Proof of Concept of a Binary Blood Assay for Predicting Radiosensitivity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data
2.1.1. Training Cohort
2.1.2. Validation Cohort
2.2. Toxicity Endpoint Definition
- “radioresistant” (RR) patients with early side effects graded <2.
- “radiosensitive” (RS) patients with early side effects graded ≥2.
2.3. RADIODTECT® Assay
- Isolation and Treatment of Human Lymphocytes
- 2.
- Cell Lysis
- 3.
- ELISA Assay
2.4. Statistical Analysis
2.4.1. Determination of Threshold
2.4.2. Performances of the Assay
3. Results
3.1. Population
3.2. pATM Threshold Determination
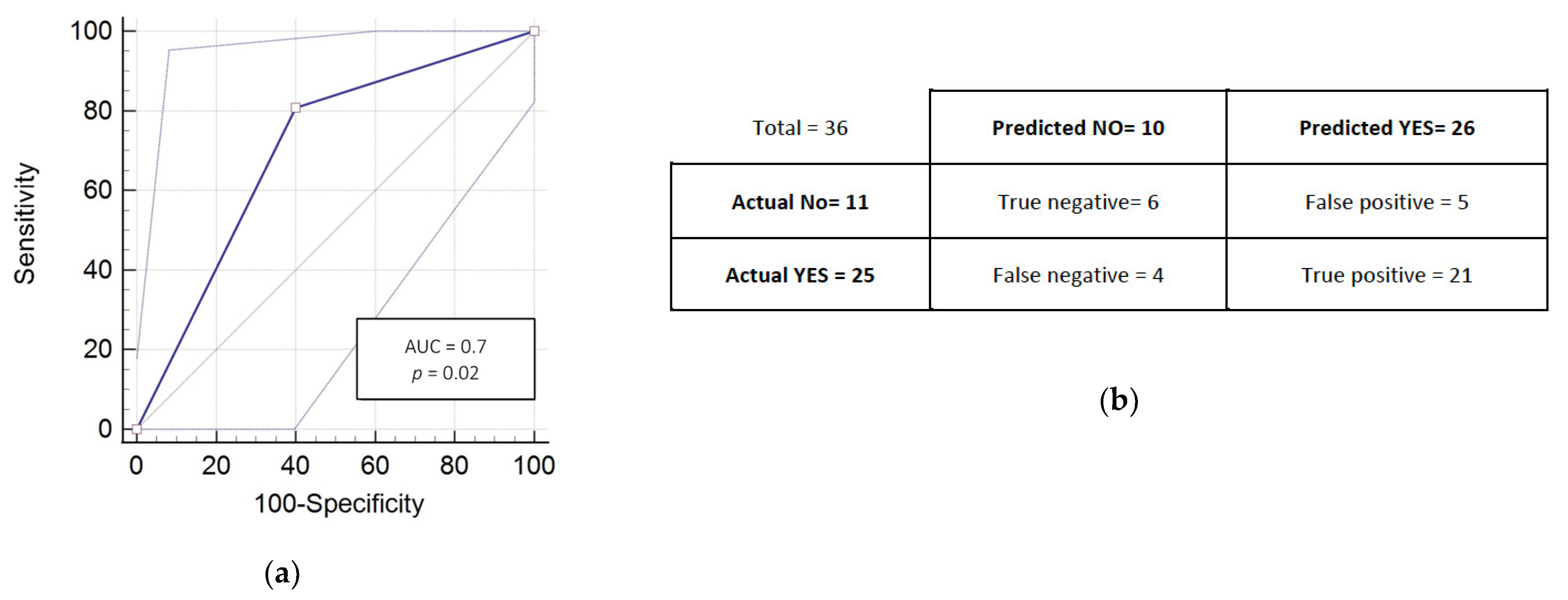
3.3. RADIODTECT® Assay Performances on Validation Cohort
3.4. Prediction of Radiosensitivity Considering RadioDtect and the Addition of Concurrent Chemotherapy as a Binary Variable
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litwin, M.S.; Tan, H.-J. The diagnosis and treatment of prostate cancer: A review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Carlotto, A.; Hogsett, V.L.; Maiorini, E.M.; Razulis, J.G.; Sonis, S.T. The economic burden of toxicities associated with cancer treatment: Review of the literature and analysis of nausea and vomiting, diarrhoea, oral mucositis and fatigue. Pharmacoeconomics 2013, 31, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Choy, H.; Kim, D.W. Chemotherapy and irradiation interaction. Semin. Oncol. 2003, 30 (Suppl. 9), 3–10. [Google Scholar] [CrossRef]
- Henk, J.M. Controlled trials of synchronous chemotherapy with radiotherapy in head and neck cancer: Overview of radiation morbidity. Clin. Oncol. (R Coll. Radiol.) 1997, 9, 308–312. [Google Scholar] [CrossRef]
- Averbeck, D.; Candéias, S.; Chandna, S.; Foray, N.; Friedl, A.A.; Haghdoost, S.; Jeggo, P.A.; Lumniczky, K.; Paris, F.; Quintens, R.; et al. Establishing mechanisms affecting the individual response to ionizing radiation. Int. J. Radiat. Biol. 2020, 96, 297–323. [Google Scholar] [CrossRef] [Green Version]
- Belkacemi, Y.; Colson-Durand, L.; Granzotto, A.; Husheng, S.; To, N.H.; Majdoul, S.; Guet, S.; Hervé, M.L.; Fonteneau, G.; Diana, C.; et al. The Henri Mondor Procedure of Morbidity and Mortality Review Meetings: Prospective Registration of Clinical, Dosimetric, and Individual Radiosensitivity Data of Patients with Severe Radiation Toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 629–636. [Google Scholar] [CrossRef]
- Turesson, I.; Nyman, J.; Holmberg, E.; Odén, A. Prognostic factors for acute and late skin reactions in radiotherapy patients. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 1065–1075. [Google Scholar] [CrossRef]
- Gatti, R.A. The inherited basis of human radiosensitivity. Acta Oncol. 2001, 40, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Joubert, A.; Zimmerman, K.M.; Bencokova, Z.; Gastaldo, J.; Chavaudra, N.; Favaudon, V.; Arlett, C.F.; Foray, N. DNA double-strand break repair defects in syndromes associated with acute radiation response: At least two different assays to predict intrinsic radiosensitivity? IJRB 2008, 84, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Bodgi, L.; Foray, N. The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: Resolution of the linear-quadratic model. Int. J. Radiat. Biol. 2016, 92, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Berthel, E.; Foray, N.; Ferlazzo, M. The nucleoshuttling of the ATM protein: A unified model to describe the individual response to high and low dose of radiation. Cancers 2019, 11, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granzotto, A.; Benadjaoud, M.A.; Vogin, G.; Devic, C.; Ferlazzo, M.L.; Bodgi, L.; Pereira, S.; Sonzogni, L.; Forcheron, F.; Viau, M.; et al. Influence of Nucleoshuttling of the ATM Protein in the Healthy Tissues Response to Radiation Therapy: Toward a Molecular Classification of Human Radiosensitivity. Int. J. Radiat. Biol. 2016, 94, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Vogin, G.; Bastogne, T.; Bodgi, L.; Gillet-Daubin, J.; Canet, A.; Pereira, S.; Foray, N. The Phosphorylated ATM Immunofluorescence Assay: A High-performance Radiosensitivity Assay to Predict Postradiation Therapy Overreactions. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 690–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, S.; Bodgi, L.; Duclos, M.; Canet, A.; Ferlazzo, M.L.; Devic, C.; Granzotto, A.; Deneuve, S.; Vogin, G.; Foray, N. Fast and Binary Assay for Predicting Radiosensitivity Based on the Theory of ATM Nucleo-Shuttling: Development, Validation, and Performance. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 353–360. [Google Scholar] [CrossRef]
- Ferlazzo, M.L.; Sonzogni, L.; Granzotto, A.; Bodgi, L.; Lartin, O.; Devic, C.; Vogin, G.; Pereira, S.; Foray, N. Mutations of the Huntington’s disease protein impact on the ATM-dependent signaling and repair pathways of the radiation-induced DNA double-strand breaks: Corrective effect of statins and bisphosphonates. Mol. Neurobiol. 2014, 49, 1200–1211. [Google Scholar] [CrossRef]
- Ferlazzo, M.L.; Bach-Tobdji, M.K.E.; Djerad, A.; Sonzogni, L.; Devic, C.; Granzotto, A.; Bodgi, L.; Bachelet, J.T.; Djefal-Kerrar, A.; Hennequin, C.; et al. Radiobiological Characterization of Tuberous Sclerosis: A Delay in the Nucleo-Shuttling of ATM May Be Responsible for Radiosensitivity. Mol. Neurobiol. 2018, 55, 4973–4983. [Google Scholar] [CrossRef]
- Maalouf, M.; Granzotto, A.; Devic, C.; Bodgi, L.; Ferlazzo, M.; Peaucelle, C.; Bajard, M.; Giraud, J.Y.; Balosso, J.; Hérault, J.; et al. Influence of Linear Energy Transfer on the Nucleo-shuttling of the ATM Protein: A Novel Biological Interpretation Relevant for Particles and Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 709–718. [Google Scholar] [CrossRef]
- Bakkenist, C.J.; Czambel, R.K.; Lin, Y.; Yates, N.A.; Zeng, X.; Shogan, J.; Schmitz, J.C. Quantitative analysis of ATM phosphorylation in lymphocytes. DNA Repair (Amst.) 2019, 80, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, M.L.; Bourguignon, M.; Foray, N. Functional Assays for Individual Radiosensitivity: A Critical Review. Semin. Radiat. Oncol. 2017, 27, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Azria, D.; Riou, O.; Castan, F.; Nguyen, T.D.; Peignaux, K.; Lemanski, C.; Lagrange, J.L.; Kirova, Y.; Lartigau, E.; Belkacemi, Y.; et al. Radiation-induced CD8 T-lymphocyte apoptosis as a predictor of breast fibrosis after radiotherapy: Results of the prospective multicenter French trial. EBioMedicine 2015, 2, 1965–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baijer, J.; Déchamps, N.; Perdry, H.; Morales, P.; Kerns, S.; Vasilescu, A.; Baulande, S.; Azria, D.; Roméo, P.H. TNFSF10/TRAIL regulates human T4 effector memory lymphocyte radiosensitivity and predicts radiation-induced acute and subacute dermatitis. Oncotarget 2016, 7, 21416–21427. [Google Scholar] [CrossRef] [Green Version]
- Rosenstein, B.S.; West, C.M.; Bentzen, S.M.; Alsner, J.; Andreassen, C.N.; Azria, D.; Barnett, G.C.; Baumann, M.; Burnet, N.; Chang-Claude, J.; et al. Radiogenomics: Radiobiology enters the era of big data and team science. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 709–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozsahin, M.; Crompton, N.E.; Gourgou, S.; Kramar, A.; Li, L.; Shi, Y.; Sozzi, W.J.; Zouhair, A.; Mirimanoff, R.O.; Azria, D. CD4 and CD8 T-lymphocyte apoptosis can predict radiation-induced late toxicity: A prospective study in 399 patients. Clin. Cancer Res. 2005, 11, 7426–7433. [Google Scholar] [CrossRef] [Green Version]
- Azria, D.; Ozsahin, M.; Kramar, A.; Peters, S.; Atencio, D.P.; Crompton, N.E.; Mornex, F.; Pèlegrin, A.; Dubois, J.B.; Mirimanoff, R.O.; et al. Single nucleotide poly- morphisms, apoptosis, and the development of severe late adverse effects after radiotherapy. Clin. Cancer Res. 2008, 14, 6284–6288. [Google Scholar] [CrossRef] [Green Version]
- Barnett, G.C.; Coles, C.E.; Elliott, R.M.; Baynes, C.; Luccarini, C.; Conroy, D.; Wilkinson, J.S.; Tyrer, J.; Misra, V.; Platte, R.; et al. Independent validation of genes and polymorphisms reported to be associated with radiation toxicity: A prospective analysis study. Lancet Oncol. 2012, 13, 65–77. [Google Scholar] [CrossRef]


| Patients’ Characteristics and Treatments | Head and Neck Patients N = 53 | Prostate Patients N = 63 | Breast Patients N = 24 | Rectum Patients N = 5 | Others (Brain, Lung, Lymph Node, Esophagus) N = 5 | Total N = 150 | |
|---|---|---|---|---|---|---|---|
| Gender | Female | 13 | 0 | 24 | 3 | 3 | 43 |
| Male | 40 | 63 | 0 | 2 | 2 | 107 | |
| Median Age (Range) | 61 (31, 84) | 64 (32, 83) | 69 (49, 89) | 59 (49, 69) | 66.5 (56, 77) | 62.5 (31; 84) | |
| Type of Radiotherapy | Definitive | 4 | 52 | 0 | 2 | 1 | 59 |
| Adjuvant | 49 | 11 | 24 | 3 | 4 | 91 | |
| Mean Dose | Tumor (range) | 62 (50–70) | 70 (62–80) | 54.2 (42.4–66) | 47.4 (36–59.4) | 45 (24–66) | 52 (24–80) |
| Concurrent Chemotherapy | Yes | 19 (Cisplatin) | 0 | 0 | 3 (2 capecitabin mitomycin, 1 Cisplatin) | 2 (1 Folfox, 1 carboplatin, 5FU) | 24 |
| No | 34 | 63 | 24 | 2 | 3 | 126 | |
| Concurrent Hormonotherapy | Yes | - | 16 | - | - | - | 16 |
| No | 47 | 134 | |||||
| CTCAE Highest Score | 1 | 17 | 55 | 14 | 1 | 1 | 89 |
| 2 | 20 | 7 | 8 | 2 | 1 | 38 | |
| 3 | 13 | 1 | 2 | 2 | 2 | 20 | |
| 4 | 3 | 0 | 0 | 0 | 0 | 3 | |
| Patients’ Characteristics and Treatments | Head and Neck Patients N = 36 | |
|---|---|---|
| Gender | Female | 4 |
| Male | 32 | |
| Median Age (Range) | - | 57 (32–85) |
| Type of radiotherapy | VMAT | 28 |
| IMRT | 7 | |
| Tomotherapy | 1 | |
| Mean Dose | Tumor (range) | 60 Gy (50–70 Gy) |
| Concurrent Chemotherapy (Cisplatin and TPF) | Yes | 20 |
| No | 16 | |
| CTCAE Highest Score for Acute Toxicities | 1 | 11 |
| 2 | 11 | |
| 3 | 10 | |
| 4 | 4 | |
| Performances | Training Cohort | Validation Cohort | ||
|---|---|---|---|---|
| RadioDtect | RadioDtect + Chemotherapy | RadioDtect | RadioDtect + Chemotherapy | |
| AUC | 0.71 | 0.77 | 0.70 | 0.72 |
| 95% CI | 0.63 to 0.77 | 0.70 to 0.84 | 0.53 to 0.84 | 0.59 to 0.85 |
| p-value | <0.001 | <0.001 | 0.02 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deneuve, S.; Mirjolet, C.; Bastogne, T.; Duclos, M.; Retif, P.; Zrounba, P.; Roux, P.-E.; Poupart, M.; Vogin, G.; Foray, N.; et al. Proof of Concept of a Binary Blood Assay for Predicting Radiosensitivity. Cancers 2021, 13, 2477. https://doi.org/10.3390/cancers13102477
Deneuve S, Mirjolet C, Bastogne T, Duclos M, Retif P, Zrounba P, Roux P-E, Poupart M, Vogin G, Foray N, et al. Proof of Concept of a Binary Blood Assay for Predicting Radiosensitivity. Cancers. 2021; 13(10):2477. https://doi.org/10.3390/cancers13102477
Chicago/Turabian StyleDeneuve, Sophie, Céline Mirjolet, Thierry Bastogne, Mirlande Duclos, Paul Retif, Philippe Zrounba, Pierre-Eric Roux, Marc Poupart, Guillaume Vogin, Nicolas Foray, and et al. 2021. "Proof of Concept of a Binary Blood Assay for Predicting Radiosensitivity" Cancers 13, no. 10: 2477. https://doi.org/10.3390/cancers13102477










