Predictive and Prognostic Value of Microsatellite Instability in Gynecologic Cancer (Endometrial and Ovarian)
Abstract
Simple Summary
Abstract
1. Introduction
2. Clinical Classifications of Endometrial and Ovarian Cancers
2.1. Endometrial Cancer
2.2. Ovarian Cancer
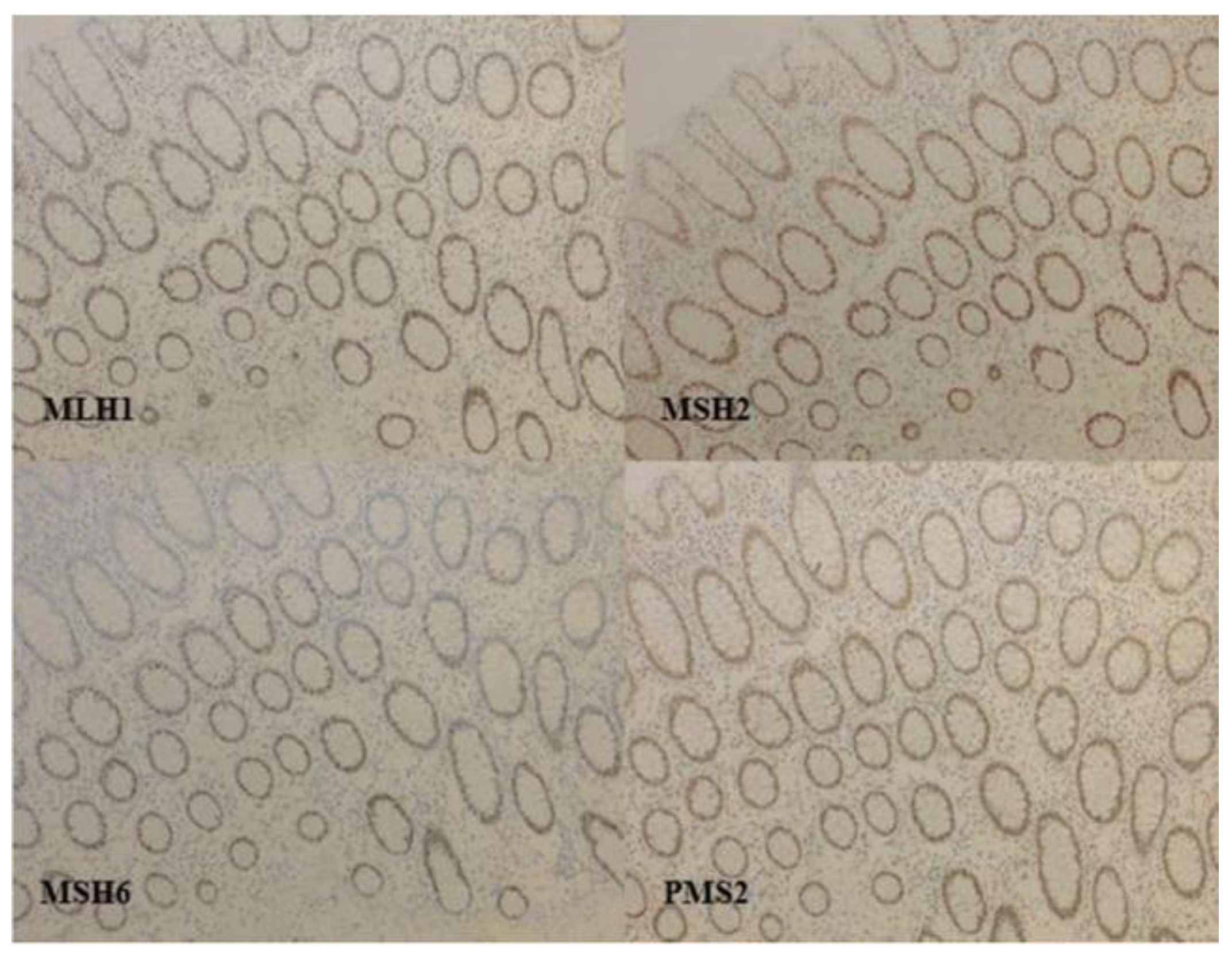
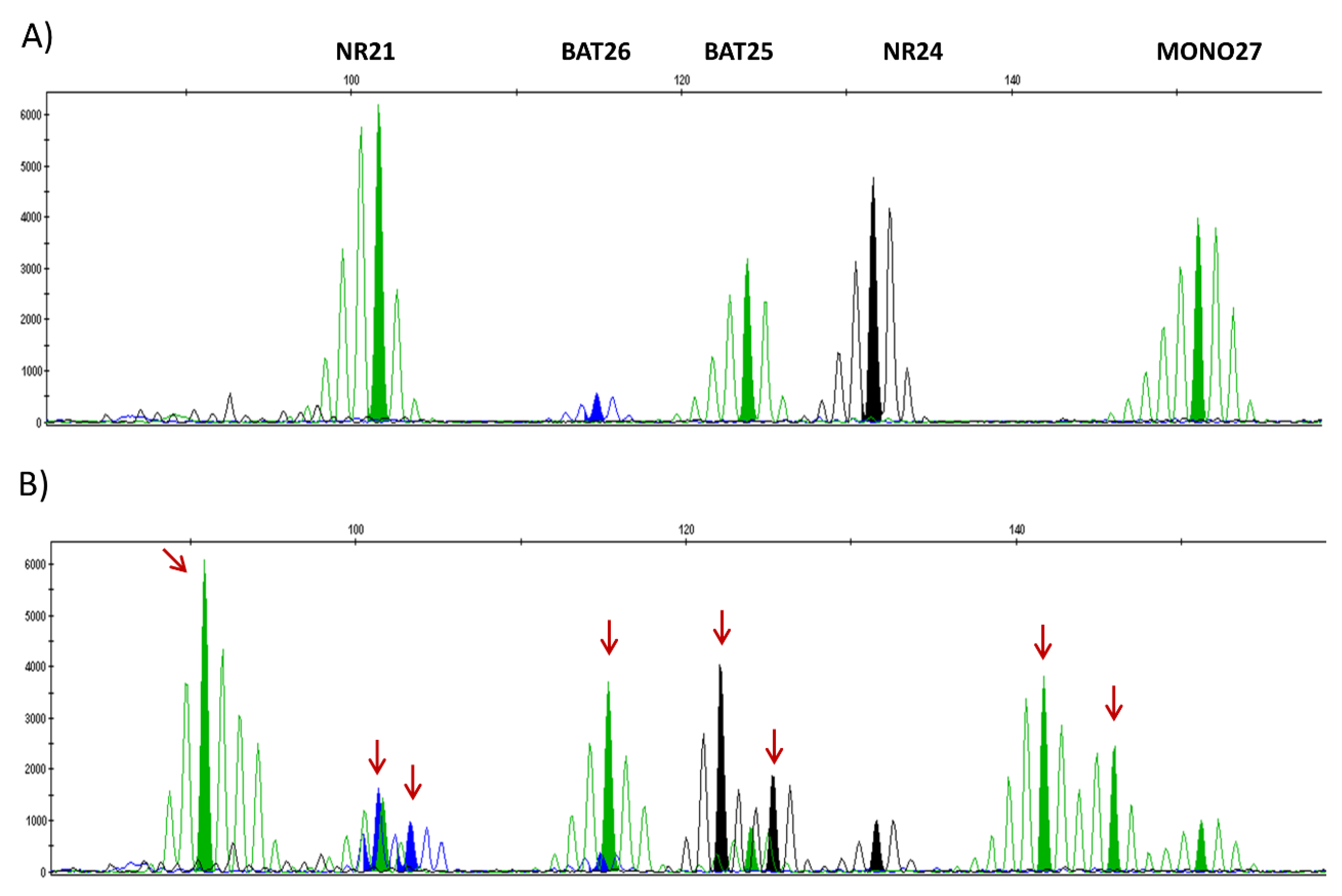
3. Detection of dMMR/MSI Gynecologic Tumors
3.1. Detection Techniques
3.2. Specificity of Detection Techniques in Endometrial Cancers and Discrepancies
3.3. Prevalence of dMMR Phenotype among Gynecologic Cancers
4. Characteristics of dMMR Endometrial Cancer
- ultramuted associated with an inactivating mutation of the exonuclease domain of POLE (5%) and 232 × 10−6 mutations per Megabase (Mb) average.
- hypermuted dMMR (30%) with 18 × 10−6 mutations per Mb and most with MLH1 promoter methylation.
- “serous-like” (20%) characterized by a mutation of TP53 and a high number of alterations in the number of copies of genes (“copy number high”).
- “copy number low” with few mutations and a low number of alterations in the number of copy, MSS, and without mutation of TP53 or POLE (45%) [18].
4.1. Molecular Alterations Associated with dMMR Phenotype
4.2. Age of Women
4.3. Family History
4.4. Body Mass Index
4.5. Tumor Characteristics
4.6. Prognostic Value of dMMR Status
4.7. Effectiveness of Adjuvant Treatments and MMR Status
- Pelvic radiotherapy in high intermediate risk tumors
- Adjuvant chemotherapy in high-risk tumors
5. Use of MMR Status in Current Practice in Endometrial Cancers
Predictive Benefit of Response to Immunotherapy in Advanced/Metastatic Stages
6. Ovarian Cancer and MSI: Description and Prognostic Value
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate |
| CTLA4 | cytotoxic T-lymphocyte-associated protein 4 |
| CRC | colorectal cancer |
| dMMR | deficient mismatch repair |
| EC | endometrial cancers |
| ESGO | European Society of Gynaecological Oncology |
| ESMO | European Society for Medical Oncology |
| ESTRO | European Society for Radiotherapy and Oncology |
| FIGO | Federation of Gynecology and Obstetrics |
| HGSC | High-grade serous carcinoma |
| HNPCC | Hereditary Non-Polyposis Colorectal Cancer |
| IHC | immunohistochemistry |
| LS | Lynch syndrome |
| LVSI | lymphovascular space invasion |
| MMR | mismatch repair |
| MSI | microsatellite instable |
| MSS | microsatellite stable |
| NGS | next generation sequencing |
| NSMP | non specific molecular profile |
| OC | ovarian cancer |
| PARP | poly ADP ribose polymerase |
| PCR | polymerase chain reaction |
| PD-1 | program death 1 |
| PD-L1 | program death ligand 1 |
| pMMR | proficient mismatch repair |
| POLE | polymerase ε |
| ProMisE | Proactive Molecular Risk Classifier for Endometrial Cancer |
References
- Stelloo, E.; Jansen, A.M.L.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Ruano, D.; Church, D.N.; Morreau, H.; Smit, V.T.H.B.M.; van Wezel, T.; et al. Practical Guidance for Mismatch Repair-Deficiency Testing in Endometrial Cancer. Ann. Oncol. 2017, 28, 96–102. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Endometrial Carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- di Silvestro, P.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Efficacy of Maintenance Olaparib for Patients With Newly Diagnosed Advanced Ovarian Cancer With a BRCA Mutation: Subgroup Analysis Findings From the SOLO1 Trial. J. Clin. Oncol. 2020, 38, 3528–3537. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, A.R.; Hamilton, S.R.; Allegra, C.J.; Grody, W.; Cushman-Vokoun, A.M.; Funkhouser, W.K.; Kopetz, S.E.; Lieu, C.; Lindor, N.M.; Minsky, B.D.; et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. Arch. Pathol. Lab. Med. 2017, 141, 625–657. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for Cancer Detection and Familial Predisposition: Development of International Criteria for the Determination of Microsatellite Instability in Colorectal Cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar]
- Siemanowski, J.; Schömig-Markiefka, B.; Buhl, T.; Haak, A.; Siebolts, U.; Dietmaier, W.; Arens, N.; Pauly, N.; Ataseven, B.; Büttner, R.; et al. Managing Difficulties of Microsatellite Instability Testing in Endometrial Cancer-Limitations and Advantages of Four Different PCR-Based Approaches. Cancers 2021, 13, 1268. [Google Scholar] [CrossRef]
- Suraweera, N.; Duval, A.; Reperant, M.; Vaury, C.; Furlan, D.; Leroy, K.; Seruca, R.; Iacopetta, B.; Hamelin, R. Evaluation of Tumor Microsatellite Instability Using Five Quasimonomorphic Mononucleotide Repeats and Pentaplex PCR. Gastroenterology 2002, 123, 1804–1811. [Google Scholar] [CrossRef]
- Wong, Y.F.; Cheung, T.H.; Lo, K.W.K.; Yim, S.F.; Chan, L.K.Y.; Buhard, O.; Duval, A.; Chung, T.K.H.; Hamelin, R. Detection of Microsatellite Instability in Endometrial Cancer: Advantages of a Panel of Five Mononucleotide Repeats over the National Cancer Institute Panel of Markers. Carcinogenesis 2006, 27, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Evrard, C.; Tachon, G.; Randrian, V.; Karayan-Tapon, L.; Tougeron, D. Microsatellite Instability: Diagnosis, Heterogeneity, Discordance, and Clinical Impact in Colorectal Cancer. Cancers 2019, 11, 1567. [Google Scholar] [CrossRef]
- Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 25 August 2020).
- Wang, Y.; Shi, C.; Eisenberg, R.; Vnencak-Jones, C.L. Differences in Microsatellite Instability Profiles between Endometrioid and Colorectal Cancers. J. Mol. Diagn. 2017, 19, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Cerbone, M.; Gencarelli, A.; Mollo, A.; Insabato, L.; Zullo, F. Diagnostic Accuracy of Immunohistochemistry for Mismatch Repair Proteins as Surrogate of Microsatellite Instability Molecular Testing in Endometrial Cancer. Pathol. Oncol. Res. 2020, 26, 1417–1427. [Google Scholar] [CrossRef]
- Wortman, B.G.; Creutzberg, C.L.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.H.W.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; et al. Ten-Year Results of the PORTEC-2 Trial for High-Intermediate Risk Endometrial Carcinoma: Improving Patient Selection for Adjuvant Therapy. Br. J. Cancer 2018, 119, 1067–1074. [Google Scholar] [CrossRef]
- Soslow, R.A.; Tornos, C.; Park, K.J.; Malpica, A.; Matias-Guiu, X.; Oliva, E.; Parkash, V.; Carlson, J.; McCluggage, W.G.; Gilks, C.B. Endometrial Carcinoma Diagnosis: Use of FIGO Grading and Genomic Subcategories in Clinical Practice: Recommendations of the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2019, 38, S64–S74. [Google Scholar] [CrossRef]
- Rossi, L.; Frere-Belda, M.-A.L.; Laurent-Puig, P.; Buecher, B.; Pauw, A.D.; Stoppa-Lyonnet, D.; Canlorbe, G.; Caron, O.; Borghese, B.; Colas, C.; et al. Clinicopathologic Characteristics of Endometrial Cancer in Lynch Syndrome: A French Multicenter Study. Int. J. Gynecol. Cancer 2017, 27, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.-Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 2017, PO.17.00073. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A. Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Stransky, N.; McCord, C.L.; Cerami, E.; Lagowski, J.; Kelly, D.; Angiuoli, S.V.; Sausen, M.; Kann, L.; Shukla, M.; et al. Genomic Analyses of Gynaecologic Carcinosarcomas Reveal Frequent Mutations in Chromatin Remodelling Genes. Nat. Commun. 2014, 5, 5006. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Gencarelli, A.; Mollo, A.; Guida, M.; Insabato, L.; Santoro, A.; Zannoni, G.F.; Zullo, F. TCGA Classification of Endometrial Cancer: The Place of Carcinosarcoma. Pathol. Oncol. Res. 2020, 26, 2067–2073. [Google Scholar] [CrossRef]
- de Lair, D.F.; Burke, K.A.; Selenica, P.; Lim, R.S.; Scott, S.N.; Middha, S.; Mohanty, A.S.; Cheng, D.T.; Berger, M.F.; Soslow, R.A.; et al. The Genetic Landscape of Endometrial Clear Cell Carcinomas. J. Pathol. 2017, 243, 230–241. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Mascolo, M.; Guida, M.; Insabato, L.; Zannoni, G.F.; Zullo, F. TCGA Molecular Subgroups in Endometrial Undifferentiated/Dedifferentiated Carcinoma. Pathol. Oncol. Res. 2020, 26, 1411–1416. [Google Scholar] [CrossRef]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final Validation of the ProMisE Molecular Classifier for Endometrial Carcinoma in a Large Population-Based Case Series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, P.J.; Billingsley, C.C.; Lankes, H.A.; Ali, S.; Cohn, D.E.; Broaddus, R.J.; Ramirez, N.; Pritchard, C.C.; Hampel, H.; Chassen, A.S.; et al. Combined Microsatellite Instability, MLH1 Methylation Analysis, and Immunohistochemistry for Lynch Syndrome Screening in Endometrial Cancers From GOG210: An NRG Oncology and Gynecologic Oncology Group Study. J. Clin. Oncol. 2015, 33, 4301–4308. [Google Scholar] [CrossRef]
- Latham, A.; Srinivasan, P.; Kemel, Y.; Shia, J.; Bandlamudi, C.; Mandelker, D. Microsatellite Instability is Associated with the Presence of Lynch Syndrome Pan-Cancer. Available online: https://www-ncbi-nlm-nih-gov.proxy.insermbiblio.inist.fr/pmc/articles/PMC6553803/ (accessed on 6 October 2020).
- Dondi, G.; Coluccelli, S.; de Leo, A.; Ferrari, S.; Gruppioni, E.; Bovicelli, A.; Godino, L.; Coadă, C.A.; Morganti, A.G.; Giordano, A.; et al. An Analysis of Clinical, Surgical, Pathological and Molecular Characteristics of Endometrial Cancer According to Mismatch Repair Status. A Multidisciplinary Approach. Int. J. Mol. Sci. 2020, 21, 7188. [Google Scholar] [CrossRef]
- Kwon, J.S.; Scott, J.L.; Gilks, C.B.; Daniels, M.S.; Sun, C.C.; Lu, K.H. Testing Women With Endometrial Cancer to Detect Lynch Syndrome. J. Clin. Oncol. 2011, 29, 2247–2252. [Google Scholar] [CrossRef]
- McMeekin, D.S.; Tritchler, D.L.; Cohn, D.E.; Mutch, D.G.; Lankes, H.A.; Geller, M.A.; Powell, M.A.; Backes, F.J.; Landrum, L.M.; Zaino, R.; et al. Clinicopathologic Significance of Mismatch Repair Defects in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2016, 34, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.K.; Broaddus, R.R. Gynecologic Cancers in Lynch Syndrome/HNPCC. Fam. Cancer 2005, 4, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Rabban, J.T.; Calkins, S.M.; Karnezis, A.N.; Grenert, J.P.; Blanco, A.; Crawford, B.; Chen, L. Association of Tumor Morphology With Mismatch-Repair Protein Status in Older Endometrial Cancer Patients: Implications for Universal Versus Selective Screening Strategies for Lynch Syndrome. Am. J. Surg. Pathol. 2014, 38, 793–800. [Google Scholar] [CrossRef]
- Gallon, R.; Gawthorpe, P.; Phelps, R.L.; Hayes, C.; Borthwick, G.M.; Santibanez-Koref, M.; Jackson, M.S.; Burn, J. How Should We Test for Lynch Syndrome? A Review of Current Guidelines and Future Strategies. Cancers 2021, 13, 406. [Google Scholar] [CrossRef]
- Westin, S.N.; Lacour, R.A.; Urbauer, D.L.; Luthra, R.; Bodurka, D.C.; Lu, K.H.; Broaddus, R.R. Carcinoma of the Lower Uterine Segment: A Newly Described Association With Lynch Syndrome. J. Clin. Oncol. 2008, 26, 5965–5971. [Google Scholar] [CrossRef]
- Garg, K.; Soslow, R.A. Lynch Syndrome (Hereditary Non-Polyposis Colorectal Cancer) and Endometrial Carcinoma. J. Clin. Pathol. 2009, 62, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Mascolo, M.; Carotenuto, C.; Guida, M.; Mollo, A.; Insabato, L.; Zullo, F. Histopathological Characterization of ProMisE Molecular Groups of Endometrial Cancer. Gynecol. Oncol. 2020, 157, 252–259. [Google Scholar] [CrossRef]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. Adjuvant Chemoradiotherapy versus Radiotherapy Alone in Women with High-Risk Endometrial Cancer (PORTEC-3): Patterns of Recurrence and Post-Hoc Survival Analysis of a Randomised Phase 3 Trial. Lancet Oncol. 2019, 20, 1273–1285. [Google Scholar] [CrossRef]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Murali, R.; Davidson, B.; Fadare, O.; Carlson, J.A.; Crum, C.P.; Gilks, C.B.; Irving, J.A.; Malpica, A.; Matias-Guiu, X.; McCluggage, W.G.; et al. High-Grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations. Int. J. Gynecol. Pathol. 2019, 38, S40–S63. [Google Scholar] [CrossRef]
- Bosse, T.; Nout, R.A.; McAlpine, J.N.; McConechy, M.K.; Britton, H.; Hussein, Y.; Gonzalez, C.; Ganesan, R.; Steele, J.C.; Harrison, B.T.; et al. Molecular Classification of Grade 3 Endometrioid Endometrial Cancers Identifies Distinct Prognostic Subgroups. Am. J. Surg. Pathol. 2018, 42, 561–568. [Google Scholar] [CrossRef]
- Beinse, G.; Rance, B.; Just, P.-A.; Izac, B.; Letourneur, F.; Saidu, N.E.B.; Chouzenoux, S.; Nicco, C.; Goldwasser, F.; Batteux, F.; et al. Identification of TP53 Mutated Group Using a Molecular and Immunohistochemical Classification of Endometrial Carcinoma to Improve Prognostic Evaluation for Adjuvant Treatments. Int. J. Gynecol. Cancer 2020, 30, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Soumerai, T.E.; Donoghue, M.T.A.; Bandlamudi, C.; Srinivasan, P.; Chang, M.T.; Zamarin, D.; Cadoo, K.A.; Grisham, R.N.; O’Cearbhaill, R.E.; Tew, W.P.; et al. Clinical Utility of Prospective Molecular Characterization in Advanced Endometrial Cancer. Clin. Cancer Res. 2018, 24, 5939–5947. [Google Scholar] [CrossRef]
- Germano, G.; Lamba, S.; Rospo, G.; Barault, L.; Magrì, A.; Maione, F.; Russo, M.; Crisafulli, G.; Bartolini, A.; Lerda, G.; et al. Inactivation of DNA Repair Triggers Neoantigen Generation and Impairs Tumour Growth. Nature 2017, 552, 116–120. [Google Scholar] [CrossRef]
- Pakish, J.B.; Zhang, Q.; Chen, Z.; Liang, H.; Chisholm, G.B.; Yuan, Y.; Mok, S.C.; Broaddus, R.R.; Lu, K.H.; Yates, M.S. Immune Microenvironment in Microsatellite-Instable Endometrial Cancers: Hereditary or Sporadic Origin Matters. Clin. Cancer Res. 2017, 23, 4473–4481. [Google Scholar] [CrossRef]
- Talhouk, A.; Derocher, H.; Schmidt, P.; Leung, S.; Milne, K.; Gilks, C.B.; Anglesio, M.S.; Nelson, B.H.; McAlpine, J.N. Molecular Subtype Not Immune Response Drives Outcomes in Endometrial Carcinoma. Clin. Cancer Res. 2019, 25, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. Available online: https://www-nejm-org.gate2.inist.fr/doi/10.1056/NEJMoa1500596?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dwww.ncbi.nlm.nih.gov (accessed on 22 June 2019).
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; di Giacomo, A.M.; de Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Bang, Y.-J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Mehnert, J.M.; Aung, K.L.; Lopez, J.; et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1–Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 2535–2541. [Google Scholar] [CrossRef]
- Oaknin, A.; Duska, L.R.; Sullivan, R.J.; Pothuri, B.; Ellard, S.L.; Leath, C.A.; Moreno, V.; Kristeleit, R.S.; Guo, W.; Danaee, H.; et al. Preliminary Safety, Efficacy, and Pharmacokinetic/Pharmacodynamic Characterization from GARNET, a Phase I/II Clinical Trial of the Anti–PD-1 Monoclonal Antibody, TSR-042, in Patients with Recurrent or Advanced MSI-h and MSS Endometrial Cancer. Gynecol. Oncol. 2019, 154, 17. [Google Scholar] [CrossRef]
- Oaknin, A.; Gilbert, L.; Tinker, A.V.; Sabatier, R.; Boni, V.; O’Malley, D.M.; Ghamande, S.; Duska, L.; Ghatage, P.; Guo, W.; et al. LBA36 Safety and Antitumor Activity of Dostarlimab in Patients (Pts) with Advanced or Recurrent DNA Mismatch Repair Deficient (DMMR) or Proficient (MMRp) Endometrial Cancer (EC): Results from GARNET. Ann. Oncol. 2020, 31, S1166. [Google Scholar] [CrossRef]
- Antill, Y.C.; Kok, P.S.; Robledo, K.; Barnes, E.; Friedlander, M.; Baron-Hay, S.E.; Shannon, C.M.; Coward, J.; Beale, P.J.; Goss, G.; et al. Activity of Durvalumab in Advanced Endometrial Cancer (AEC) According to Mismatch Repair (MMR) Status: The Phase II PHAEDRA Trial (ANZGOG1601). J. Clin. Oncol. 2019, 37, 5501. [Google Scholar] [CrossRef]
- Cohen, R.; Hain, E.; Buhard, O.; Guilloux, A.; Bardier, A.; Kaci, R.; Bertheau, P.; Renaud, F.; Bibeau, F.; Fléjou, J.-F.; et al. Association of Primary Resistance to Immune Checkpoint Inhibitors in Metastatic Colorectal Cancer With Misdiagnosis of Microsatellite Instability or Mismatch Repair Deficiency Status. JAMA Oncol. 2019, 5, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Fraune, C.; Rosebrock, J.; Simon, R.; Hube-Magg, C.; Makrypidi-Fraune, G.; Kluth, M.; Büscheck, F.; Höflmayer, D.; Schmalfeldt, B.; Müller, V.; et al. High Homogeneity of MMR Deficiency in Ovarian Cancer. Gynecol. Oncol. 2020, 156, 669–675. [Google Scholar] [CrossRef]
- Soliman, P.T.; Broaddus, R.R.; Schmeler, K.M.; Daniels, M.S.; Gonzalez, D.; Slomovitz, B.M.; Gershenson, D.M.; Lu, K.H. Women With Synchronous Primary Cancers of the Endometrium and Ovary: Do They Have Lynch Syndrome? J. Clin. Oncol. 2005, 23, 9344–9350. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Nakamura, K.; Nomura, H.; Banno, K.; Irie, H.; Adachi, M.; Iida, M.; Umene, K.; Nogami, Y.; Masuda, K.; et al. Clinicopathologic Analysis With Immunohistochemistry for DNA Mismatch Repair Protein Expression in Synchronous Primary Endometrial and Ovarian Cancers. Int. J. Gynecol. Cancer 2015, 25, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Dong, D.; He, W.; Song, L.; Wang, Q.; Yue, J.; Xie, L. Mismatch Repair Deficiency Is Associated with MSI Phenotype, Increased Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Immune Cells in Ovarian Cancer. Gynecol. Oncol. 2018, 149, 146–154. [Google Scholar] [CrossRef]
- Aysal, A.; Karnezis, A.; Medhi, I.; Grenert, J.P.; Zaloudek, C.J.; Rabban, J.T. Ovarian Endometrioid Adenocarcinoma: Incidence and Clinical Significance of the Morphologic and Immunohistochemical Markers of Mismatch Repair Protein Defects and Tumor Microsatellite Instability. Am. J. Surg. Pathol. 2012, 36, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Rambau, P.F.; Duggan, M.A.; Ghatage, P.; Warfa, K.; Steed, H.; Perrier, R.; Kelemen, L.E.; Köbel, M. Significant Frequency of MSH2/MSH6 Abnormality in Ovarian Endometrioid Carcinoma Supports Histotype-Specific Lynch Syndrome Screening in Ovarian Carcinomas. Histopathology 2016, 69, 288–297. [Google Scholar] [CrossRef]
- Hollis, R.L.; Thomson, J.P.; Stanley, B.; Churchman, M.; Meynert, A.M.; Rye, T.; Bartos, C.; Iida, Y.; Croy, I.; Mackean, M.; et al. Molecular Stratification of Endometrioid Ovarian Carcinoma Predicts Clinical Outcome. Nat. Commun. 2020, 11, 4995. [Google Scholar] [CrossRef]
- Kramer, P.; Talhouk, A.; Brett, M.A. Endometrial Cancer Molecular Risk Stratification is Equally Prognostic for Endometrioid Ovarian Carcinoma|Clinical Cancer Research. Available online: https://clincancerres-aacrjournals-org.proxy.insermbiblio.inist.fr/content/26/20/5400.long (accessed on 16 November 2020).
- Willis, B.C.; Sloan, E.A.; Atkins, K.A.; Stoler, M.H.; Mills, A.M. Mismatch Repair Status and PD-L1 Expression in Clear Cell Carcinomas of the Ovary and Endometrium. Mod. Pathol. 2017, 30, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, P.; Paula, A.D.C.; Tseng, J.; Leitao, M.M.; Bashashati, A.; Huntsman, D.G.; Nazeran, T.M.; Aghajanian, C.; Abu-Rustum, N.R.; DeLair, D.F.; et al. Molecular Profiling and Molecular Classification of Endometrioid Ovarian Carcinomas. Gynecol. Oncol. 2019, 154, 516–523. [Google Scholar] [CrossRef]
- Akbari, M.R.; Zhang, S.; Cragun, D.; Lee, J.-H.; Coppola, D.; McLaughlin, J.; Risch, H.A.; Rosen, B.; Shaw, P.; Sellers, T.A.; et al. Correlation between Germline Mutations in MMR Genes and Microsatellite Instability in Ovarian Cancer Specimens. Fam. Cancer 2017, 16, 351–355. [Google Scholar] [CrossRef]

| Risk Group | 2016 ESMO Classification Based on Clinical Characteristics [2] | 2021 ESGO ESTRO ESP Classification Based on Clinical and Molecular Characteristics [3] |
|---|---|---|
| Low |
|
|
| ||
| Intermediate |
|
|
| ||
| ||
| High-intermediate |
|
|
|
| |
| ||
| High |
|
|
|
| |
|
| |
| Advanced Metastatic |
|
|
|
|
| Tumoral Type | % of dMMR Tumor | ||
|---|---|---|---|
| NGS [17] | PCR (MSI) | IHC | |
| Endometrial carcinoma | 32% (n = 542) | 24% (n = 696) [1] | 28% (n = 696) [1] |
| Endometrioid | 25% (n = 679) [1] | ||
| Serous | 0 (n = 53) [18] | 6% (n = 17) [1] | |
| Carcinosarcoma | 3.5% (n = 57) | 18% (n = 22) [19] | |
| 7% (n = 231) [20] | |||
| Clear cells | 19% (n = 32) [21] | ||
| Un- and dedifferenciated | 44% (n = 73) [22] | ||
| Trials | Line of Treatment | Evaluated Treatments | Population | Number of Patients | Objective Response Rate (%) (95% CI) | Duration of Response (Months) | PFS (Months) | OS (Months) |
|---|---|---|---|---|---|---|---|---|
| Marabelle et al., 2020 [47] | ≥2 | Pembrolizumab | dMMR | 49 | 57.1% (42.2 to 71.2) | NR (2.9 to 27.0+) | 25.7 (4.9 to NR) | NR (27.2 to NR) |
| Oaknin et al., 2020 [50] | ≥2 | Dostarlimab | dMMR | 179 | 44.7% (34.9–54.8) | NR (2.6–28.9) | / | NR |
| pMMR | 161 | 13.4% (8.3–20.1) | NR (1.5–30.4) | / | NR | |||
| Antill et al., 2019 [51] | ≥1 | Durvalumab | dMMR | 35 | 40% (26–56) | / | / | / |
| pMMR | 36 | 3% (1–14) | / | / | / |
| Trial | Evaluable Patients | All Comers | Endometrioid OC |
|---|---|---|---|
| Fraune et al., 2020 [53] | 478 | 10/478 (2.1%) (IHC) | 8/35 (22.8%) (IHC) |
| 9/478 (1.8%) (PCR) | 8/35 (22.8%) (PCR) | ||
| Xiao et al., 2018 [56] * | 419 | 29/419 (6.9%) (IHC) | 15/98 (15.3%) (IHC) |
| Aysal et al., 2012 [57] | 71 | / | 7/71 (10.0%) (IHC) |
| 7/71 (10.0%) (PCR) | |||
| Rambau et al., 2016 [58] | 612 | 29/612 (4.7%) (IHC) | 25/181 (13.8%) (IHC) |
| Hollis et al., 2020 [59] | 112 | / | 20/112 (17.5%) (NGS) |
| Kramer P et al., 2020 [60] | 511 | / | 13.7% (IHC) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evrard, C.; Alexandre, J. Predictive and Prognostic Value of Microsatellite Instability in Gynecologic Cancer (Endometrial and Ovarian). Cancers 2021, 13, 2434. https://doi.org/10.3390/cancers13102434
Evrard C, Alexandre J. Predictive and Prognostic Value of Microsatellite Instability in Gynecologic Cancer (Endometrial and Ovarian). Cancers. 2021; 13(10):2434. https://doi.org/10.3390/cancers13102434
Chicago/Turabian StyleEvrard, Camille, and Jérôme Alexandre. 2021. "Predictive and Prognostic Value of Microsatellite Instability in Gynecologic Cancer (Endometrial and Ovarian)" Cancers 13, no. 10: 2434. https://doi.org/10.3390/cancers13102434
APA StyleEvrard, C., & Alexandre, J. (2021). Predictive and Prognostic Value of Microsatellite Instability in Gynecologic Cancer (Endometrial and Ovarian). Cancers, 13(10), 2434. https://doi.org/10.3390/cancers13102434





