Identification of Lifestyle Behaviors Associated with Recurrence and Survival in Colorectal Cancer Patients Using Random Survival Forests
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Lifestyle Assessment
2.3. Assessment of Background Variables
2.4. Outcome Assessment
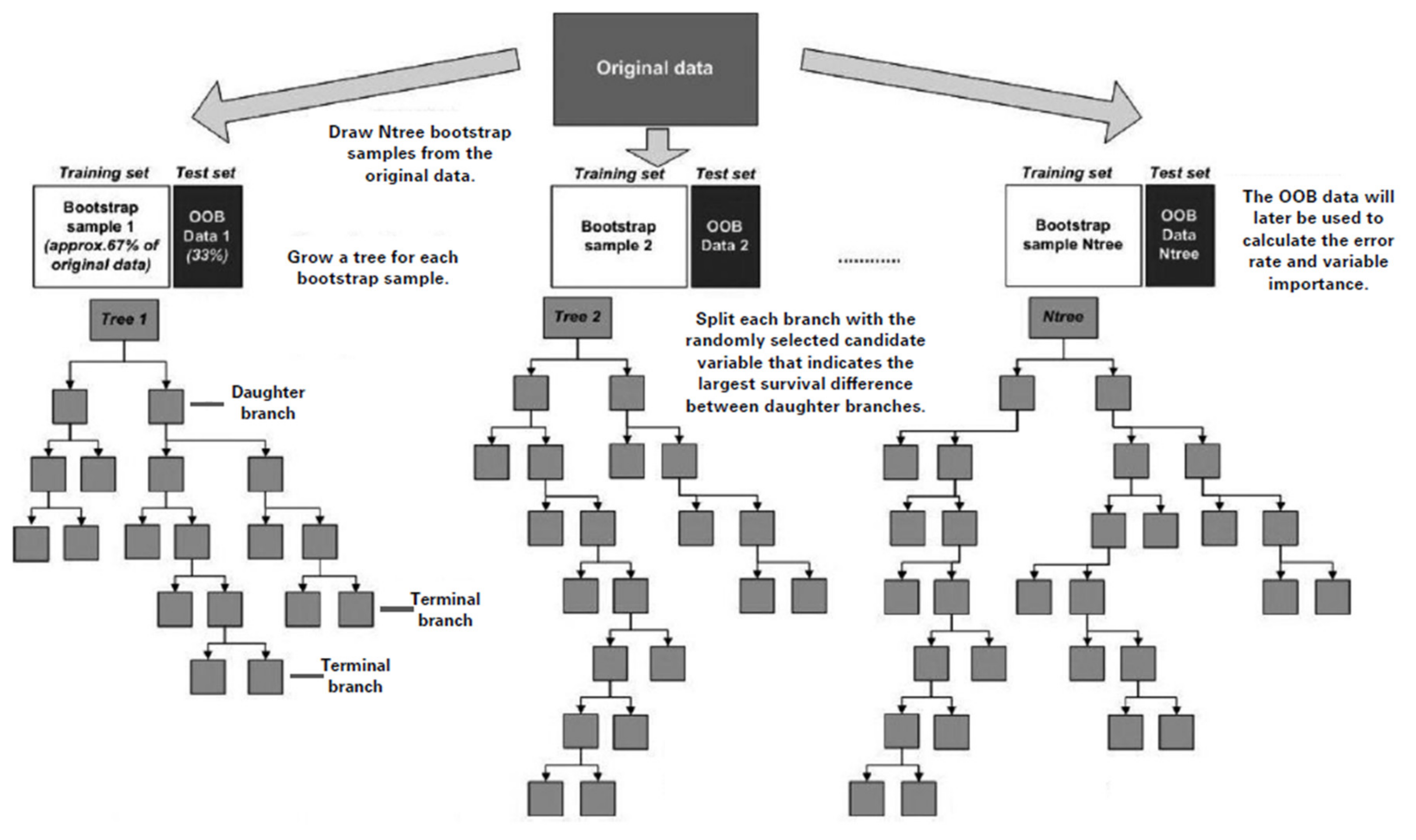
2.5. Random Survival Forests
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brouwer, N.P.M.; Bos, A.; Lemmens, V.; Tanis, P.J.; Hugen, N.; Nagtegaal, I.D.; de Wilt, J.H.W.; Verhoeven, R.H.A. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int. J. Cancer 2018, 143, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018. Available online: dietandcancerreport.org (accessed on 1 January 2021).
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [PubMed]
- Des Guetz, G.; Uzzan, B.; Bouillet, T.; Nicolas, P.; Chouahnia, K.; Zelek, L.; Morere, J.F. Impact of Physical Activity on Cancer-Specific and Overall Survival of Patients with Colorectal Cancer. Gastroenterol. Res. Pract. 2013, 2013, 340851. [Google Scholar] [CrossRef]
- Je, Y.; Jeon, J.Y.; Giovannucci, E.L.; Meyerhardt, J.A. Association between physical activity and mortality in colorectal cancer: A meta-analysis of prospective cohort studies. Int. J. Cancer 2013, 133, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Leitzmann, M.F. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann. Oncol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Guo, F.; Ye, J.; Li, Y.; Shi, D.; Fang, D.; Guo, J.; Li, L. Pre- and post-diagnosis physical activity is associated with survival benefits of colorectal cancer patients: A systematic review and meta-analysis. Oncotarget 2016, 7, 52095–52103. [Google Scholar] [CrossRef]
- Qiu, S.; Jiang, C.; Zhou, L. Physical activity and mortality in patients with colorectal cancer: A meta-analysis of prospective cohort studies. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. (ECP) 2020, 29, 15–26. [Google Scholar] [CrossRef]
- Van Zutphen, M.; Kampman, E.; Giovannucci, E.L.; van Duijnhoven, F.J.B. Lifestyle after Colorectal Cancer Diagnosis in Relation to Survival and Recurrence: A Review of the Literature. Curr. Colorectal Cancer Rep. 2017, 13, 370–401. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Meyerhardt, J.A. Role of physical activity and diet after colorectal cancer diagnosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1825–1834. [Google Scholar] [CrossRef]
- Lee, J.; Jeon, J.Y.; Meyerhardt, J.A. Diet and lifestyle in survivors of colorectal cancer. Hematol. Oncol. Clin. N. Am. 2015, 29, 1–27. [Google Scholar] [CrossRef]
- Lee, J.; Meyerhardt, J.A.; Giovannucci, E.; Jeon, J.Y. Association between Body Mass Index and Prognosis of Colorectal Cancer: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2015, 10, e0120706. [Google Scholar] [CrossRef] [PubMed]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Association of Survival With Adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors After Colon Cancer Diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncol. 2018, 4, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Kashambwa, R.; Sato, K.; Chiuve, S.E.; Fuchs, C.S.; Wu, K.; Giovannucci, E.; Ogino, S.; Hu, F.B.; Meyerhardt, J.A. Post diagnosis diet quality and colorectal cancer survival in women. PLoS ONE 2014, 9, e115377. [Google Scholar] [CrossRef] [PubMed]
- Tamakoshi, A.; Nakamura, K.; Ukawa, S.; Okada, E.; Hirata, M.; Nagai, A.; Matsuda, K.; Kamatani, Y.; Muto, K.; Kiyohara, Y.; et al. Characteristics and prognosis of Japanese colorectal cancer patients: The BioBank Japan Project. J. Epidemiol. 2017, 27, S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Zhang, S.; Niedzwiecki, D.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; Atienza, D.; et al. Grain Intake and Clinical Outcome in Stage III Colon Cancer: Results From CALGB 89803 (Alliance). JNCI Cancer Spectr. 2018, 2, pky017. [Google Scholar] [CrossRef]
- Song, M.; Wu, K.; Meyerhardt, J.A.; Ogino, S.; Wang, M.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncol. 2018, 4, 71–79. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Niedzwiecki, D.; Hollis, D.; Saltz, L.B.; Hu, F.B.; Mayer, R.J.; Nelson, H.; Whittom, R.; Hantel, A.; Thomas, J.; et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA J. Am. Med. Assoc. 2007, 298, 754–764. [Google Scholar] [CrossRef]
- Guinter, M.A.; McCullough, M.L.; Gapstur, S.M.; Campbell, P.T. Associations of Pre- and Postdiagnosis Diet Quality With Risk of Mortality Among Men and Women With Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, JCO1800714. [Google Scholar] [CrossRef]
- Fuchs, M.A.; Sato, K.; Niedzwiecki, D.; Ye, X.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Sugar-sweetened beverage intake and cancer recurrence and survival in CALGB 89803 (Alliance). PLoS ONE 2014, 9, e99816. [Google Scholar] [CrossRef]
- McCullough, M.L.; Gapstur, S.M.; Shah, R.; Jacobs, E.J.; Campbell, P.T. Association between red and processed meat intake and mortality among colorectal cancer survivors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Robien, K.; Lazovich, D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. In Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology; American Association for Cancer Research: Philadelphia, PA, USA, 2013; Volume 22, pp. 792–802. [Google Scholar] [CrossRef]
- Van Zutphen, M.; Boshuizen, H.C.; Kenkhuis, M.F.; Wesselink, E.; Geijsen, A.; de Wilt, J.H.W.; van Halteren, H.K.; Spillenaar Bilgen, E.J.; Keulen, E.T.P.; Janssen-Heijnen, M.L.G.; et al. Lifestyle after colorectal cancer diagnosis in relation to recurrence and all-cause mortality. Am. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ishwaran, H.; Kogalur, U.B.; Blackstone, E.H.; Lauer, M.S. Random Survival Forests. Ann. Appl. Stat. 2008, 2, 841–860. [Google Scholar] [CrossRef]
- Datema, F.R.; Moya, A.; Krause, P.; Back, T.; Willmes, L.; Langeveld, T.; Baatenburg de Jong, R.J.; Blom, H.M. Novel head and neck cancer survival analysis approach: Random survival forests versus Cox proportional hazards regression. Head Neck 2012, 34, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Hsich, E.; Gorodeski, E.Z.; Blackstone, E.H.; Ishwaran, H.; Lauer, M.S. Identifying important risk factors for survival in patient with systolic heart failure using random survival forests. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 39–45. [Google Scholar] [CrossRef]
- Miao, F.; Cai, Y.P.; Zhang, Y.X.; Li, Y.; Zhang, Y.T. Risk Prediction of One-Year Mortality in Patients with Cardiac Arrhythmias Using Random Survival Forest. Comput. Math. Methods Med. 2015, 2015, 303250. [Google Scholar] [CrossRef]
- Adham, D.; Abbasgholizadeh, N.; Abazari, M. Prognostic Factors for Survival in Patients with Gastric Cancer using a Random Survival Forest. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 129–134. [Google Scholar] [CrossRef]
- Hsich, E.M.; Thuita, L.; McNamara, D.M.; Rogers, J.G.; Valapour, M.; Goldberg, L.R.; Yancy, C.W.; Blackstone, E.H.; Ishwaran, H.; HEarts to MaxImize Survival (THEMIS) Investigators. Variables of importance in the Scientific Registry of Transplant Recipients database predictive of heart transplant waitlist mortality. Am. J. Transplant. 2019, 19, 2067–2076. [Google Scholar] [CrossRef]
- Kalin, M.; Cima, I.; Schiess, R.; Fankhauser, N.; Powles, T.; Wild, P.; Templeton, A.; Cerny, T.; Aebersold, R.; Krek, W.; et al. Novel prognostic markers in the serum of patients with castration-resistant prostate cancer derived from quantitative analysis of the pten conditional knockout mouse proteome. Eur. Urol. 2011, 60, 1235–1243. [Google Scholar] [CrossRef]
- Winkels, R.M.; Heine-Broring, R.C.; van Zutphen, M.; van Harten-Gerritsen, S.; Kok, D.E.; van Duijnhoven, F.J.; Kampman, E. The COLON study: Colorectal cancer: Longitudinal, Observational study on Nutritional and lifestyle factors that may influence colorectal tumour recurrence, survival and quality of life. BMC Cancer 2014, 14, 374. [Google Scholar] [CrossRef]
- Siebelink, E.; Geelen, A.; de Vries, J.H. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br. J. Nutr. 2011, 106, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.T.; de Vries, J.H.; Meijboom, S.; Beekman, M.; de Craen, A.J.; Slagboom, P.E.; Feskens, E.J. Relative validity of the food frequency questionnaire used to assess dietary intake in the Leiden Longevity Study. Nutr. J. 2013, 12, 75. [Google Scholar] [CrossRef]
- RIVM/Netherlands Nutrition Center. Dutch Food Composition (NEVO) Table 2011; Netherlands Nutrition Center: The Hague, The Netherlands, 2011. [Google Scholar]
- Kromhout, D.; Spaaij, C.J.; de Goede, J.; Weggemans, R.M. The 2015 Dutch food-based dietary guidelines. Eur. J. Clin. Nutr. 2016, 70, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Looman, M.; Feskens, E.J.; de Rijk, M.; Meijboom, S.; Biesbroek, S.; Temme, E.H.; de Vries, J.; Geelen, A. Development and evaluation of the Dutch Healthy Diet index 2015. Public Health Nutr. 2017, 20, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Wendel-Vos, G.C.; Schuit, A.J.; Saris, W.H.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- Wagenmakers, R.; van den Akker-Scheek, I.; Groothoff, J.W.; Zijlstra, W.; Bulstra, S.K.; Kootstra, J.W.; Wendel-Vos, G.C.; van Raaij, J.J.; Stevens, M. Reliability and validity of the short questionnaire to assess health-enhancing physical activity (SQUASH) in patients after total hip arthroplasty. BMC Musculoskelet. Disord. 2008, 9, 141. [Google Scholar] [CrossRef]
- De Hollander, E.L.; Zwart, L.; de Vries, S.I.; Wendel-Vos, W. The SQUASH was a more valid tool than the OBiN for categorizing adults according to the Dutch physical activity and the combined guideline. J. Clin. Epidemiol. 2012, 65, 73–81. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Van Leersum, N.J.; Snijders, H.S.; Henneman, D.; Kolfschoten, N.E.; Gooiker, G.A.; ten Berge, M.G.; Eddes, E.H.; Wouters, M.W.; Tollenaar, R.A.; Dutch Surgical Colorectal Cancer Audit Group. The Dutch surgical colorectal audit. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2013, 39, 1063–1070. [Google Scholar] [CrossRef]
- Strobl, C.; Malley, J.; Tutz, G. An introduction to recursive partitioning: Rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol. Methods 2009, 14, 323–348. [Google Scholar] [CrossRef]
- Ishwaran, H.; Kogalur, U.B. Random survival forest for R. R News 2007, 7, 25–31. [Google Scholar]
- Aune, D.; Lau, R.; Chan, D.S.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology 2011, 141, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Van Blarigan, E.L.; Ou, F.S.; Niedzwiecki, D.; Zhang, S.; Fuchs, C.S.; Saltz, L.; Mayer, R.J.; Venook, A.; Ogino, S.; Song, M.; et al. Dietary Fat Intake after Colon Cancer Diagnosis in Relation to Cancer Recurrence and Survival: CALGB 89803 (Alliance). In Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology; American Association for Cancer Research: Philadelphia, PA, USA, 2018; Volume 27, pp. 1227–1230. [Google Scholar] [CrossRef]
- Song, M.; Wu, K.; Meyerhardt, J.A.; Yilmaz, O.; Wang, M.; Ogino, S.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Low-Carbohydrate Diet Score and Macronutrient Intake in Relation to Survival After Colorectal Cancer Diagnosis. JNCI Cancer Spectr. 2018, 2, pky077. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; McCullough, M.L.; Gapstur, S.M.; Jacobs, E.J.; Bostick, R.M.; Fedirko, V.; Flanders, W.D.; Campbell, P.T. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: The Cancer Prevention Study-II Nutrition Cohort. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Mele, M.C.; Cintoni, M.; Raoul, P.; Ianiro, G.; Salerno, L.; Pozzo, C.; Bria, E.; Muscaritoli, M.; Molfino, A.; et al. The Facts about Food after Cancer Diagnosis: A Systematic Review of Prospective Cohort Studies. Nutrients 2020, 12, 2345. [Google Scholar] [CrossRef]
- Ford, K.L.; Sawyer, M.B.; Trottier, C.F.; Ghosh, S.; Deutz, N.E.P.; Siervo, M.; Porter Starr, K.N.; Bales, C.W.; Disi, I.R.; Prado, C.M. Protein Recommendation to Increase Muscle (PRIMe): Study protocol for a randomized controlled pilot trial investigating the feasibility of a high protein diet to halt loss of muscle mass in patients with colorectal cancer. Clin. Nutr. ESPEN 2021, 41, 175–185. [Google Scholar] [CrossRef]
- Omurlu, I.K.; Ture, M.; Tokatli, F. The comparisons of random survival forests and Cox regression analysis with simulation and an application related to breast cancer. Expert Syst. Appl. 2009, 36, 8582–8588. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Reichmann, R.; Kaaks, R.; Jenab, M.; Bueno-de-Mesquita, H.B.; Dahm, C.C.; Eriksen, A.K.; Tjonneland, A.; Artaud, F.; Boutron-Ruault, M.C.; et al. Development and validation of a lifestyle-based model for colorectal cancer risk prediction: The LiFeCRC score. BMC Med. 2021, 19, 1. [Google Scholar] [CrossRef]
- Van Baar, H.; Winkels, R.M.; Brouwer, J.G.M.; Posthuma, L.; Bours, M.J.L.; Weijenberg, M.P.; Boshuizen, H.C.; van Zutphen, M.; van Duijnhoven, F.J.B.; Kok, D.E.; et al. Associations of Abdominal Skeletal Muscle Mass, Fat Mass, and Mortality among Men and Women with Stage I-III Colorectal Cancer. In Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology; American Association for Cancer Research: Philadelphia, PA, USA, 2020; Volume 29, pp. 956–965. [Google Scholar] [CrossRef]


| Background Variables, n(%) or Median (IQR) | n = 1180 |
|---|---|
| Age at diagnosis, y | 66 (61–71) |
| Men | 747 (63%) |
| Education (missing n = 9) | |
| Low | 482 (41%) |
| Medium | 314 (27%) |
| High | 375 (32%) |
| Living with partner (missing n = 7) | 988 (84%) |
| Tumor stage | |
| I | 307 (26%) |
| II | 356 (30%) |
| III | 517 (44%) |
| Tumor site | |
| Colon | 796 (67%) |
| Rectum | 384 (33%) |
| Neo-adjuvant treatment | 272 (23%) |
| Adjuvant chemotherapy | 284 (24%) |
| ASA physical performance classification (missing n = 51) | |
| I | 354 (30%) |
| II | 653 (55%) |
| III | 122 (10%) |
| Daily NSAID use | 102 (9%) |
| Smoking at diagnosis (missing n = 8) | |
| Yes | 119 (10%) |
| Former | 694 (59%) |
| Never | 359 (31%) |
| Lifestyle six months post-diagnosis, n(%) or median (IQR) | |
| Body Mass Index, kg/m2 (missing n = 6) | 25.9 (23.9–28.5) |
| Physical activity 1, min/wk. | 480 (240–840) |
| Diet | |
| Fruits and vegetables, g/day | 248 (147–350) |
| Red and processed meat, g/day | 63 (38–85) |
| Sugary drinks, g/day | 70 (13–176) |
| Dietary fiber, g/day | 19 (15–24) |
| Energy intake, kcal/day | 1765 (1472–2112) |
| Alcohol intake | |
| Non-drinker 2 | 293 (25%) |
| Amount (g/d) among drinkers | 9 (3–21) |
| Amount (g/d) among all | 5 (0–16) |
| Current smoker (missing n = 2) | 80 (7%) |
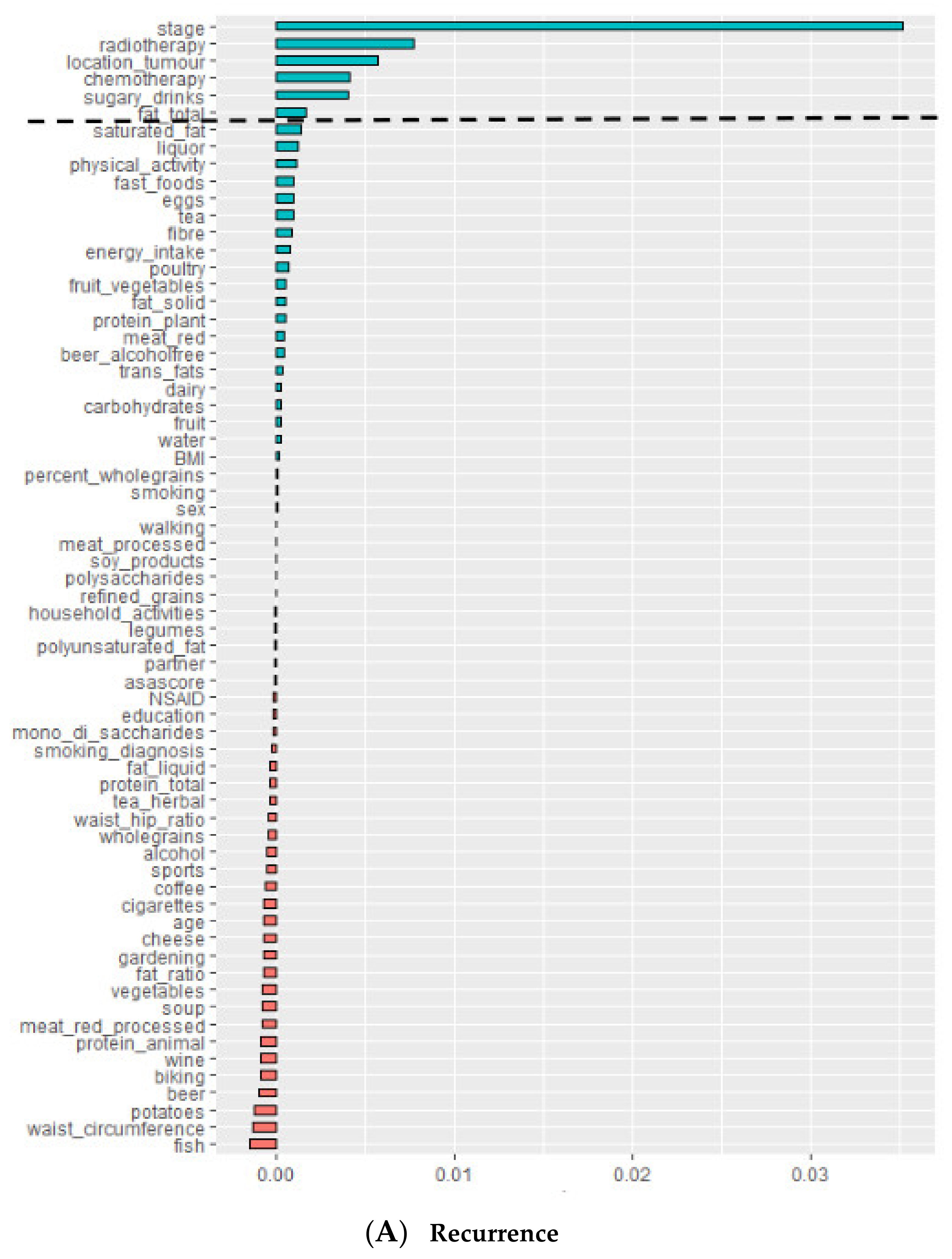
| Variables Predictive of Recurrence | Number of Times Selected as Predictive Variable in 10 Repetitions of RSF Model |
|---|---|
| Stage | 10 |
| Tumor location | 10 |
| Adjuvant chemotherapy | 10 |
| Neo-adjuvant therapy | 10 |
| Sugary drinks | 10 |
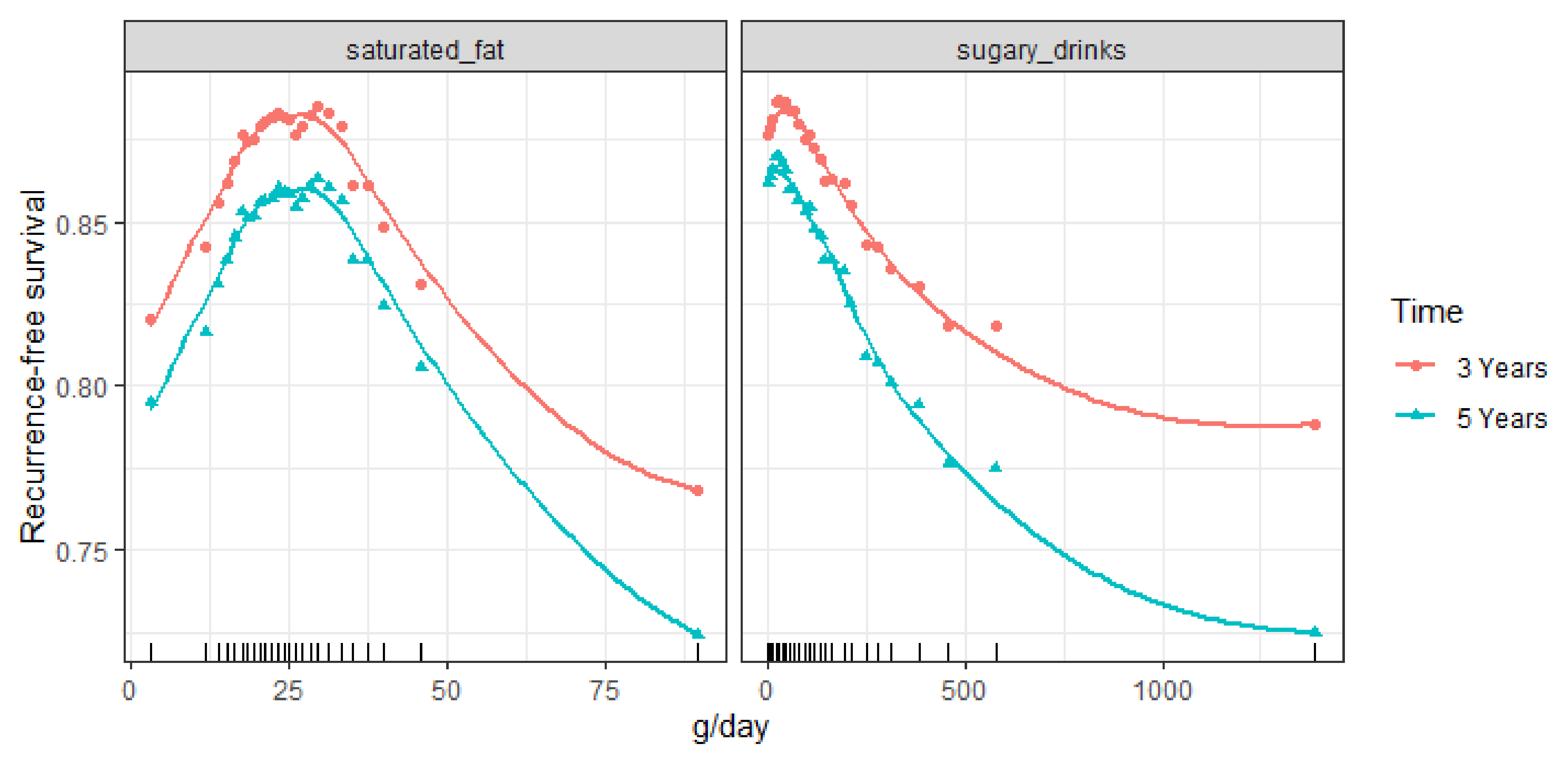
| Saturated fat | 8 |
| Fruit | 6 |
| Total fat | 4 |
| Trans-fats | 3 |
| Eggs | 3 |
| Polyunsaturated fat | 3 |
| Carbohydrates | 3 |
| Fiber | 2 |
| Liquor | 2 |
| Energy intake | 2 |
| Variables predictive of all-cause mortality | |
| Age | 10 |
| Stage | 10 |
| Liquid fat & oils | 10 |
| Fruit & vegetables | 10 |
| Animal protein | 10 |
| Fruit | 9 |
| Polyunsaturated fat | 9 |
| Potato | 8 |
| Processed meat | 8 |
| ASA classification | 7 |
| Herbal tea | 6 |
| Sugary drinks | 6 |
| Soup | 6 |
| Adjuvant chemotherapy | 6 |
| Alcohol | 5 |
| BMI | 4 |
| Beer | 4 |
| Education | 4 |
| Plant protein | 4 |
| Neo-adjuvant therapy | 2 |
| Dietary fiber | 2 |
| RSF Model | Prediction Error Rate 1 (Mean ± SE) | |
|---|---|---|
| Recurrence | All-Cause Mortality | |
| Final model (background and identified lifestyle variables) | 0.3376 ± 0.0005 | 0.3452 ± 0.0006 |
| Only background variables | 0.3570 ± 0.0005 | 0.3483 ± 0.0004 |
| Full model (background and lifestyle variables) | 0.3777 ± 0.0006 | 0.3964 ± 0.0009 |
| Only lifestyle variables | 0.4858 ± 0.0014 | 0.4309 ± 0.0007 |
| Only noise (benchmark model) | 0.5706 ± 0.0014 | 0.4886 ± 0.0011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Zutphen, M.; van Duijnhoven, F.J.B.; Wesselink, E.; Schrauwen, R.W.M.; Kouwenhoven, E.A.; van Halteren, H.K.; de Wilt, J.H.W.; Winkels, R.M.; Kok, D.E.; Boshuizen, H.C. Identification of Lifestyle Behaviors Associated with Recurrence and Survival in Colorectal Cancer Patients Using Random Survival Forests. Cancers 2021, 13, 2442. https://doi.org/10.3390/cancers13102442
van Zutphen M, van Duijnhoven FJB, Wesselink E, Schrauwen RWM, Kouwenhoven EA, van Halteren HK, de Wilt JHW, Winkels RM, Kok DE, Boshuizen HC. Identification of Lifestyle Behaviors Associated with Recurrence and Survival in Colorectal Cancer Patients Using Random Survival Forests. Cancers. 2021; 13(10):2442. https://doi.org/10.3390/cancers13102442
Chicago/Turabian Stylevan Zutphen, Moniek, Fränzel J. B. van Duijnhoven, Evertine Wesselink, Ruud W. M. Schrauwen, Ewout A. Kouwenhoven, Henk K. van Halteren, Johannes H. W. de Wilt, Renate M. Winkels, Dieuwertje E. Kok, and Hendriek C. Boshuizen. 2021. "Identification of Lifestyle Behaviors Associated with Recurrence and Survival in Colorectal Cancer Patients Using Random Survival Forests" Cancers 13, no. 10: 2442. https://doi.org/10.3390/cancers13102442
APA Stylevan Zutphen, M., van Duijnhoven, F. J. B., Wesselink, E., Schrauwen, R. W. M., Kouwenhoven, E. A., van Halteren, H. K., de Wilt, J. H. W., Winkels, R. M., Kok, D. E., & Boshuizen, H. C. (2021). Identification of Lifestyle Behaviors Associated with Recurrence and Survival in Colorectal Cancer Patients Using Random Survival Forests. Cancers, 13(10), 2442. https://doi.org/10.3390/cancers13102442







