Patterns of Recurrence after Neoadjuvant Therapy in Early Breast Cancer, according to the Residual Cancer Burden Index and Reductions in Neoadjuvant Treatment Intensity
Abstract
Simple Summary
Abstract
1. Introduction
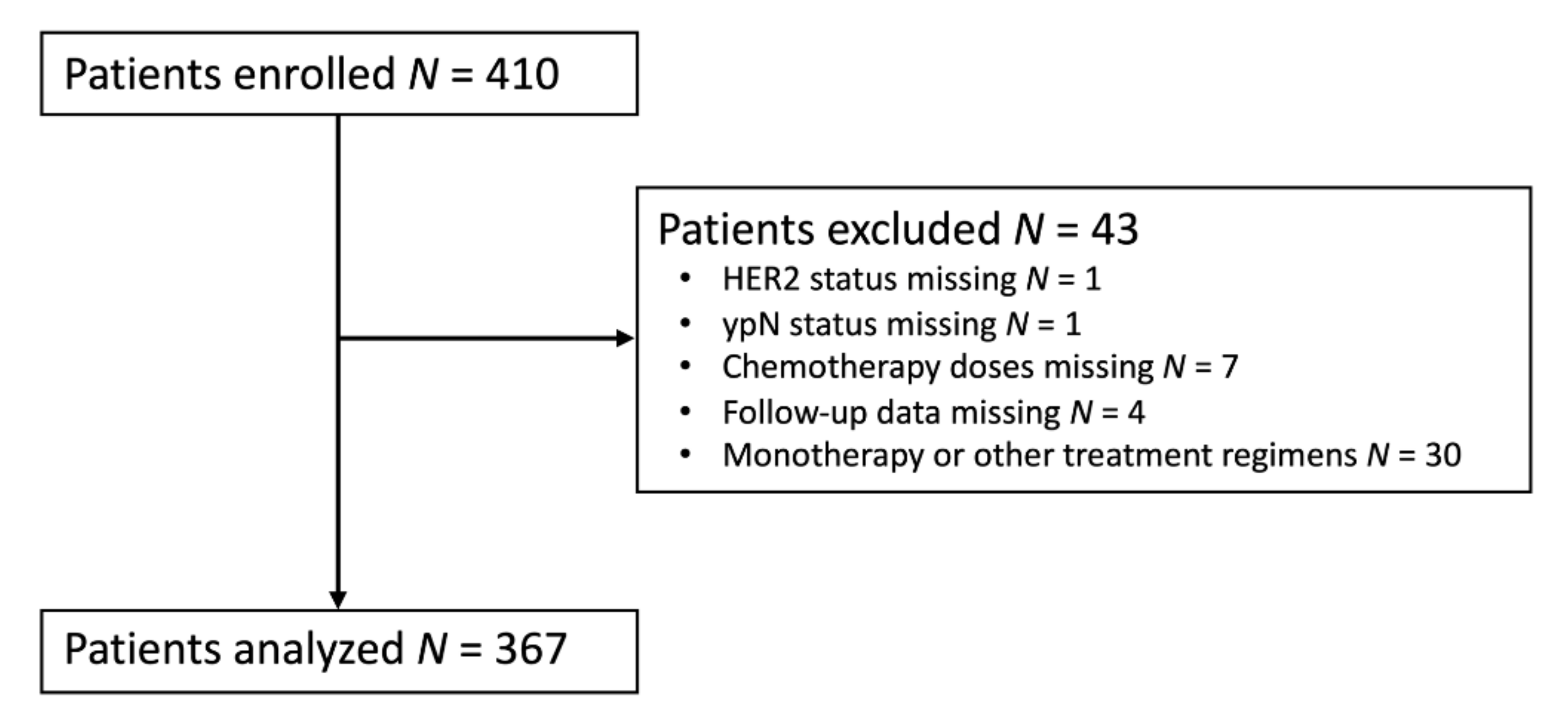
2. Materials and Methods
2.1. Study Design
2.2. Pathology and RCB Evaluation
2.3. Statistical Methods
3. Results
3.1. Patient Characteristics
3.2. Association of RCB Score with Clinical Outcome
3.3. Analysis of RCB Score and Association of A/T Dose Reduction with RCB Score and Clinical Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broglio, K.R.; Quintana, M.; Foster, M.; Olinger, M.; McGlothlin, A.; Berry, S.M.; Boileau, J.F.; Brezden-Masley, C.; Chia, S.; Dent, S.; et al. Association of Pathologic Complete Response to Neoadjuvant Therapy in HER2-Positive Breast Cancer With Long-Term Outcomes: A Meta-Analysis. JAMA Oncol. 2016, 2, 751–760. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Bossuyt, V.; Provenzano, E.; Symmans, W.F.; Boughey, J.C.; Coles, C.; Curigliano, G.; Dixon, J.M.; Esserman, L.J.; Fastner, G.; Kuehn, T.; et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann. Oncol. 2015, 26, 1280–1291. [Google Scholar] [CrossRef]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef]
- Symmans, W.F.; Wei, C.; Gould, R.; Yu, X.; Zhang, Y.; Liu, M.; Walls, A.; Bousamra, A.; Ramineni, M.; Sinn, B.; et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J. Clin. Oncol. 2017, 35, 1049–1060. [Google Scholar] [CrossRef]
- Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Taylor, C.; et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar] [CrossRef]
- Bonadonna, G.; Zambetti, M.; Valagussa, P. Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes. Ten-year results. JAMA 1995, 273, 542–547. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: A patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 2019, 393, 1440–1452. [Google Scholar] [CrossRef]
- Hryniuk, W.M.; Levine, M.N.; Levin, L. Analysis of dose intensity for chemotherapy in early (stage II) and advanced breast cancer. NCI Monogr 1986, 1, 87–94. [Google Scholar]
- Budman, D.R. Dose and schedule as determinants of outcomes in chemotherapy for breast cancer. Semin. Oncol. 2004, 31, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chirivella, I.; Bermejo, B.; Insa, A.; Pérez-Fidalgo, A.; Magro, A.; Rosello, S.; García-Garre, E.; Martín, P.; Bosch, A.; Lluch, A. Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res. Treat. 2009, 114, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.D.; Posch, F.; Suppan, C.; Bargfrieder, U.; Gumpoldsberger, M.; Hammer, R.; Hauser, H.; Dandachi, N.; Prein, K.; Stoeger, H.; et al. Validation of Residual Cancer Burden as Prognostic Factor for Breast Cancer Patients After Neoadjuvant Therapy. Ann. Surg. Oncol. 2019, 26, 4274–4283. [Google Scholar] [CrossRef] [PubMed]
- Hudis, C.A.; Barlow, W.E.; Costantino, J.P.; Gray, R.J.; Pritchard, K.I.; Chapman, J.A.; Sparano, J.A.; Hunsberger, S.; Enos, R.A.; Gelber, R.D.; et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J. Clin. Oncol. 2007, 25, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- MD Anderson Cancer Center Residual Cancer Burden Calculator. The University of Texas. Available online: www.mdanderson.org/breastcancer_RCB (accessed on 8 June 2018).
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Royston, P.; Parmar, M.K. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat. Med. 2002, 21, 2175–2197. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24, S26–S35. [Google Scholar] [CrossRef]
- Berruti, A.; Amoroso, V.; Gallo, F.; Bertaglia, V.; Simoncini, E.; Pedersini, R.; Ferrari, L.; Bottini, A.; Bruzzi, P.; Sormani, M.P. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: A meta-regression of 29 randomized prospective studies. J. Clin. Oncol. 2014, 32, 3883–3891. [Google Scholar] [CrossRef]
- Campbell, J.I.; Yau, C.; Krass, P.; Moore, D.; Carey, L.A.; Au, A.; Chhieng, D.; Giri, D.; Livasy, C.; Mies, C.; et al. Comparison of residual cancer burden, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: Results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res. Treat. 2017, 165, 181–191. [Google Scholar] [CrossRef]
- Choi, M.; Park, Y.H.; Ahn, J.S.; Im, Y.H.; Nam, S.J.; Cho, S.Y.; Cho, E.Y. Assessment of pathologic response and long-term outcome in locally advanced breast cancers after neoadjuvant chemotherapy: Comparison of pathologic classification systems. Breast Cancer Res. Treat. 2016, 160, 475–489. [Google Scholar] [CrossRef]
- Corben, A.D.; Abi-Raad, R.; Popa, I.; Teo, C.H.; Macklin, E.A.; Koerner, F.C.; Taghian, A.G.; Brachtel, E.F. Pathologic response and long-term follow-up in breast cancer patients treated with neoadjuvant chemotherapy: A comparison between classifications and their practical application. Arch. Pathol. Lab. Med. 2013, 137, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.; van der Noordaa, M.; Wei, J.; Osdoit, M.; Reyal, F.; Hamy, A.-S.; Lae, M.; Martin, M.; del Monte, M.; Boughey, J.C.; et al. Abstract GS5-01: Residual cancer burden after neoadjuvant therapy and long-term survival outcomes in breast cancer: A multi-center pooled analysis. Cancer Res. 2020, 80, GS5-01. [Google Scholar] [CrossRef]
- Cossetti, R.J.; Tyldesley, S.K.; Speers, C.H.; Zheng, Y.; Gelmon, K.A. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J. Clin. Oncol. 2015, 33, 65–73. [Google Scholar] [CrossRef]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Medeiros Alencar, V.H.; Badran, A.; Bonfill, X.; et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef]
- Hamy, A.-S.; Darrigues, L.; Laas, E.; De Croze, D.; Topciu, L.; Lam, G.-T.; Evrevin, C.; Rozette, S.; Laot, L.; Lerebours, F.; et al. Prognostic value of the Residual Cancer Burden index according to breast cancer subtype: Validation on a cohort of BC patients treated by neoadjuvant chemotherapy. PLoS ONE 2020, 15, e0234191. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Lluch, A.; Barrios, C.H.; Torrecillas, L.; Ruiz-Borrego, M.; Bines, J.; Segalla, J.; Guerrero-Zotano, Á.; García-Sáenz, J.A.; Torres, R.; Haba, J.D.L.; et al. Phase III Trial of Adjuvant Capecitabine After Standard Neo-/Adjuvant Chemotherapy in Patients With Early Triple-Negative Breast Cancer (GEICAM/2003-11_CIBOMA/2004-01). J. Clin. Oncol. 2020, 38, 203–213. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Norton, L. A Gompertzian model of human breast cancer growth. Cancer Res. 1988, 48, 7067–7071. [Google Scholar]
- Skipper, H.E. Kinetics of mammary tumor cell growth and implications for therapy. Cancer 1971, 28, 1479–1499. [Google Scholar] [CrossRef]
- De Azambuja, E.; Paesmans, M.; Beauduin, M.; Vindevoghel, A.; Cornez, N.; Finet, C.; Ries, F.; Closon-Dejardin, M.T.; Kerger, J.; Gobert, P.; et al. Long-term benefit of high-dose epirubicin in adjuvant chemotherapy for node-positive breast cancer: 15-year efficacy results of the Belgian multicentre study. J. Clin. Oncol. 2009, 27, 720–725. [Google Scholar] [CrossRef]
- Liutkauskiene, S.; Grizas, S.; Jureniene, K.; Suipyte, J.; Statnickaite, A.; Juozaityte, E. Retrospective analysis of the impact of anthracycline dose reduction and chemotherapy delays on the outcomes of early breast cancer molecular subtypes. BMC Cancer 2018, 18, 453. [Google Scholar] [CrossRef]
- Lyman, G.H.; Dale, D.C.; Crawford, J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: A nationwide study of community practices. J. Clin. Oncol. 2003, 21, 4524–4531. [Google Scholar] [CrossRef]



| n | Total | No RFS Event during Follow-Up | RFS Event during Follow-Up | p-Value | |
|---|---|---|---|---|---|
| (% miss.) | n = 367 | n = 307 | n = 60 | ||
| Age at neoadjuvant treatment start (years) | 367 (0) | 54.6 (47.0–63.3) | 54.7 (47.5–63.4) | 53.4 (45.0–59.5) | 0.149 |
| Female gender | 367 (0) | 365 (99.5%) | 306 (99.7%) | 59 (98.3%) | 0.301 |
| Molecular breast cancer subtype | 367 (0) | 0.146 | |||
| HR-positive/HER2- | 132 (36.0%) | 110 (35.8%) | 22 (36.7%) | ||
| HER2+ | 127 (34.6%) | 112 (36.5%) | 15 (25.0%) | ||
| Triple-negative | 108 (29.4%) | 85 (27.7%) | 23 (38.3%) | ||
| Histological grade | 358 (2.5) | 0.349 | |||
| G1 | 3 (0.8%) | 3 (1.0%) | 0 (0.0%) | ||
| G2 | 121 (33.8%) | 106 (35.2%) | 15 (26.3%) | ||
| G3 | 234 (65.4%) | 192 (63.8%) | 42 (73.7%) | ||
| Ki67 labeling index (%) | 366 (0.3) | 40.0 (27.5–70.0) | 40.0 (25.0–70.0) | 40.0 (30.0–70.0) | 0.357 |
| Surgical outcome | 367 (0) | 0.040 | |||
| Mastectomy | 112 (30.5%) | 87 (28.3%) | 25 (41.7%) | ||
| Breast conservation | 255 (69.5%) | 220 (71.7%) | 35 (58.3%) | ||
| Definitive axillary procedure | 367 (0) | <0.001 | |||
| Sentinel node biopsy (SNB) | 126 (34.3%) | 118 (38.4%) | 8 (13.3%) | ||
| Axillary lymph node dissection (ALND) | 241 (65.7%) | 189 (61.6%) | 52 (86.7%) | ||
| Post-neoadjuvant tumor category (ypT) | 367 (0) | <0.001 | |||
| ypTis-ypT0 | 140 (38.1%) | 127 (41.4%) | 13 (21.7%) | ||
| ypT1 | 157 (42.8%) | 132 (43.0%) | 25 (41.7%) | ||
| ypT2 | 51 (13.9%) | 37 (12.0%) | 14 (23.3%) | ||
| ypT3-ypT4 | 19 (5.2%) | 11 (3.6%) | 8 (13.3%) | ||
| Post-neoadjuvant nodal status (ypN) | 367 (0) | <0.001 | |||
| ypN0 | 265 (72.2%) | 235 (76.6%) | 30 (50.0%) | ||
| ypN1 | 64 (17.4%) | 51 (16.6%) | 13 (21.7%) | ||
| ypN2 | 32 (8.7%) | 19 (6.2%) | 13 (21.7%) | ||
| ypN3 | 6 (1.6%) | 2 (0.7%) | 4 (6.6%) | ||
| Number of positive nodes | 367 (0) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 1.0 (0.0–4.0) | <0.001 |
| Adjuvant endocrine therapy | 367 (0) | 191 (52.0%) | 162 (52.8%) | 29 (48.3%) | 0.529 |
| Adjuvant chemotherapy ± anti-HER2 | 367 (0) | 145 (39.5%) | 126 (41.0%) | 19 (31.7%) | 0.174 |
| RCB score | 367 (0) | 1.52 (0.00–2.34) | 1.33 (0.00–2.10) | 2.21 (1.54–3.60) | <0.001 |
| RCB class | 367 (0) | <0.001 | |||
| RCB Class 0 | 123 (33.5%) | 116 (37.8%) | 7 (11.7%) | ||
| RCB Class 1 | 47 (12.8%) | 41 (13.4%) | 6 (10.0%) | ||
| RCB Class 2 | 143 (39.0%) | 117 (38.1%) | 26 (43.3%) | ||
| RCB Class 3 | 54 (14.7%) | 33 (10.7%) | 21 (35.0%) |
| Variable | RFS (Events = 60) | DDFS (Events = 56) | OS (Events = 43) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| RCB score (by one-point increase) | 1.60 | 1.33–1.93 | <0.0001 | 1.70 | 1.39–2.05 | <0.0001 | 1.67 | 1.34–2.08 | <0.0001 |
| RCB class | |||||||||
| RCB Class 0 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| RCB Class 1 | 2.18 | 0.73–6.50 | 0.161 | 3.11 | 0.95–10.19 | 0.061 | 2.53 | 0.63–10.12 | 0.189 |
| RCB Class 2 | 3.15 | 1.37–7.27 | 0.007 | 4.23 | 1.62–11.07 | 0.003 | 4.23 | 1.47–12.55 | 0.008 |
| RCB Class 3 | 7.44 | 3.16–17.50 | <0.0001 | 10.23 | 3.84–27.25 | <0.0001 | 9.13 | 3.03–27.51 | <0.0001 |
| Age at treatment start (per five-year increase) | 0.92 | 0.82–1.03 | 0.134 | 0.92 | 0.82–1.04 | 0.166 | 0.88 | 0.77–1.01 | 0.065 |
| Molecular breast cancer subtype | |||||||||
| HR-positive/HER2- | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| HER2+ | 0.78 | 0.40–1.50 | 0.454 | 0.74 | 0.36–1.48 | 0.385 | 0.86 | 0.38–1.97 | 0.727 |
| Triple-negative | 1.46 | 0.82–2.63 | 0.202 | 1.58 | 0.87–2.88 | 0.135 | 2.08 | 1.04–4.19 | 0.040 |
| Tumor grade G3 | 1.35 | 0.75–2.43 | 0.321 | 1.69 | 0.89–3.21 | 0.108 | 1.38 | 0.70–2.75 | 0.353 |
| Ki67 (per 10% increase) | 1.05 | 0.94–1.17 | 0.392 | 1.07 | 0.96–1.20 | 0.215 | 1.10 | 0.97–1.25 | 0.137 |
| Breast conservation | 0.59 | 0.36–0.99 | 0.046 | 0.61 | 0.36–1.03 | 0.065 | 0.67 | 0.36–1.23 | 0.199 |
| Axillary lymph node dissection | 2.38 | 1.13–5.03 | 0.023 | 2.66 | 1.20–5.89 | 0.016 | 3.60 | 1.28–10.11 | 0.015 |
| Post-neoadjuvant tumor category (ypT) | |||||||||
| ypTis-ypT0 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| ypT1 | 1.67 | 0.85–3.26 | 0.135 | 1.91 | 0.93–3.90 | 0.076 | 2.12 | 0.93–4.84 | 0.075 |
| ypT2 | 3.10 | 1.46–6.60 | 0.003 | 3.40 | 1.52–7.59 | 0.003 | 3.72 | 1.47–9.43 | 0.006 |
| ypT3-ypT4 | 4.95 | 2.05–11.95 | <0.0001 | 6.35 | 2.55–15.80 | <0.0001 | 5.63 | 1.95–16.25 | 0.001 |
| Number of positive nodes (per 1 increase) | 1.15 | 1.09–1.20 | <0.0001 | 1.16 | 1.10–1.21 | <0.0001 | 1.15 | 1.08–1.22 | <0.0001 |
| Post-neoadjuvant nodal status (ypN) | |||||||||
| ypN0 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| ypN1 | 1.86 | 0.97–3.56 | 0.062 | 1.91 | 0.97–3.77 | 0.062 | 1.77 | 0.81–3.84 | 0.150 |
| ypN2 | 4.20 | 2.19–8.06 | <0.0001 | 4.67 | 2.41–9.06 | <0.0001 | 4.40 | 2.13–9.08 | <0.0001 |
| ypN3 | 7.37 | 2.59–20.98 | <0.0001 | 11.01 | 3.83–31.67 | <0.0001 | 2.47 | 0.33–18.30 | 0.378 |
| Adjuvant endocrine therapy | 0.81 | 0.49–1.34 | 0.406 | 0.88 | 0.52–1.48 | 0.627 | 0.63 | 0.34–1.15 | 0.132 |
| Adjuvant chemotherapy ± anti-HER2 | 0.83 | 0.48–1.42 | 0.489 | 0.77 | 0.43–1.35 | 0.359 | 0.84 | 0.44–1.58 | 0.584 |
| NAC dose modification | 1.25 | 0.75–2.08 | 0.392 | 1.16 | 0.68–1.97 | 0.584 | 1.18 | 0.65–2.15 | 0.587 |
| Cumulative A/T doses (per 100 units increase) | 0.99 | 0.91–1.06 | 0.713 | 1.02 | 0.95–1.11 | 0.534 | 1.01 | 0.93–1.1 | 0.793 |
| Models | Variable | Regression Coefficient β | 95% CI | p-Value |
|---|---|---|---|---|
| Univariable models | Age at treatment start (per five-year increase) | 0.04 | −0.02–0.10 | 0.179 |
| Molecular subtype | ||||
| HR+ | Ref. | Ref. | Ref. | |
| HER2+ | −1.40 | −1.69 to (−1.10) | <0.0001 | |
| Triple-negative | −1.07 | −1.38 to (−0.77) | <0.0001 | |
| Tumor grade G3 | −0.79 | −1.07 to (−0.51) | <0.0001 | |
| Ki67 index (per 10% increase) | −0.17 | −0.22 to (−0.11) | <0.0001 | |
| Dose modification | 0.06 | −0.22–0.33 | 0.689 | |
| Cumulative A + T dose (per 100 units increase) | −0.01 | −0.06–0.03 | 0.532 | |
| Multi-variable model #1 | Molecular subtype | |||
| HR+ | Ref. | Ref. | Ref. | |
| HER2+ | −1.41 | −1.68 to (−1.13) | <0.0001 | |
| Triple-negative | −0.67 | −0.99 to (−0.36) | <0.0001 | |
| Ki67 index (per 10% increase) | −0.17 | −0.23 to (−0.12) | <0.0001 | |
| Multi-variable model #2 | Dose modification | 1.11 | −0.01–2.24 | 0.053 |
| Cumulative A + T dose (per 100 units increase) | 0.04 | −0.03–0.12 | 0.262 | |
| Dose modification # cumulative A + T dose a | −0.10 | −0.20–0.00 | 0.047 | |
| Multi-variable model #3 | Molecular subtype | |||
| HR+ | Ref. | Ref. | Ref. | |
| HER2+ | −1.42 | −1.69 to (−1.14) | <0.0001 | |
| Triple-negative | −0.68 | −1.00 to (−0.36) | <0.0001 | |
| Ki67 index (per 10% increase) | −0.17 | −0.23 to (−0.11) | <0.0001 | |
| Dose modification | 0.95 | −0.01–1.92 | 0.052 | |
| Cumulative A + T dose (per 100 units increase) | 0.03 | −0.05–0.09 | 0.392 | |
| Dose modification # cumulative A + T dose a | −0.09 | −0.17–0.00 | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suppan, C.; Posch, F.; Mueller, H.D.; Mischitz, N.; Steiner, D.; Klocker, E.V.; Setaffy, L.; Bargfrieder, U.; Hammer, R.; Hauser, H.; et al. Patterns of Recurrence after Neoadjuvant Therapy in Early Breast Cancer, according to the Residual Cancer Burden Index and Reductions in Neoadjuvant Treatment Intensity. Cancers 2021, 13, 2492. https://doi.org/10.3390/cancers13102492
Suppan C, Posch F, Mueller HD, Mischitz N, Steiner D, Klocker EV, Setaffy L, Bargfrieder U, Hammer R, Hauser H, et al. Patterns of Recurrence after Neoadjuvant Therapy in Early Breast Cancer, according to the Residual Cancer Burden Index and Reductions in Neoadjuvant Treatment Intensity. Cancers. 2021; 13(10):2492. https://doi.org/10.3390/cancers13102492
Chicago/Turabian StyleSuppan, Christoph, Florian Posch, Hannah Deborah Mueller, Nina Mischitz, Daniel Steiner, Eva Valentina Klocker, Lisa Setaffy, Ute Bargfrieder, Robert Hammer, Hubert Hauser, and et al. 2021. "Patterns of Recurrence after Neoadjuvant Therapy in Early Breast Cancer, according to the Residual Cancer Burden Index and Reductions in Neoadjuvant Treatment Intensity" Cancers 13, no. 10: 2492. https://doi.org/10.3390/cancers13102492
APA StyleSuppan, C., Posch, F., Mueller, H. D., Mischitz, N., Steiner, D., Klocker, E. V., Setaffy, L., Bargfrieder, U., Hammer, R., Hauser, H., Jost, P. J., Dandachi, N., Lax, S., & Balic, M. (2021). Patterns of Recurrence after Neoadjuvant Therapy in Early Breast Cancer, according to the Residual Cancer Burden Index and Reductions in Neoadjuvant Treatment Intensity. Cancers, 13(10), 2492. https://doi.org/10.3390/cancers13102492







