Influence of Postoperative Changes in Sarcopenia on Long-Term Survival in Non-Metastatic Colorectal Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Treatment and Surveillance
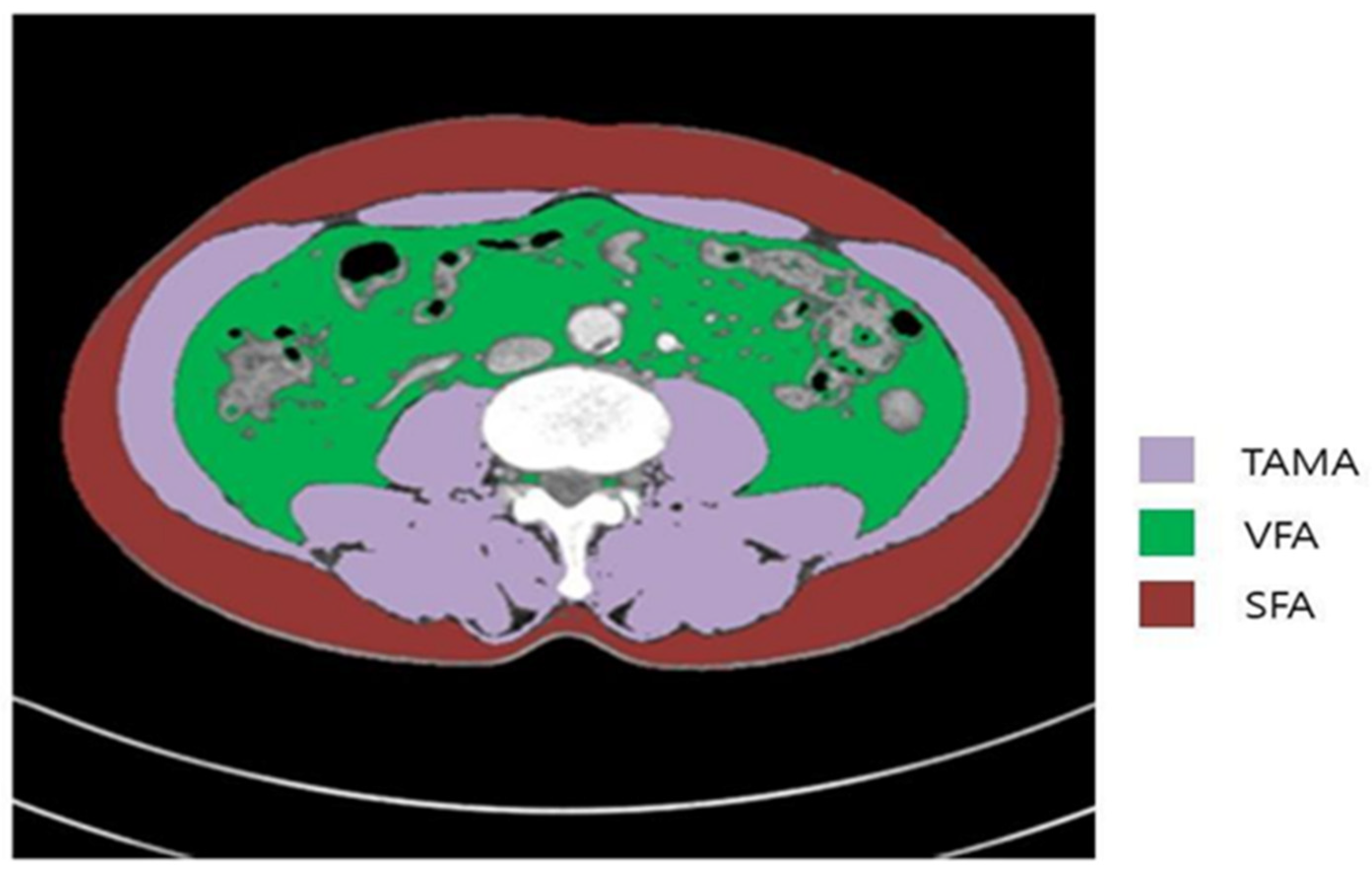
2.3. Measurements and Definitions of Body Composition Parameters
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Changes in SMI during Surveillance
3.3. Oncologic Outcomes According to Sarcopenia Status and its Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heymsfield, S.B.; Wang, Z.M.; Visser, M.; Gallagher, D.; Pierson, R.N., Jr. Techniques Used in the Measurement of Body Composition: An Overview with Emphasis on Bioelectrical Impedance Analysis. Am. J. Clin. Nutr. 1996, 64, 478S–484S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Malietzis, G.; Aziz, O.; Bagnall, N.M.; Johns, N.; Fearon, K.C.; Jenkins, J.T. The Role of Body Composition Evaluation by Computerized Tomography in Determining Colorectal Cancer Treatment Outcomes: A Systematic Review. Eur. J. Surg. Oncol. 2015, 41, 186–196. [Google Scholar] [CrossRef]
- van der Kroft, G.; Bours, D.M.J.L.; Janssen-Heijnen, D.M.; van Berlo, D.C.L.H.; Konsten, D.J.L.M. Value of Sarcopenia Assessed by Computed Tomography for the Prediction of Postoperative Morbidity Following Oncological Colorectal Resection: A Comparison with the Malnutrition Screening Tool. Clin. Nutr. ESPEN 2018, 24, 114–119. [Google Scholar] [CrossRef]
- Gibson, D.J.; Burden, S.T.; Strauss, B.J.; Todd, C.; Lal, S. The Role of Computed Tomography in Evaluating Body Composition and the Influence of Reduced Muscle Mass on Clinical Outcome in Abdominal Malignancy: A Systematic Review. Eur. J. Clin. Nutr. 2015, 69, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, M.; Yasuno, M.; Ishiguro, M.; Yamauchi, S.; Kikuchi, A.; Tokura, M.; Ishikawa, T.; Nakatani, E.; Uetake, H. Morphologic Change of the Psoas Muscle as a Surrogate Marker of Sarcopenia and Predictor of Complications After Colorectal Cancer Surgery. Int. J. Colorectal Dis. 2017, 32, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T. Perioperative Body Composition Changes in the Multimodal Treatment of Gastrointestinal Cancer. Surg. Today 2020, 50, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Yoon, S.B.; Lee, K.; Song, M.; Lee, I.S.; Lee, M.A.; Hong, T.H.; Choi, M.G. Preoperative Sarcopenia and Post-operative Accelerated Muscle Loss Negatively Impact Survival After Resection of Pancreatic Cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 326–334. [Google Scholar] [CrossRef]
- Boshier, P.R.; Heneghan, R.; Markar, S.R.; Baracos, V.E.; Low, D.E. Assessment of Body Composition and Sarcopenia in Patients with Esophageal Cancer: A Systematic Review and Meta-Analysis. Dis. Esophagus 2018, 31, 1–11. [Google Scholar]
- Hur, H.; Oh, C.M.; Won, Y.J.; Oh, J.H.; Kim, N.K. Characteristics and Survival of Korean Patients with Colorectal Cancer Based on Data From the Korea Central Cancer Registry Data. Ann. Coloproctol. 2018, 34, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, R.; Oki, E.; Sasaki, S.; Hirose, K.; Jogo, T.; Edahiro, K.; Korehisa, S.; Taniguchi, D.; Kudo, K.; Kurashige, J.; et al. Sarcopenia Is an Independent Predictor of Complications After Colorectal Cancer Surgery. Surg. Today 2018, 48, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ozoya, O.O.; Siegel, E.M.; Srikumar, T.; Bloomer, A.M.; DeRenzis, A.; Shibata, D. Quantitative Assessment of Visceral Obesity and Postoperative Colon Cancer Outcomes. J. Gastrointest. Surg. 2017, 21, 534–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, J.J.; Reif, R.; Bigam, D.; Baracos, V.E.; Eurich, D.T.; Sawyer, M.M. Change in Skeletal Muscle Following Resection of Stage I–III Colorectal Cancer Is Predictive of Poor Survival: A Cohort Study. World J. Surg. 2019, 43, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of Systemic Inflammation and Sarcopenia with Survival in NonMetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- Han, J.S.; Ryu, H.; Park, I.J.; Kim, K.W.; Shin, Y.; Kim, S.O.; Lim, S.B.; Kim, C.W.; Yoon, Y.S.; Lee, J.L.; et al. Association of Body Composition with Long-Term Survival in Non-Metastatic Rectal Cancer Patients. Cancer Res. Treat. 2020, 52, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Baba, Y.; Sakamoto, Y.; Ohuchi, M.; Tokunaga, R.; Kurashige, J.; Hiyoshi, Y.; Iwagami, S.; Yoshida, N.; Yoshida, M.; et al. Sarcopenia Is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann. Surg. Oncol. 2015, 22, 2663–2668. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Nakamura, T.; Yamane, M.; Islam, A.H.; Menju, M.; Yamasaki, K.; Arai, T.; Kotani, K.; Funahashi, T.; Yamashita, S.; et al. Abdominal Fat: Standardized Technique for Measurement at CT. Radiology 1999, 211, 283–286. [Google Scholar] [CrossRef]
- Zarinsefat, A.; Terjimanian, M.N.; Sheetz, K.H.; Stein, I.C.; Mazurek, A.A.; Waits, S.A.; Sullivan, J.A.; Wang, S.C.; Englesbe, M.J. Perioperative Changes in Trunk Musculature and Postoperative Outcomes. J. Surg. Res. 2014, 191, 106–112. [Google Scholar] [CrossRef]
- Deng, C.Y.; Lin, Y.C.; Wu, J.S.; Cheung, Y.C.; Fan, C.W.; Yeh, K.Y.; McMahon, C.J. Progressive Sarcopenia in Patients with Colorectal Cancer Predicts Survival. Am. J. Roentgenol. 2018, 210, 526–532. [Google Scholar] [CrossRef]
- Kurk, S.A.; Peeters, P.H.M.; Dorresteijn, B.; de Jong, P.A.; Jourdan, M.; Creemers, G.M.; Erdkamp, F.L.G.; de Jongh, F.E.; Kint, P.A.M.; Poppema, B.J.; et al. Loss of Skeletal Muscle Index and Survival in Patients with Metastatic Colorectal Cancer: Secondary Analysis of the phase 3 CAIRO3 Trial. Cancer Med. 2020, 9, 1033–1043. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, T.; Feng, D.; Dai, X.; Lv, T.; Wnag, X.; Gong, J.; Zhu, W.; Li, J. A new diagnostic index for sarcopenia and its association with short-term postoperative complications in patients undergoing surgery for colorectal cancer. Colorectal Dis. 2019, 21, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Caan, B.J.; CespedesFeliciano, E.M.; Meyerhardt, J.A.; Peng, P.D.; Baracos, V.E.; Lee, V.S.; Ely, S.; Gologorsky, R.C.; Weltzien, E.; et al. Association of Low Muscle Mass and Low Muscle Radiodensity with Morbidity and Mortality for Colon Cancer Surgery. JAMA Surg. 2020, 155, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.J.G.; Senadeera, S.C.; Fruzekke, F.A. Sarcopenia, as Assessed by Psoas Cross-Sectional Area, Is Predictive of Adverse Postoperative Outcomes in Patients Undergoing Colorectal Cancer Surgery. Dis. Colon Rectum 2020, 63, 807–815. [Google Scholar] [CrossRef]
- Lau, E.M.; Lynn, H.S.; Woo, J.W.; Kwok, T.C.; Melton, L.J., III. Prevalence of and Risk Factors for Sarcopenia in Elderly Chinese Men and Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Kim, K.W.; Shin, Y.; Lee, J.; Ko, Y.; Kim, Y.J.; Lee, M.J.; Bae, S.J.; Park, S.W.; Choe, J.; et al. Reference Data and T-Scores of Lumbar Skeletal Muscle Area and Its Skeletal Muscle Indices Measured by CT Scan in a Healthy Korean Population. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 265–271. [Google Scholar] [CrossRef]
- Matsuoka, K.; Yamada, T.; Matsuoka, T.; Nagai, S.; Ueda, M.; Miyamoto, Y. Significance of Body Mass Index for Postoperative Outcomes After Lung Cancer Surgery in Elderly Patients. World J. Surg. 2018, 42, 153–160. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Neugebauer, R.; Meyerhardt, J.; Prado, C.M.; Weltzien, E.; Kwan, M.L.; Xiao, J.; Caan, B.J. Analysis of Body Mass Index and Mortality in Patients with Colorectal Cancer Using Causal Diagrams. JAMA Oncol. 2016, 2, 1137–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cakir, H.; Heus, C.; Verduin, W.M.; Lak, A.; Doodeman, H.J.; Bemelman, W.A.; Houdijk, A.P. Visceral Obesity, Body Mass Index and Risk of Complications After Colon Cancer Resection: A Retrospective Cohort Study. Surgery 2015, 157, 909–915. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, H.L.; Doo, E.Y.; Lee, K.N.; Jun, D.W.; Lee, O.Y.; Han, D.S.; Yoon, B.C.; Choi, H.S.; Lee, K.H. Visceral Obesity Predicts Fewer Lymph Node Metastases and Better Overall Survival in Colon Cancer. J. Gastrointest. Surg. 2015, 19, 1513–1521. [Google Scholar] [CrossRef] [Green Version]
- van Roekel, E.H.; Bours, M.J.L.; Te Molder, M.E.M.; Breedveld-Peters, J.J.L.; Olde Damink, S.W.M.; Schouten, L.J.; Sanduleanu, S.; Beets, G.L.; Weijenberg, M.P. Associations of Adipose and Muscle Tissue Parameters at Colorectal Cancer Diagnosis with Long-Term Health-Related Quality of Life. Qual. Life Res. 2017, 26, 1745–1759. [Google Scholar] [CrossRef] [Green Version]




| Variables | Mean ± SD, or No (%) |
|---|---|
| Age, years, mean ± SD | 60.43 ± 10.70 |
| Sex | |
| Male | 1373 (58.8) |
| Female | 960 (41.1) |
| Location | |
| Colon | 1728 (74.1) |
| Rectum | 605 (25.9) |
| Pathologic stage | |
| Stage (y) 0–II | 1546 (66.3) |
| Stage (y) III | 787 (33.7) |
| Adjuvant chemotherapy | |
| Yes | 1387 (59.5) |
| No | 946 (40.6) |
| PCRT in rectal cancer patients | |
| Yes | 261 (43.1) |
| No | 344 (56.9) |
| Preoperative Sarcopenia | |
| Yes | 1155 (49.5) |
| No | 1178 (50.5) |
| Surgery | |
| Right hemicolectomy | 687 (29.5) |
| Left hemicolectomy | 146 (6.3) |
| Anterior resection | 867 (37.2) |
| Low anterior resection | 419 (18.0) |
| Ultra-low anterior resection | 155 (6.6) |
| Abdominoperineal resection | 57 (2.4) |
| Hartmann’s operation | 2 (0.1) |
| Number of harvested lymph nodes | |
| <12 | 128 (5.5) |
| ≥12 | 2205 (94.5) |
| Lymphovascular invasion | |
| Yes | 476 (20.4) |
| No | 1857 (79.6) |
| Perineural invasion | |
| Yes | 380 (16.3) |
| No | 1953 (83.7) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Hazard Ratio (95%CI) | p-Value | Hazard Ratio (95%CI) | p-Value |
| Preoperative sarcopenia status | ||||
| Normal | 1 | 1 | ||
| Sarcopenia | 1.571 (1.245–1.983) | <0.001 | 1.177 (0.880–1.574) | 0.271 |
| Sarcopenia status at 2–3 years postoperatively | ||||
| Normal | 1 | 1 | ||
| Sarcopenia | 1.769 (1.408–2.222) | <0.001 | 1.551 (1.157–2.078) | 0.003 |
| Increase in SMI | 0.213 (0.067–0.678) | 0.009 | 0.246 (0.073–0.827) * | 0.023 |
| Lymphovascular invasion | ||||
| No | 1 | <0.001 | 1 | <0.001 |
| Yes | 2.915 (2.310–3.678) | 1.887 (1.304–2.181) | ||
| Perineural invasion | ||||
| No | 1 | <0.001 | 1 | <0.001 |
| Yes | 3.305 (2.607–4.189) | 2.076 (1.614–2.671) | ||
| Age, years | 0.990 (0.979–1.000) | 0.053 | 0.99(0.985–1.006) | 0.364 |
| Stage | ||||
| Stage (y) 0–II | 1 | 1 | ||
| Stage (y) III | 2.982 (2.366–3.758) | <0.001 | 1.618 (1.230–2.219) | 0.001 |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 0.732 (0.576–0.931) | 0.011 | 0.875 (0.679–1.126) | 0.299 |
| Adjuvant chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 3.501(2.576–4.758) | <0.001 | 1.911 (1.346–2.713) | 0.001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Hazard Ratio (95%CI) | p-Value | Hazard Ratio (95%CI) | p-Value |
| Preoperative sarcopenia status | ||||
| Normal | 1 | 1 | ||
| Sarcopenia | 2.234 (1.700–2.934) | <0.001 | 1.447 (1.032–2.027) | 0.032 |
| Sarcopenia status at 2–3 years postoperatively | ||||
| Normal | 1 | 1 | ||
| Sarcopenia | 2.444 (1.887–3.167) | <0.001 | 1.806 (1.298–2.513) | <0.001 |
| Increase in SMI | 0.079 (0.021–0.304) | <0.001 | 0.110 (0.027–0.449) * | 0.002 |
| Lymphovascular invasion | ||||
| No | 1 | <0.001 | 1 | 0.001 |
| Yes | 2.885 (2.225–3.739) | 1.653 (1.240–2.204) | ||
| Perineural invasion | ||||
| No | 1 | <0.001 | 1 | <0.001 |
| Yes | 3.277 (2.516–4.268) | 2.176 (1.640–2.887) | ||
| Age | 1.013 (1.001–1.025) | 0.041 | 1.014 (1.002–1.027) | 0.021 |
| Stage | ||||
| Stage (y) 0–II | 1 | 1 | ||
| Stage (y) III | 3.200 (2.467–4.151) | <0.001 | 2.080 (1.514–2.859) | <0.001 |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 0.679 (0.517–0.891) | 0.005 | 0.910 (0.682–1.214) | 0.520 |
| Adjuvant chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 2.511 (1.841–3.424) | <0.001 | 1.282 (0.888–1.850) | 0.347 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Park, I.-J.; Kim, K.-W.; Shin, Y.; Lim, S.-B.; Kim, C.-W.; Yoon, Y.-S.; Lee, J.-L.; Yu, C.-S.; Kim, J.-C. Influence of Postoperative Changes in Sarcopenia on Long-Term Survival in Non-Metastatic Colorectal Cancer Patients. Cancers 2021, 13, 2410. https://doi.org/10.3390/cancers13102410
Lee C, Park I-J, Kim K-W, Shin Y, Lim S-B, Kim C-W, Yoon Y-S, Lee J-L, Yu C-S, Kim J-C. Influence of Postoperative Changes in Sarcopenia on Long-Term Survival in Non-Metastatic Colorectal Cancer Patients. Cancers. 2021; 13(10):2410. https://doi.org/10.3390/cancers13102410
Chicago/Turabian StyleLee, Chungyeop, In-Ja Park, Kyung-Won Kim, Yongbin Shin, Seok-Byung Lim, Chan-Wook Kim, Yong-Sik Yoon, Jong-Lyul Lee, Chang-Sik Yu, and Jin-Cheon Kim. 2021. "Influence of Postoperative Changes in Sarcopenia on Long-Term Survival in Non-Metastatic Colorectal Cancer Patients" Cancers 13, no. 10: 2410. https://doi.org/10.3390/cancers13102410






