentially Targets Lung Cancer Stem-like Cells. Oncotarget 2016, 7, 11332–11348. [Google Scholar] [CrossRef] [Green Version]
- Park, W.J.; Ma, E. Inhibition of PCAF Histone Acetyltransferase, Cytotoxicity and Cell Permeability of 2-Acylamino-1-(3- or 4-Carboxy-Phenyl)Benzamides. Molecules 2012, 17, 13116–13131. [Google Scholar] [CrossRef]
- Baell, J.B.; Leaver, D.J.; Hermans, S.J.; Kelly, G.L.; Brennan, M.S.; Downer, N.L.; Nguyen, N.; Wichmann, J.; McRae, H.M.; Yang, Y.; et al. Inhibitors of Histone Acetyltransferases KAT6A/B Induce Senescence and Arrest Tumour Growth. Nature 2018, 560, 253–257. [Google Scholar] [CrossRef]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting Epigenetic Regulators for Cancer Therapy: Mechanisms and Advances in Clinical Trials. Signal Transduct. Target. Ther. 2019, 4, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanyam, K.; Swaminathan, V.; Ranganathan, A.; Kundu, T.K. Small Molecule Modulators of Histone Acetyltransferase P300. J. Biol. Chem. 2003, 278, 19134–19140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grazzini, R.; Hesk, D.; Heininger, E.; Hildenbrandt, G.; Reddy, C.C.; Cox-Foster, D.; Medford, J.; Craig, R.; Mumma, R.O. Inhibition of Lipoxygenase and Prostaglandin Endoperoxide Synthase by Anacardic Acids. Biochem. Biophys. Res. Commun. 1991, 176, 775–780. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Hori, I.; Yokokawa, Y. Tyrosinase Inhibitors from Anacardium Occidentale Fruits. J. Nat. Prod. 1994, 57, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Cho, Y.S.; Park, E.J.; Kim, J.; Oh, W.K.; Lee, H.S.; Ahn, J.S. Phospholipase Cγ1 Inhibitory Principles from the Sarcotestas of Ginkgo Biloba. J. Nat. Prod. 1998, 61, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Kubo, J.; Lee, J.R.; Kubo, I. Anti-Helicobacter Pylori Agents from the Cashew Apple. J. Agric. Food Chem. 1999, 47, 533–537. [Google Scholar] [CrossRef]
- Kubo, I.; Nitoda, T.; Tocoli, F.E.; Green, I.R. Multifunctional Cytotoxic Agents from Anacardium Occidentale. Phytother. Res. 2011, 25, 38–45. [Google Scholar] [CrossRef]
- Wisastra, R.; Kok, P.A.M.; Eleftheriadis, N.; Baumgartner, M.P.; Camacho, C.J.; Haisma, H.J.; Dekker, F.J. Discovery of a Novel Activator of 5-Lipoxygenase from an Anacardic Acid Derived Compound Collection. Bioorg. Med. Chem. 2013, 21, 7763–7778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghizzoni, M.; Boltjes, A.; de Graaf, C.; Haisma, H.J.; Dekker, F.J. Improved Inhibition of the Histone Acetyltransferase PCAF by an Anacardic Acid Derivative. Bioorg. Med. Chem. 2010, 18, 5826–5834. [Google Scholar] [CrossRef] [PubMed]
- Ghizzoni, M.; Wu, J.; Gao, T.; Haisma, H.J.; Dekker, F.J.; Zheng, Y.G. 6-Alkylsalicylates Are Selective Tip60 Inhibitors and Target the Acetyl-CoA Binding Site. Eur. J. Med. Chem. 2012, 47, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Bosch, T.; Leus, N.G.J.; Wapenaar, H.; Boichenko, A.; Hermans, J.; Bischoff, R.; Haisma, H.J.; Dekker, F.J. A 6-Alkylsalicylate Histone Acetyltransferase Inhibitor Inhibits Histone Acetylation and pro-Inflammatory Gene Expression in Murine Precision-Cut Lung Slices. Pulm. Pharmacol. Ther. 2017, 44, 88–95. [Google Scholar] [CrossRef]
- Srivastava, G.; Mehta, J.L. Currying the Heart: Curcumin and Cardioprotection. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 22–27. [Google Scholar] [CrossRef]
- Coban, D.; Milenkovic, D.; Chanet, A.; Khallou-Laschet, J.; Sabbe, L.; Palagani, A.; Berghe, W.V.; Mazur, A.; Morand, C. Dietary Curcumin Inhibits Atherosclerosis by Affecting the Expression of Genes Involved in Leukocyte Adhesion and Transendothelial Migration. Mol. Nutr. Food Res. 2012, 56, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Maradana, M.R.; Thomas, R.; O’Sullivan, B.J. Targeted Delivery of Curcumin for Treating Type 2 Diabetes. Mol. Nutr. Food Res. 2013, 57, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Sun, Y.; Huang, K.; Zheng, L. Curcumin, a Potential Therapeutic Candidate for Retinal Diseases. Mol. Nutr. Food Res. 2013, 57, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Pradhan, S.K.; Thanuja, G.R.; Vedamurthy, B.M.; Agrawal, S.; Dasgupta, D.; Kundu, T.K. Mechanism of P300 Specific Histone Acetyltransferase Inhibition by Small Molecules. J. Med. Chem. 2009, 52, 267–277. [Google Scholar] [CrossRef]
- Shin, I.-S.; Kim, J.-M.; Kim, K.L.; Jang, S.Y.; Jeon, E.-S.; Choi, S.H.; Kim, D.-K.; Suh, W.; Kim, Y.-W. Early Growth Response Factor-1 Is Associated With Intraluminal Thrombus Formation in Human Abdominal Aortic Aneurysm. J. Am. Coll. Cardiol. 2009, 53, 792–799. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Wang, Z.; Ali, R.; Maitah, M.Y.; Kong, D.; Banerjee, S.; Padhye, S.; Sarkar, F.H. Apoptosis-Inducing Effect of Garcinol Is Mediated by NF-KappaB Signaling in Breast Cancer Cells. J. Cell Biochem. 2010, 109, 1134–1141. [Google Scholar] [CrossRef]
- Saadat, N.; Gupta, S.V. Potential Role of Garcinol as an Anticancer Agent. Available online: https://www.hindawi.com/journals/jo/2012/647206/ (accessed on 6 March 2021).
- Liu, C.; Ho, P.C.-L.; Wong, F.C.; Sethi, G.; Wang, L.Z.; Goh, B.C. Garcinol: Current Status of Its Anti-Oxidative, Anti-Inflammatory and Anti-Cancer Effects. Cancer Lett. 2015, 362, 8–14. [Google Scholar] [CrossRef]
- Aggarwal, S.; Das, S.N. Garcinol Inhibits Tumour Cell Proliferation, Angiogenesis, Cell Cycle Progression and Induces Apoptosis via NF-ΚB Inhibition in Oral Cancer. Tumor Biol. 2016, 37, 7175–7184. [Google Scholar] [CrossRef]
- Mantelingu, K.; Reddy, B.A.A.; Swaminathan, V.; Kishore, A.H.; Siddappa, N.B.; Kumar, G.V.P.; Nagashankar, G.; Natesh, N.; Roy, S.; Sadhale, P.P.; et al. Specific Inhibition of P300-HAT Alters Global Gene Expression and Represses HIV Replication. Chem. Biol. 2007, 14, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.-C.; Park, S.; Lim, B.J.; Seong, A.-R.; Sung, A.-R.; Lee, Y.-H.; Shiota, M.; Yokomizo, A.; Naito, S.; Na, Y.; et al. Procyanidin B3, an Inhibitor of Histone Acetyltransferase, Enhances the Action of Antagonist for Prostate Cancer Cells via Inhibition of P300-Dependent Acetylation of Androgen Receptor. Biochem. J. 2011, 433, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Seong, A.-R.; Yoo, J.-Y.; Choi, K.; Lee, M.-H.; Lee, Y.-H.; Lee, J.; Jun, W.; Kim, S.; Yoon, H.-G. Delphinidin, a Specific Inhibitor of Histone Acetyltransferase, Suppresses Inflammatory Signaling via Prevention of NF-ΚB Acetylation in Fibroblast-like Synoviocyte MH7A Cells. Biochem. Biophys. Res. Commun. 2011, 410, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, S.; Tomura, A.; Ikeda, N.; Hatano, M.; Odanaka, J.; Kubota, Y.; Umekita, M.; Igarashi, M.; Sawa, R.; Morino, T. Discovery and Characterization of NK13650s, Naturally Occurring P300-Selective Histone Acetyltransferase Inhibitors. J. Org. Chem. 2012, 77, 9044–9052. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Bourke, E.; Scobie, M.; Famme, M.A.; Koolmeister, T.; Helleday, T.; Eriksson, L.A.; Lowndes, N.F.; Brown, J.A.L. Rational Design and Validation of a Tip60 Histone Acetyltransferase Inhibitor. Sci. Rep. 2014, 4, 5372. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.P.; Robaa, D.; Alhalabi, Z.; Sippl, W.; Jung, M. KATching-Up on Small Molecule Modulators of Lysine Acetyltransferases. J. Med. Chem. 2016, 59, 1249–1270. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Cassel, R.; Schneider-Anthony, A.; Merienne, K.; Cosquer, B.; Tzeplaeff, L.; Sinha, S.H.; Kumar, M.; Chaturbedy, P.; Eswaramoorthy, M.; et al. Reinstating Plasticity and Memory in a Tauopathy Mouse Model with an Acetyltransferase Activator. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Mulder, K.W.; Wang, X.; Escriu, C.; Ito, Y.; Schwarz, R.F.; Gillis, J.; Sirokmány, G.; Donati, G.; Uribe-Lewis, S.; Pavlidis, P.; et al. Diverse Epigenetic Strategies Interact to Control Epidermal Differentiation. Nat. Cell Biol. 2012, 14, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, A.Y.; Chesnelong, C.; Yang, Y.; Nabbi, A.; Thalappilly, S.; Alekseev, V.; Riabowol, K. ING5 Activity in Self-Renewal of Glioblastoma Stem Cells via Calcium and Follicle Stimulating Hormone Pathways. Oncogene 2018, 37, 286–301. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [Green Version]
| Name on Original Publication | Inhibited Complex | Biological Effects Noted | Reference |
|---|---|---|---|
| Anacardic acid | p300 PCAF | Targeted tyrosinase, tissue factor VIIa, xanthine oxidase, phosphatidylinositol-specific phospholipase C, lipoxygenase, and cyclooxygenase | [83,84,85,86,87,88,89] |
| MG149 | Tip60 MOF p300/PCAF | Inhibited the expression of pro-inflammatory genes | [90,91,92] |
| 2-acylamino-1-(3- or 4-carboxyphenyl)benzamides (small molecule inhibitors) | PCAF | Cytotoxic to several cancer cell lines | [80] |
| Curcumin | p300/CBP | Was capable of alleviating ventricular hypertrophy, microangiopathy and heart failure | [93,94,95,96] |
| Garcinol | P300 PCAF | Takes part in the response to oxidative-stress inflammatory and apoptosis processes, regulation of early growth response protein 1 (EGR-1), proliferation, metastasis and angiogenesis, and in addition epigenetic pathways | [83,97,98,99,100,101,102] |
| LTK-14 | P300 | Inhibited the multiplication of the HIV virus while being nontoxic for T-cells. | [103] |
| Pro-B3 | p300 60% inhibition Tip60/PCAF/CBP < 40% inhibition | Inhibition of the activation of the androgen receptor (AR) by p300-mediated both in vitro and in vivo; resulted in the suppression of prostate cancer cell growth | [104] |
| Delphinidin | P300 | The inhibition of expression of inflammatory cytokines in MH7A cells along with the release of cytokines in Jurkat T lymphocyte cell line. | [105] |
| NK13650A | P300 | Supressed the transcription of the AR and slowed the growth of prostate cancer cells | [106] |
| WM-8014 | KAT6A | Inhibited cell proliferation and induced senescence | [81] |
| TH1834 | Tip60 | Induced DNA damage and cell cycle dysregulation into cancer cells. | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Dantas, A.; Riabowol, K. Histone Acetyltransferases and Stem Cell Identity. Cancers 2021, 13, 2407. https://doi.org/10.3390/cancers13102407
He R, Dantas A, Riabowol K. Histone Acetyltransferases and Stem Cell Identity. Cancers. 2021; 13(10):2407. https://doi.org/10.3390/cancers13102407
Chicago/Turabian StyleHe, Ruicen, Arthur Dantas, and Karl Riabowol. 2021. "Histone Acetyltransferases and Stem Cell Identity" Cancers 13, no. 10: 2407. https://doi.org/10.3390/cancers13102407
APA StyleHe, R., Dantas, A., & Riabowol, K. (2021). Histone Acetyltransferases and Stem Cell Identity. Cancers, 13(10), 2407. https://doi.org/10.3390/cancers13102407






