Harnessing CD16-Mediated NK Cell Functions to Enhance Therapeutic Efficacy of Tumor-Targeting mAbs
Abstract
:Simple Summary
Abstract
1. Introduction
2. NK Cells as Participants to Antitumor Immunosurveillance
3. CD16 as a Relevant Receptor for Tumor-Targeting mAb Therapeutic Efficacy: The Contribution of NK Cells
4. NK Cells as Players in Tumor-Targeting mAb-Dependent “Vaccinal Effect”
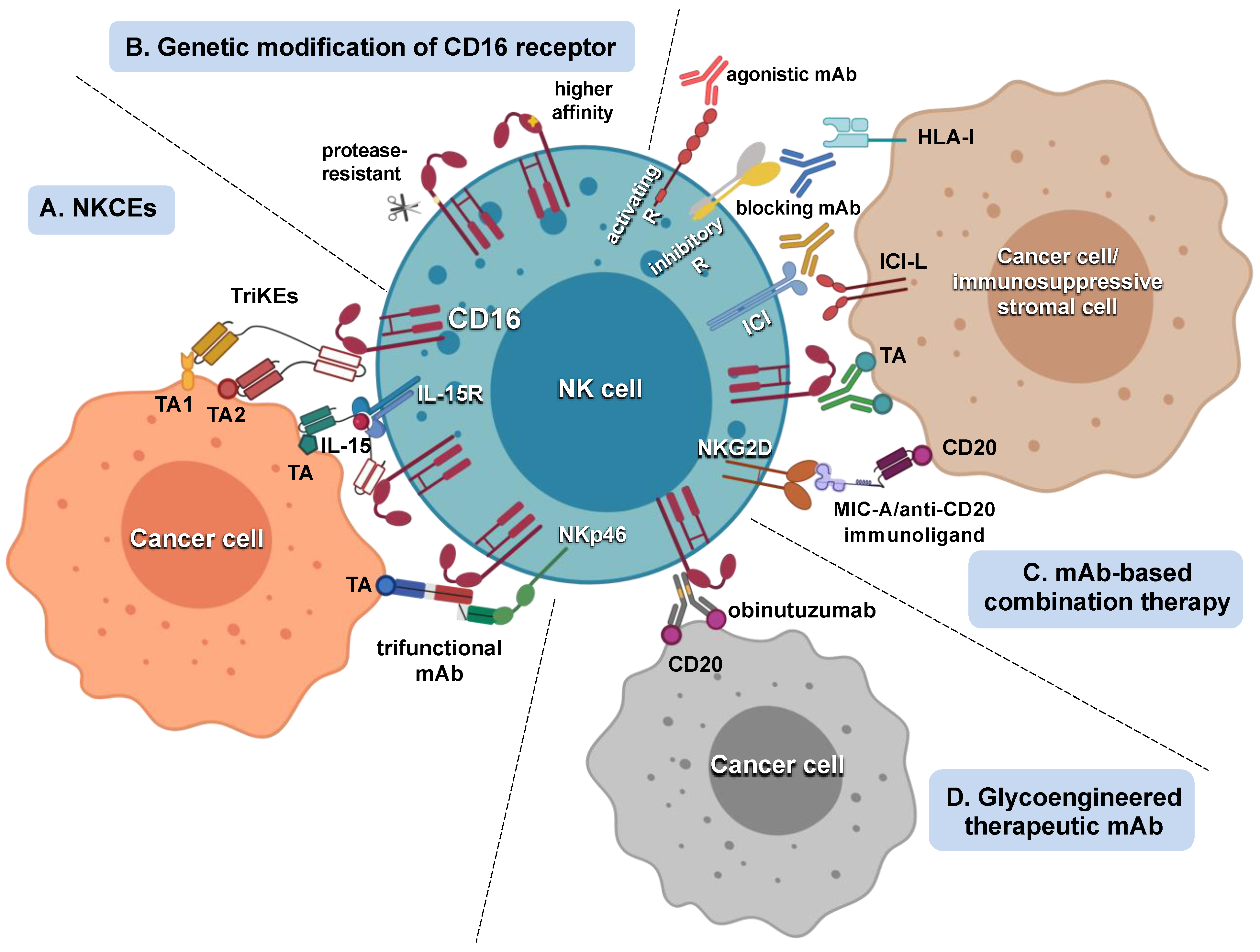
5. Strategies Aimed at Potentiating NK Cell Response to Tumor-Targeting mAbs
5.1. Strategies That Improve mAb Interaction with CD16
5.2. Strategies That Amplify NK Cell Responsiveness to CD16 Engagement
5.3. Tumor-Targeting mAb/Adoptive NK Cell Therapy Combination
6. Memory NK Cells as Emergent Effectors in mAb-Based Antitumor Approaches
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | antibody-dependent cell-mediated cytotoxicity |
| BiKE | bispecific killer cell engager |
| TriKE | trispecific killer cell engager |
| CLL | chronic lymphocytic leukemia |
| CRC | colorectal cancer |
| CSC | cancer stem cells |
| DC | dendritic cell |
| (ADAM)17 | disintegrin and metalloproteinase |
| FcγR | Fc gamma receptor |
| FL | follicular lymphoma |
| ITAM | immunoreceptor tyrosine-based activation motif |
| HCMV | human cytomegalovirus |
| HNSCC | head and neck squamous cell carcinoma |
| HSCT | hematopoietic stem-cell transplantation |
| IFNγ | interferon gamma |
| KIR | killer-cell immunoglobulin-like receptor |
| mAb | monoclonal antibody |
| NCR | natural cytotoxicity receptor |
| NK | natural killer |
| NKCE | NK cell engagers |
| NKG2D | natural killer group 2D |
| TA | tumor-associated antigen |
| TME | tumor microenvironment |
References
- Ferris, R.L.; Jaffee, E.M.; Ferrone, S. Tumor Antigen-Targeted, Monoclonal Antibody-Based Immunotherapy: Clinical Response, Cellular Immunity, and Immunoescape. J. Clin. Oncol. 2010, 28, 4390–4399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody Therapy of Cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Sliwkowski, M.X.; Mellman, I. Antibody Therapeutics in Cancer. Science 2013, 341, 1192–1198. [Google Scholar] [CrossRef]
- Wang, W.; Erbe, A.K.; Hank, J.A.; Morris, Z.S.; Sondel, P.M. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front. Immunol. 2015, 6, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinchieri, G.; Valiante, N. Receptors for the Fc Fragment of IgG on Natural Killer Cells. Nat. Immun. 1993, 12, 218–234. [Google Scholar]
- Long, E.O.; Sik Kim, H.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling Natural Killer Cell Responses: Integration of Signals for Activation and Inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef] [Green Version]
- Yeap, W.H.; Wong, K.L.; Shimasaki, N.; Teo, E.C.Y.; Quek, J.K.S.; Yong, H.X.; Diong, C.P.; Bertoletti, A.; Linn, Y.C.; Wong, S.C. CD16 Is Indispensable for Antibody-Dependent Cellular Cytotoxicity by Human Monocytes. Sci. Rep. 2016, 6, 34310. [Google Scholar] [CrossRef]
- Lanier, L.L. Up on the Tightrope: Natural Killer Cell Activation and Inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and Granzymes: Function, Dysfunction and Human Pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Wallin, R.P.A.; Screpanti, V.; Michaëlsson, J.; Grandien, A.; Ljunggren, H.-G. Regulation of Perforin-Independent NK Cell-Mediated Cytotoxicity. Eur. J. Immunol. 2003, 33, 2727–2735. [Google Scholar] [CrossRef]
- Smyth, M.J.; Cretney, E.; Kelly, J.M.; Westwood, J.A.; Street, S.E.A.; Yagita, H.; Takeda, K.; van Dommelen, S.L.H.; Degli-Esposti, M.A.; Hayakawa, Y. Activation of NK Cell Cytotoxicity. Mol. Immunol. 2005, 42, 501–510. [Google Scholar] [CrossRef]
- Cretney, E.; Takeda, K.; Yagita, H.; Glaccum, M.; Peschon, J.J.; Smyth, M.J. Increased Susceptibility to Tumor Initiation and Metastasis in TNF-Related Apoptosis-Inducing Ligand-Deficient Mice. J. Immunol. 2002, 168, 1356–1361. [Google Scholar] [CrossRef] [Green Version]
- Prager, I.; Watzl, C. Mechanisms of Natural Killer Cell-mediated Cellular Cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef]
- Warren, H.S.; Kinnear, B.F. Quantitative Analysis of the Effect of CD16 Ligation on Human NK Cell Proliferation. J. Immunol. 1999, 162, 735–742. [Google Scholar]
- Ortaldo, J.R.; Mason, A.T.; O’Shea, J.J. Receptor-Induced Death in Human Natural Killer Cells: Involvement of CD16. J. Exp. Med. 1995, 181, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Morvan, M.G.; Lanier, L.L. NK Cells and Cancer: You Can Teach Innate Cells New Tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Battella, S.; Cox, M.C.; Santoni, A.; Palmieri, G. Natural Killer (NK) Cells and Anti-Tumor Therapeutic MAb: Unexplored Interactions. J. Leukoc. Biol. 2016, 99, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of CDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef] [Green Version]
- Martín-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced Recruitment of NK Cells to Lymph Nodes Provides IFN-Gamma for T(H)1 Priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef]
- Walzer, T.; Dalod, M.; Robbins, S.H.; Zitvogel, L.; Vivier, E. Natural-Killer Cells and Dendritic Cells: “L’union Fait La Force”. Blood 2005, 106, 2252–2258. [Google Scholar] [CrossRef] [Green Version]
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D. Interferons, Immunity and Cancer Immunoediting. Nat. Rev. Immunol. 2006, 6, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I.S.; Coudert, J.D.; Andoniou, C.E.; Degli-Esposti, M.A. “Natural Regulators”: NK Cells as Modulators of T Cell Immunity. Front. Immunol. 2016, 7, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abès, R.; Gélizé, E.; Fridman, W.H.; Teillaud, J.-L. Long-Lasting Antitumor Protection by Anti-CD20 Antibody through Cellular Immune Response. Blood 2010, 116, 926–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiLillo, D.J.; Ravetch, J.V. Fc-Receptor Interactions Regulate Both Cytotoxic and Immunomodulatory Therapeutic Antibody Effector Functions. Cancer Immunol. Res. 2015, 3, 704–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahl, J.; Cerwenka, A. Tricking the Balance: NK Cells in Anti-Cancer Immunity. Immunobiology 2017, 222, 11–20. [Google Scholar] [CrossRef]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting Natural Killer Cells in Cancer Immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Malmberg, K.J.; Carlsten, M.; Björklund, A.; Sohlberg, E.; Bryceson, Y.T.; Ljunggren, H.G. Natural Killer Cell-Mediated Immunosurveillance of Human Cancer. Semin. Immunol. 2017, 31, 20–29. [Google Scholar] [CrossRef]
- Shimasaki, N.; Jain, A.; Campana, D. NK Cells for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Trinchieri, G. Biology of Natural Killer Cells. Adv. Immunol. 1989, 47, 187–376. [Google Scholar] [CrossRef]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Activation, Coactivation, and Costimulation of Resting Human Natural Killer Cells. Immunol. Rev. 2006, 214, 73–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinet, L.; Smyth, M.J. Balancing Natural Killer Cell Activation through Paired Receptors. Nat. Rev. Immunol. 2015, 15, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Chester, C.; Fritsch, K.; Kohrt, H.E. Natural Killer Cell Immunomodulation: Targeting Activating, Inhibitory, and Co-Stimulatory Receptor Signaling for Cancer Immunotherapy. Front. Immunol. 2015, 6, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Arooj, S.; Wang, H. NK Cell-Based Immune Checkpoint Inhibition. Front. Immunol. 2020, 11, 167. [Google Scholar] [CrossRef]
- Dougall, W.C.; Kurtulus, S.; Smyth, M.J.; Anderson, A.C. TIGIT and CD96: New Checkpoint Receptor Targets for Cancer Immunotherapy. Immunol. Rev. 2017, 276, 112–120. [Google Scholar] [CrossRef]
- Muntasell, A.; Ochoa, M.C.; Cordeiro, L.; Berraondo, P.; López-Díaz de Cerio, A.; Cabo, M.; López-Botet, M.; Melero, I. Targeting NK-Cell Checkpoints for Cancer Immunotherapy. Curr. Opin. Immunol. 2017, 45, 73–81. [Google Scholar] [CrossRef]
- Beldi-Ferchiou, A.; Lambert, M.; Dogniaux, S.; Vély, F.; Vivier, E.; Olive, D.; Dupuy, S.; Levasseur, F.; Zucman, D.; Lebbé, C.; et al. PD-1 Mediates Functional Exhaustion of Activated NK Cells in Patients with Kaposi Sarcoma. Oncotarget 2016, 7, 72961–72977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Shmuel, A.; Biber, G.; Barda-Saad, M. Unleashing Natural Killer Cells in the Tumor Microenvironment—The Next Generation of Immunotherapy? Front. Immunol. 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerwenka, A.; Lanier, L.L. Natural Killer Cell Memory in Infection, Inflammation and Cancer. Nat. Rev. Immunol. 2016, 16, 112–123. [Google Scholar] [CrossRef]
- Rölle, A.; Brodin, P. Immune Adaptation to Environmental Influence: The Case of NK Cells and HCMV. Trends Immunol. 2016, 37, 233–243. [Google Scholar] [CrossRef]
- Huntington, N.D.; Cursons, J.; Rautela, J. The Cancer–Natural Killer Cell Immunity Cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar] [CrossRef]
- Pross, H.F.; Lotzová, E. Role of Natural Killer Cells in Cancer. Nat. Immun. 1993, 12, 279–292. [Google Scholar]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural Cytotoxic Activity of Peripheral-Blood Lymphocytes and Cancer Incidence: An 11-Year Follow-up Study of a General Population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Habif, G.; Crinier, A.; André, P.; Vivier, E.; Narni-Mancinelli, E. Targeting Natural Killer Cells in Solid Tumors. Cell. Mol. Immunol. 2019, 16, 415–422. [Google Scholar] [CrossRef]
- Kruse, P.H.; Matta, J.; Ugolini, S.; Vivier, E. Natural Cytotoxicity Receptors and Their Ligands. Immunol. Cell Biol. 2014, 92, 221–229. [Google Scholar] [CrossRef]
- Miller, J.S.; Lanier, L.L. Natural Killer Cells in Cancer Immunotherapy. Annu. Rev. Cancer Biol. 2019, 3, 77–103. [Google Scholar] [CrossRef] [Green Version]
- Chretien, A.S.; Le Roy, A.; Vey, N.; Prebet, T.; Blaise, D.; Fauriat, C.; Olive, D. Cancer-Induced Alterations of NK-Mediated Target Recognition: Current and Investigational Pharmacological Strategies Aiming at Restoring NK-Mediated Anti-Tumor Activity. Front. Immunol. 2014, 5, 122. [Google Scholar] [CrossRef] [Green Version]
- Marcus, A.; Gowen, B.G.; Thompson, T.W.; Iannello, A.; Ardolino, M.; Deng, W.; Wang, L.; Shifrin, N.; Raulet, D.H. Recognition of Tumors by the Innate Immune System and Natural Killer Cells. Adv. Immunol. 2014, 122, 91–128. [Google Scholar] [CrossRef] [Green Version]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural Killer Cells and Other Innate Lymphoid Cells in Cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Barrow, A.D.; Martin, C.J.; Colonna, M. The Natural Cytotoxicity Receptors in Health and Disease. Front. Immunol. 2019, 10, 909. [Google Scholar] [CrossRef] [Green Version]
- Luna, J.I.; Grossenbacher, S.K.; Murphy, W.J.; Canter, R.J. Targeting Cancer Stem Cells with Natural Killer Cell Immunotherapy. Expert Opin. Biol. Ther. 2017, 17, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Nanut, M.P.; Ko, M.W.; Safaie, T.; Kos, J.; Jewett, A. Natural Killer Cells Target and Differentiate Cancer Stem-like Cells/Undifferentiated Tumors: Strategies to Optimize Their Growth and Expansion for Effective Cancer Immunotherapy. Curr. Opin. Immunol. 2018, 51, 170–180. [Google Scholar] [CrossRef]
- Tallerico, R.; Garofalo, C.; Carbone, E. A New Biological Feature of Natural Killer Cells: The Recognition of Solid Tumor-Derived Cancer Stem Cells. Front. Immunol. 2016, 7, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonangeli, F.; Zingoni, A.; Soriani, A.; Santoni, A. Senescent Cells: Living or Dying Is a Matter of NK Cells. J. Leukoc. Biol. 2019, 105, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.M.; Seligson, N.D.; Ahmad, S.M.; Rasool, R.U.; Gandhi, S.G.; Bhagat, M.; Goswami, A. Convergence of Therapy-Induced Senescence (TIS) and EMT in Multistep Carcinogenesis: Current Opinions and Emerging Perspectives. Cell Death Discov. 2020, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, B.C.; Hsu, K.C. Selection of Allogeneic Hematopoietic Cell Transplant Donors to Optimize Natural Killer Cell Alloreactivity. Semin. Hematol. 2020, 57, 167–174. [Google Scholar] [CrossRef]
- Yao, X.; Matosevic, S. Chemokine Networks Modulating Natural Killer Cell Trafficking to Solid Tumors. Cytokine Growth Factor Rev. 2021, 59, 36–45. [Google Scholar] [CrossRef]
- Bernardini, G.; Antonangeli, F.; Bonanni, V.; Santoni, A. Dysregulation of Chemokine/Chemokine Receptor Axes and NK Cell Tissue Localization during Diseases. Front. Immunol. 2016, 7, 402. [Google Scholar] [CrossRef]
- Castriconi, R.; Carrega, P.; Dondero, A.; Bellora, F.; Casu, B.; Regis, S.; Ferlazzo, G.; Bottino, C. Molecular Mechanisms Directing Migration and Retention of Natural Killer Cells in Human Tissues. Front. Immunol. 2018, 9, 2324. [Google Scholar] [CrossRef]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK Cell–Cancer Cycle: Advances and New Challenges in NK Cell–Based Immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef]
- Bald, T.; Pedde, A.-M.; Corvino, D.; Böttcher, J.P. The Role of NK Cell as Central Communicators in Cancer Immunity. Adv. Immunol. 2020, 147, 61–88. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.; Hu, B.; Wang, P.; Lv, X.; Chen, S.; Shao, Z. Prognostic Significance of Tumor-Infiltrating Natural Killer Cells in Solid Tumors: A Systematic Review and Meta-Analysis. Front. Immunol. 2020, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Nersesian, S.; Schwartz, S.L.; Grantham, S.R.; MacLean, L.K.; Lee, S.N.; Pugh-Toole, M.; Boudreau, J.E. NK Cell Infiltration Is Associated with Improved Overall Survival in Solid Cancers: A Systematic Review and Meta-Analysis. Transl. Oncol. 2021, 14, 100930. [Google Scholar] [CrossRef] [PubMed]
- Shembrey, C.; Huntington, N.D.; Hollande, F. Impact of Tumor and Immunological Heterogeneity on the Anti-Cancer Immune Response. Cancers 2019, 11, 1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cózar, B.; Greppi, M.; Carpentier, S.; Narni-Mancinelli, E.; Chiossone, L.; Vivier, E. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2020, 11, 34–44. [Google Scholar] [CrossRef]
- Ali, T.H.; Pisanti, S.; Ciaglia, E.; Mortarini, R.; Anichini, A.; Garofalo, C.; Tallerico, R.; Santinami, M.; Gulletta, E.; Ietto, C.; et al. Enrichment of CD56dimKIR+CD57+ Highly Cytotoxic NK Cells in Tumour-Infiltrated Lymph Nodes of Melanoma Patients. Nat. Commun. 2014, 5, 5639. [Google Scholar] [CrossRef] [PubMed]
- Messaoudene, M.; Fregni, G.; Fourmentraux-Neves, E.; Chanal, J.; Maubec, E.; Mazouz-Dorval, S.; Couturaud, B.; Girod, A.; Sastre-Garau, X.; Albert, S.; et al. Mature Cytotoxic CD56Bright/CD16+ Natural Killer Cells Can Infiltrate Lymph Nodes Adjacent to Metastatic Melanoma. Cancer Res. 2014, 74, 81–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, E.; Carlomagno, S.; Pesce, S.; Muccio, L.; Obino, V.; Greppi, M.; Solari, A.; Setti, C.; Marcenaro, E.; Della Chiesa, M.; et al. Role of the Main Non HLA-Specific Activating NK Receptors in Pancreatic, Colorectal and Gastric Tumors Surveillance. Cancers 2020, 12, 3705. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C.; Smyth, M.J. NK Cells and Cancer Immunoediting. Curr. Top. Microbiol. Immunol. 2016, 395, 115–145. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Maurer, S.; Ferrari de Andrade, L. NK Cell Interaction with Platelets and Myeloid Cells in the Tumor Milieu. Front. Immunol. 2020, 11, 608849. [Google Scholar] [CrossRef]
- Gaggero, S.; Witt, K.; Carlsten, M.; Mitra, S. Cytokines Orchestrating the Natural Killer-Myeloid Cell Crosstalk in the Tumor Microenvironment: Implications for Natural Killer Cell-Based Cancer Immunotherapy. Front. Immunol. 2021, 11, 621225. [Google Scholar] [CrossRef]
- Regis, S.; Dondero, A.; Caliendo, F.; Bottino, C.; Castriconi, R. NK Cell Function Regulation by TGF-β-Induced Epigenetic Mechanisms. Front. Immunol. 2020, 11, 311. [Google Scholar] [CrossRef] [Green Version]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Hasmim, M.; Messai, Y.; Ziani, L.; Thiery, J.; Bouhris, J.H.; Noman, M.Z.; Chouaib, S. Critical Role of Tumor Microenvironment in Shaping NK Cell Functions: Implication of Hypoxic Stress. Front. Immunol. 2015, 6, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrén, I.; Orrantia, A.; Vitallé, J.; Zenarruzabeitia, O.; Borrego, F. NK Cell Metabolism and Tumor Microenvironment. Front. Immunol. 2019, 10, 2278. [Google Scholar] [CrossRef] [PubMed]
- Devillier, R.; Chrétien, A.; Pagliardini, T.; Salem, N.; Blaise, D.; Olive, D. Mechanisms of NK Cell Dysfunction in the Tumor Microenvironment and Current Clinical Approaches to Harness NK Cell Potential for Immunotherapy. J. Leukoc. Biol. 2020. (online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.R.; Trindade, E.S.; Souza-Fonseca-Guimaraes, F. Tumor Microenvironment-Associated Extracellular Matrix Components Regulate NK Cell Function. Front. Immunol. 2020, 11, 73. [Google Scholar] [CrossRef]
- Seidel, U.J.E.; Schlegel, P.; Lang, P. Natural Killer Cell Mediated Antibody-Dependent Cellular Cytotoxicity in Tumor Immunotherapy with Therapeutic Antibodies. Front. Immunol. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, G.J. Building Better Monoclonal Antibody-Based Therapeutics. Nat. Rev. Cancer 2015, 15, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Marshall, M.J.E.; Stopforth, R.J.; Cragg, M.S. Therapeutic Antibodies: What Have We Learnt from Targeting CD20 and Where Are We Going? Front. Immunol. 2017, 8, 1245. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.P.; Lindorfer, M.A. Immunotherapeutic Mechanisms of Anti-CD20 Monoclonal Antibodies. Curr. Opin. Immunol. 2008, 20, 444–449. [Google Scholar] [CrossRef] [Green Version]
- VanDerMeid, K.R.; Elliott, M.R.; Baran, A.M.; Barr, P.M.; Chu, C.C.; Zent, C.S. Cellular Cytotoxicity of Next-Generation CD20 Monoclonal Antibodies. Cancer Immunol. Res. 2018, 6, 1150–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golay, J. Direct Targeting of Cancer Cells with Antibodies: What Can We Learn from the Successes and Failure of Unconjugated Antibodies for Lymphoid Neoplasias? J. Autoimmun. 2017, 85, 6–19. [Google Scholar] [CrossRef]
- Nagelkerke, S.Q.; Schmidt, D.E.; de Haas, M.; Kuijpers, T.W. Genetic Variation in Low-To-Medium-Affinity Fcγ Receptors: Functional Consequences, Disease Associations, and Opportunities for Personalized Medicine. Front. Immunol. 2019, 10, 2237. [Google Scholar] [CrossRef] [Green Version]
- Kaifu, T.; Nakamura, A. Polymorphisms of Immunoglobulin Receptors and the Effects on Clinical Outcome in Cancer Immunotherapy and Other Immune Diseases: A General Review. Int. Immunol. 2017, 29, 319–325. [Google Scholar] [CrossRef]
- Zhang, W.; Gordon, M.; Schultheis, A.M.; Yang, D.Y.; Nagashima, F.; Azuma, M.; Chang, H.M.; Borucka, E.; Lurje, G.; Sherrod, A.E.; et al. FCGR2A and FCGR3A Polymorphisms Associated With Clinical Outcome of Epidermal Growth Factor Receptor–Expressing Metastatic Colorectal Cancer Patients Treated With Single-Agent Cetuximab. JCO 2007, 25, 3712–3718. [Google Scholar] [CrossRef] [PubMed]
- Persky, D.O.; Dornan, D.; Goldman, B.H.; Braziel, R.M.; Fisher, R.I.; LeBlanc, M.; Maloney, D.G.; Press, O.W.; Miller, T.P.; Rimsza, L.M. Fc Gamma Receptor 3a Genotype Predicts Overall Survival in Follicular Lymphoma Patients Treated on SWOG Trials with Combined Monoclonal Antibody plus Chemotherapy but Not Chemotherapy Alone. Haematologica 2012, 97, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.M.; Ottaiano, A.; Romano, C.; Nasti, G.; Nappi, A.; De Divitiis, C.; Napolitano, M.; Zanotta, S.; Casaretti, R.; D’Alterio, C.; et al. Antibody-Dependent Cell Cytotoxicity in Metastatic Colorectal Cancer Patients Predicts Treatment Efficacy. Cancer Immunol. Res. 2016, 4, 4366–4374. [Google Scholar] [CrossRef] [Green Version]
- Mellor, J.D.; Brown, M.P.; Irving, H.R.; Zalcberg, J.R.; Dobrovic, A. A Critical Review of the Role of Fc Gamma Receptor Polymorphisms in the Response to Monoclonal Antibodies in Cancer. J. Hematol. Oncol. 2013, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Ghesquières, H.; Cartron, G.; Seymour, J.F.; Delfau-Larue, M.H.; Offner, F.; Soubeyran, P.; Perrot, A.; Brice, P.; Bouabdallah, R.; Sonet, A.; et al. Clinical Outcome of Patients with Follicular Lymphoma Receiving Chemoimmunotherapy in the PRIMA Study Is Not Affected by FCGR3A and FCGR2A Polymorphisms. Blood 2012, 120, 2650–2657. [Google Scholar] [CrossRef]
- Carlotti, E.; Palumbo, G.A.; Oldani, E.; Tibullo, D.; Salmoiraghi, S.; Rossi, A.; Golay, J.; Pulsoni, A.; Foa, R.; Rambaldi, A. FcgammaRIIIA and FcgammaRIIA Polymorphisms Do Not Predict Clinical Outcome of Follicular Non-Hodgkin’s Lymphoma Patients Treated with Sequential CHOP and Rituximab. Haematologica 2007, 92, 1127–1130. [Google Scholar] [CrossRef] [Green Version]
- Mitrovic, Z.; Aurer, I.; Radman, I.; Ajdukovic, R.; Sertic, J.; Labar, B. FCgammaRIIIA and FCgammaRIIA Polymorphisms Are Not Associated with Response to Rituximab and CHOP in Patients with Diffuse Large B-Cell Lymphoma. Haematologica 2007, 92, 998–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bournazos, S.; Woof, J.M.; Hart, S.P.; Dransfield, I. Functional and Clinical Consequences of Fc Receptor Polymorphic and Copy Number Variants. Clin. Exp. Immunol. 2009, 157, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Ahlgrimm, M.; Pfreundschuh, M.; Kreuz, M.; Regitz, E.; Preuss, K.-D.; Bittenbring, J. The Impact of Fc-γ Receptor Polymorphisms in Elderly Patients with Diffuse Large B-Cell Lymphoma Treated with CHOP with or without Rituximab. Blood 2011, 118, 4657–4662. [Google Scholar] [CrossRef] [PubMed]
- Arnould, L.; Gelly, M.; Penault-Llorca, F.; Benoit, L.; Bonnetain, F.; Migeon, C.; Cabaret, V.; Fermeaux, V.; Bertheau, P.; Garnier, J.; et al. Trastuzumab-Based Treatment of HER2-Positive Breast Cancer: An Antibody-Dependent Cellular Cytotoxicity Mechanism? Br. J. Cancer 2006, 94, 259–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, W.J.; Erbe, A.K.; Schwarz, C.N.; Jaquish, A.A.; Anderson, B.R.; Sriramaneni, R.N.; Jagodinsky, J.C.; Bates, A.M.; Clark, P.A.; Le, T.; et al. Tumor-Specific Antibody, Cetuximab, Enhances the In Situ Vaccine Effect of Radiation in Immunologically Cold Head and Neck Squamous Cell Carcinoma. Front. Immunol. 2020, 11, 591139. [Google Scholar] [CrossRef] [PubMed]
- Muntasell, A.; Rojo, F.; Servitja, S.; Rubio-Perez, C.; Cabo, M.; Tamborero, D.; Costa-García, M.; Martínez-Garcia, M.; Menéndez, S.; Vazquez, I.; et al. NK Cell Infiltrates and HLA Class I Expression in Primary HER2+ Breast Cancer Predict and Uncouple Pathological Response and Disease-Free Survival. Clin. Cancer Res. 2019, 25, 1535–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muntasell, A.; Servitja, S.; Cabo, M.; Bermejo, B.; Pérez-Buira, S.; Rojo, F.; Costa-García, M.; Arpí, O.; Moraru, M.; Serrano, L.; et al. High Numbers of Circulating CD57+ NK Cells Associate with Resistance to HER2-Specific Therapeutic Antibodies in HER2+ Primary Breast Cancer. Cancer Immunol. Res. 2019, 7, 1280–1292. [Google Scholar] [CrossRef]
- Klanova, M.; Oestergaard, M.Z.; Trněný, M.; Hiddemann, W.; Marcus, R.; Sehn, L.H.; Vitolo, U.; Bazeos, A.; Goede, V.; Zeuner, H.; et al. Prognostic Impact of Natural Killer Cell Count in Follicular Lymphoma and Diffuse Large B-Cell Lymphoma Patients Treated with Immunochemotherapy. Clin. Cancer Res. 2019, 25, 4634–4643. [Google Scholar] [CrossRef] [Green Version]
- Kohrt, H.E.; Thielens, A.; Marabelle, A.; Sagiv-Barfi, I.; Sola, C.; Chanuc, F.; Fuseri, N.; Bonnafous, C.; Czerwinski, D.; Rajapaksa, A.; et al. Anti-KIR Antibody Enhancement of Anti-Lymphoma Activity of Natural Killer Cells as Monotherapy and in Combination with Anti-CD20 Antibodies. Blood 2014, 123, 678–686. [Google Scholar] [CrossRef] [Green Version]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A MAb Is a Checkpoint Inhibitor That Promotes Anti-Tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jie, H.B.; Schuler, P.J.; Lee, S.C.; Srivastava, R.M.; Argiris, A.; Ferrone, S.; Whiteside, T.L.; Ferris, R.L. CTLA-4+ Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015, 75, 2200–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolz, C.; Schuler, M. Molecular Mechanisms of Resistance to Rituximab and Pharmacologic Strategies for Its Circumvention. Leuk. Lymphoma 2009, 50, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Beers, S.A.; French, R.R.; Chan, H.T.C.; Lim, S.H.; Jarrett, T.C.; Vidal, R.M.; Wijayaweera, S.S.; Dixon, S.V.; Kim, H.; Cox, K.L.; et al. Antigenic Modulation Limits the Efficacy of Anti-CD20 Antibodies: Implications for Antibody Selection. Blood 2010, 115, 5191–5201. [Google Scholar] [CrossRef]
- Taylor, R.P.; Lindorfer, M.A. Fcγ-Receptor-Mediated Trogocytosis Impacts MAb-Based Therapies: Historical Precedence and Recent Developments. Blood 2015, 125, 762–766. [Google Scholar] [CrossRef] [Green Version]
- Valgardsdottir, R.; Cattaneo, I.; Klein, C.; Introna, M.; Figliuzzi, M.; Golay, J. Human Neutrophils Mediate Trogocytosis Rather than Phagocytosis of CLL B Cells Opsonized with Anti-CD20 Antibodies. Blood 2017, 129, 2636–2644. [Google Scholar] [CrossRef] [Green Version]
- Sordo-Bahamonde, C.; Vitale, M.; Lorenzo-Herrero, S.; López-Soto, A.; Gonzalez, S. Mechanisms of Resistance to NK Cell Immunotherapy. Cancers 2020, 12, 893. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Veeramani, S.; Racila, E.; Cagley, J.; Fritzinger, D.C.; Vogel, C.W.; St John, W.; Weiner, G.J. Depletion of the C3 Component of Complement Enhances the Ability of Rituximab-Coated Target Cells to Activate Human NK Cells and Improves the Efficacy of Monoclonal Antibody Therapy in an in Vivo Model. Blood 2009, 114, 5322–5330. [Google Scholar] [CrossRef] [Green Version]
- Romee, R.; Foley, B.; Lenvik, T.; Wang, Y.; Zhang, B.; Ankarlo, D.; Luo, X.; Cooley, S.; Verneris, M.; Walcheck, B.; et al. NK Cell CD16 Surface Expression and Function Is Regulated by a Disintegrin and Metalloprotease-17 (ADAM17). Blood 2013, 121, 3599–3608. [Google Scholar] [CrossRef]
- Wu, J.; Mishra, H.K.; Walcheck, B. Role of ADAM17 as a Regulatory Checkpoint of CD16A in NK Cells and as a Potential Target for Cancer Immunotherapy. J. Leukoc. Biol. 2019, 105, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Capuano, C.; Romanelli, M.; Pighi, C.; Cimino, G.; Rago, A.; Molfetta, R.; Paolini, R.; Santoni, A.; Galandrini, R. Anti-CD20 Therapy Acts via FcγRIIIA to Diminish Responsiveness of Human Natural Killer Cells. Cancer Res. 2015, 75, 4097–4108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, M.C.; Battella, S.; La Scaleia, R.; Pelliccia, S.; Di Napoli, A.; Porzia, A.; Cecere, F.; Alma, E.; Zingoni, A.; Mainiero, F.; et al. Tumor-Associated and Immunochemotherapy-Dependent Long-Term Alterations of the Peripheral Blood NK Cell Compartment in DLBCL Patients. Oncoimmunology 2015, 4, e990773. [Google Scholar] [CrossRef]
- Hilchey, S.P.; Hyrien, O.; Mosmann, T.R.; Livingstone, A.M.; Friedberg, J.W.; Young, F.; Fisher, R.I.; Kelleher, R.J.; Bankert, R.B.; Bernstein, S.H. Rituximab Immunotherapy Results in the Induction of a Lymphoma Idiotype-Specific T-Cell Response in Patients with Follicular Lymphoma: Support for a “Vaccinal Effect” of Rituximab. Blood 2009, 113, 3809–3812. [Google Scholar] [CrossRef]
- Knutson, K.L.; Clynes, R.; Shreeder, B.; Yeramian, P.; Kemp, K.P.; Ballman, K.; Tenner, K.S.; Erskine, C.L.; Norton, N.; Northfelt, D.; et al. Improved Survival of HER2+ Breast Cancer Patients Treated with Trastuzumab and Chemotherapy Is Associated with Host Antibody Immunity against the HER2 Intracellular Domain. Cancer Res. 2016, 76, 3702–3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.; Hershman, D.; Shah, N.; Suciu-Foca, N.; Petrylak, D.P.; Taub, R.; Vahdat, L.; Cheng, B.; Pegram, M.; Knutson, K.L.; et al. Augmented HER-2 Specific Immunity during Treatment with Trastuzumab and Chemotherapy. Clin. Cancer Res. 2007, 13, 5133–5143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Jiang, Z.; Mortenson, E.D.; Deng, L.; Radkevich-Brown, O.; Yang, X.; Sattar, H.; Wang, Y.; Brown, N.K.; Greene, M.; et al. The Therapeutic Effect of Anti-HER2/Neu Antibody Depends on Both Innate and Adaptive Immunity. Cancer Cell 2010, 18, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Disis, M.L.; Knutson, K.L.; Schiffman, K.; Rinn, K.; McNeel, D.G. Pre-Existent Immunity to the HER-2/Neu Oncogenic Protein in Patients with HER-2/Neu Overexpressing Breast and Ovarian Cancer. Breast Cancer Res. Treat. 2000, 62, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Selenko, N.; Majdic, O.; Draxler, S.; Berer, A.; Jäger, U.; Knapp, W.; Stöckl, J. CD20 Antibody (C2B8)-Induced Apoptosis of Lymphoma Cells Promotes Phagocytosis by Dendritic Cells and Cross-Priming of CD8+ Cytotoxic T Cells. Leukemia 2001, 15, 1619–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhodapkar, K.M.; Krasovsky, J.; Williamson, B.; Dhodapkar, M.V. Antitumor Monoclonal Antibodies Enhance Cross-Presentation of Cellular Antigens and the Generation of Myeloma-Specific Killer T Cells by Dendritic Cells. J. Exp. Med. 2002, 195, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Moeller, I.; Spagnoli, G.C.; Finke, J.; Veelken, H.; Houet, L. Uptake Routes of Tumor-Antigen MAGE-A3 by Dendritic Cells Determine Priming of Naïve T-Cell Subtypes. Cancer Immunol. Immunother. 2012, 61, 2079–2090. [Google Scholar] [CrossRef]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiLillo, D.J.; Ravetch, J.V. Differential Fc-Receptor Engagement Drives an Anti-Tumor Vaccinal Effect. Cell 2015, 161, 1035–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böttcher, J.P.; Reis e Sousa, C. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer 2018, 4, 784–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A Natural Killer-Dendritic Cell Axis Defines Checkpoint Therapy-Responsive Tumor Microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef]
- Kelly, J.M.; Darcy, P.K.; Markby, J.L.; Godfrey, D.I.; Takeda, K.; Yagita, H.; Smyth, M.J. Induction of Tumor-Specific T Cell Memory by NK Cell-Mediated Tumor Rejection. Nat. Immunol. 2002, 3, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Mocikat, R.; Braumüller, H.; Gumy, A.; Egeter, O.; Ziegler, H.; Reusch, U.; Bubeck, A.; Louis, J.; Mailhammer, R.; Riethmüller, G.; et al. Natural Killer Cells Activated by MHC Class I(Low) Targets Prime Dendritic Cells to Induce Protective CD8 T Cell Responses. Immunity 2003, 19, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Adam, C.; King, S.; Allgeier, T.; Braumüller, H.; Lüking, C.; Mysliwietz, J.; Kriegeskorte, A.; Busch, D.H.; Röcken, M.; Mocikat, R. DC-NK Cell Cross Talk as a Novel CD4+ T-Cell-Independent Pathway for Antitumor CTL Induction. Blood 2005, 106, 338–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouwer, A.L.; Saunderson, S.C.; Caldwell, F.J.; Damani, T.T.; Pelham, S.J.; Dunn, A.C.; Jack, R.W.; Stoitzner, P.; McLellan, A.D. NK Cells Are Required for Dendritic Cell-Based Immunotherapy at the Time of Tumor Challenge. J. Immunol. 2014, 192, 2514–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.L.; Mailliard, R.B.; Moschos, S.J.; Edington, H.; Lotze, M.T.; Kirkwood, J.M.; Kalinski, P. Helper Activity of Natural Killer Cells during the Dendritic Cell-Mediated Induction of Melanoma-Specific Cytotoxic T Cells. J. Immunother. 2011, 34, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.M.; Lee, S.C.; Andrade Filho, P.A.; Lord, C.A.; Jie, H.B.; Davidson, H.C.; López-Albaitero, A.; Gibson, S.P.; Gooding, W.E.; Ferrone, S.; et al. Cetuximab-Activated Natural Killer and Dendritic Cells Collaborate to Trigger Tumor Antigen-Specific T-Cell Immunity in Head and Neck Cancer Patients. Clin. Cancer Res. 2013, 19, 1858–1872. [Google Scholar] [CrossRef] [Green Version]
- Deligne, C.; Metidji, A.; Fridman, W.H.; Teillaud, J.L. Anti-CD20 Therapy Induces a Memory Th1 Response through the IFN-γ/IL-12 Axis and Prevents Protumor Regulatory T-Cell Expansion in Mice. Leukemia 2015, 29, 947–957. [Google Scholar] [CrossRef]
- Mastrangeli, R.; Palinsky, W.; Bierau, H. Glycoengineered Antibodies: Towards the next-Generation of Immunotherapeutics. Glycobiology 2019, 29, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenoweth, A.M.; Wines, B.D.; Anania, J.C.; Mark Hogarth, P. Harnessing the Immune System via FcγR Function in Immune Therapy: A Pathway to Next-gen MAbs. Immunol. Cell Biol. 2020, 98, 287–304. [Google Scholar] [CrossRef] [Green Version]
- Nimmerjahn, F.; Ravetch, J.V. Translating Basic Mechanisms of IgG Effector Activity into next Generation Cancer Therapies. Cancer Immun. 2012, 12, 13. [Google Scholar] [PubMed]
- Wang, T.T.; Ravetch, J.V. Functional Diversification of IgGs through Fc Glycosylation. J. Clin. Investig. 2019, 129, 3492–3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, K.J.; Wu, J.; Walcheck, B. Engineering Anti-Tumor Monoclonal Antibodies and Fc Receptors to Enhance ADCC by Human NK Cells. Cancers 2021, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mathieu, M.; Brezski, R.J. IgG Fc Engineering to Modulate Antibody Effector Functions. Protein Cell 2018, 9, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-de-Romaní, S.; et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 573. [Google Scholar] [CrossRef]
- Mössner, E.; Brünker, P.; Moser, S.; Püntener, U.; Schmidt, C.; Herter, S.; Grau, R.; Gerdes, C.; Nopora, A.; van Puijenbroek, E.; et al. Increasing the Efficacy of CD20 Antibody Therapy through the Engineering of a New Type II Anti-CD20 Antibody with Enhanced Direct and Immune Effector Cell-Mediated B-Cell Cytotoxicity. Blood 2010, 115, 4393–4402. [Google Scholar] [CrossRef]
- Niederfellner, G.; Lammens, A.; Mundigl, O.; Georges, G.J.; Schaefer, W.; Schwaiger, M.; Franke, A.; Wiechmann, K.; Jenewein, S.; Slootstra, J.W.; et al. Epitope Characterization and Crystal Structure of GA101 Provide Insights into the Molecular Basis for Type I/II Distinction of CD20 Antibodies. Blood 2011, 118, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Salles, G.; Morschhauser, F.; Lamy, T.; Milpied, N.; Thieblemont, C.; Tilly, H.; Bieska, G.; Asikanius, E.; Carlile, D.; Birkett, J.; et al. Phase 1 Study Results of the Type II Glycoengineered Humanized Anti-CD20 Monoclonal Antibody Obinutuzumab (GA101) in B-Cell Lymphoma Patients. Blood 2012, 119, 5126–5132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goede, V.; Fischer, K.; Busch, R.; Engelke, A.; Eichhorst, B.; Wendtner, C.M.; Chagorova, T.; de la Serna, J.; Dilhuydy, M.S.; Illmer, T.; et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2014, 370, 1101–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, R.; Davies, A.; Ando, K.; Klapper, W.; Opat, S.; Owen, C.; Phillips, E.; Sangha, R.; Schlag, R.; Seymour, J.F.; et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N. Engl. J. Med. 2017, 377, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wu, G.; Huang, X.; Ma, Y.; Zhang, Y.; Song, Q.; Xie, M.; Sun, Y.; Huang, Y.; Huang, Z.; et al. Efficacy and Safety of New Anti-CD20 Monoclonal Antibodies versus Rituximab for Induction Therapy of CD20+ B-Cell Non-Hodgkin Lymphomas: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 3255. [Google Scholar] [CrossRef] [PubMed]
- Prica, A.; Crump, M. Improving CD20 Antibody Therapy: Obinutuzumab in Lymphoproliferative Disorders. Leuk. Lymphoma 2019, 60, 573–582. [Google Scholar] [CrossRef]
- Herter, S.; Herting, F.; Mundigl, O.; Waldhauer, I.; Weinzierl, T.; Fauti, T.; Muth, G.; Ziegler-Landesberger, D.; Van Puijenbroek, E.; Lang, S.; et al. Preclinical Activity of the Type II CD20 Antibody GA101 (Obinutuzumab) Compared with Rituximab and Ofatumumab in Vitro and in Xenograft Models. Mol. Cancer Ther. 2013, 12, 2031–2042. [Google Scholar] [CrossRef] [Green Version]
- Terszowski, G.; Klein, C.; Stern, M. KIR/HLA Interactions Negatively Affect Rituximab- but Not GA101 (Obinutuzumab)-Induced Antibody-Dependent Cellular Cytotoxicity. J. Immunol. 2014, 192, 5618–5624. [Google Scholar] [CrossRef] [Green Version]
- Capuano, C.; Pighi, C.; Molfetta, R.; Paolini, R.; Battella, S.; Palmieri, G.; Giannini, G.; Belardinilli, F.; Santoni, A.; Galandrini, R. Obinutuzumab-Mediated High-Affinity Ligation of FcγRIIIA/CD16 Primes NK Cells for IFNγ Production. Oncoimmunology 2017, 6, e1290037. [Google Scholar] [CrossRef] [Green Version]
- Capuano, C.; Pighi, C.; Maggio, R.; Battella, S.; Morrone, S.; Palmieri, G.; Santoni, A.; Klein, C.; Galandrini, R. CD16 Pre-Ligation by Defucosylated Tumor-Targeting MAb Sensitizes Human NK Cells to gc Cytokine Stimulation via PI3K/MTOR Axis. Cancer Immunol. Immunother. 2020, 69, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Davis, Z.B.; Vallera, D.A.; Miller, J.S.; Felices, M. Natural Killer Cells Unleashed: Checkpoint Receptor Blockade and BiKE/TriKE Utilization in NK-Mediated Anti-Tumor Immunotherapy. Semin. Immunol. 2017, 31, 64–75. [Google Scholar] [CrossRef]
- Felices, M.; Lenvik, T.R.; Davis, Z.B.; Miller, J.S.; Vallera, D.A. Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. Methods Mol. Biol. 2016, 1441, 333–346. [Google Scholar] [CrossRef] [Green Version]
- Gleason, M.K.; Verneris, M.R.; Todhunter, D.A.; Zhang, B.; McCullar, V.; Zhou, S.X.; Panoskaltsis-Mortari, A.; Weiner, L.M.; Vallera, D.A.; Miller, J.S. Bispecific and Trispecific Killer Cell Engagers Directly Activate Human NK Cells through CD16 Signaling and Induce Cytotoxicity and Cytokine Production. Mol. Cancer Ther. 2012, 11, 2674–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleason, M.K.; Ross, J.A.; Warlick, E.D.; Lund, T.C.; Verneris, M.R.; Wiernik, A.; Spellman, S.; Haagenson, M.D.; Lenvik, A.J.; Litzow, M.R.; et al. CD16xCD33 Bispecific Killer Cell Engager (BiKE) Activates NK Cells against Primary MDS and MDSC CD33+ Targets. Blood 2014, 123, 3016–3026. [Google Scholar] [CrossRef] [PubMed]
- Rothe, A.; Sasse, S.; Topp, M.S.; Eichenauer, D.A.; Hummel, H.; Reiners, K.S.; Dietlein, M.; Kuhnert, G.; Kessler, J.; Buerkle, C.; et al. A Phase 1 Study of the Bispecific Anti-CD30/CD16A Antibody Construct AFM13 in Patients with Relapsed or Refractory Hodgkin Lymphoma. Blood 2015, 125, 4024–4031. [Google Scholar] [CrossRef] [PubMed]
- Schmohl, J.U.; Gleason, M.K.; Dougherty, P.R.; Miller, J.S.; Vallera, D.A. Heterodimeric Bispecific Single Chain Variable Fragments (ScFv) Killer Engagers (BiKEs) Enhance NK-Cell Activity Against CD133+ Colorectal Cancer Cells. Target. Oncol. 2016, 11, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Oberg, H.H.; Kellner, C.; Gonnermann, D.; Sebens, S.; Bauerschlag, D.; Gramatzki, M.; Kabelitz, D.; Peipp, M.; Wesch, D. Tribody [(HER2)2xCD16] Is More Effective Than Trastuzumab in Enhancing gδ T Cell and Natural Killer Cell Cytotoxicity Against HER2-Expressing Cancer Cells. Front. Immunol. 2018, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Glorius, P.; Baerenwaldt, A.; Kellner, C.; Staudinger, M.; Dechant, M.; Stauch, M.; Beurskens, F.J.; Parren, P.W.H.I.; van de Winkel, J.G.J.; Valerius, T.; et al. The Novel Tribody [(CD20)2xCD16] Efficiently Triggers Effector Cell-Mediated Lysis of Malignant B Cells. Leukemia 2013, 27, 190–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellwanger, K.; Reusch, U.; Fucek, I.; Wingert, S.; Ross, T.; Müller, T.; Schniegler-Mattox, U.; Haneke, T.; Rajkovic, E.; Koch, J.; et al. Redirected Optimized Cell Killing (ROCK®): A Highly Versatile Multispecific Fit-for-Purpose Antibody Platform for Engaging Innate Immunity. mAbs 2019, 11, 899–918. [Google Scholar] [CrossRef]
- Schmohl, J.U.; Felices, M.; Oh, F.; Lenvik, A.J.; Lebeau, A.M.; Panyam, J.; Miller, J.S.; Vallera, D.A. Engineering of Anti-CD133 Trispecific Molecule Capable of Inducing NK Expansion and Driving Antibody-Dependent Cell-Mediated Cytotoxicity. Cancer Res. Treat. 2017, 49, 1140–1152. [Google Scholar] [CrossRef] [Green Version]
- Vallera, D.A.; Felices, M.; McElmurry, R.; McCullar, V.; Zhou, X.; Schmohl, J.U.; Zhang, B.; Lenvik, A.J.; Panoskaltsis-Mortari, A.; Verneris, M.R.; et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin. Cancer Res. 2016, 22, 3440–3450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmohl, J.U.; Felices, M.; Taras, E.; Miller, J.S.; Vallera, D.A. Enhanced ADCC and NK Cell Activation of an Anticarcinoma Bispecific Antibody by Genetic Insertion of a Modified IL-15 Cross-Linker. Mol. Ther. 2016, 24, 1312–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felices, M.; Kodal, B.; Hinderlie, P.; Kaminski, M.F.; Cooley, S.; Weisdorf, D.J.; Vallera, D.A.; Miller, J.S.; Bachanova, V. Novel CD19-Targeted TriKE Restores NK Cell Function and Proliferative Capacity in CLL. Blood Adv. 2019, 3, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, D.; Brandt, L.; Felices, M.; Guldevall, K.; Lenvik, T.; Hinderlie, P.; Curtsinger, J.; Warlick, E.; Spellman, S.R.; Blazar, B.R.; et al. 161533 TriKE Stimulates NK-Cell Function to Overcome Myeloid-Derived Suppressor Cells in MDS. Blood Adv. 2018, 2, 1459–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.D.; Felices, M.; Vallera, D.A.; Hinderlie, P.; Cooley, S.; Arock, M.; Gotlib, J.; Ustun, C.; Miller, J.S. Trispecific Killer Engager CD16xIL15xCD33 Potently Induces NK Cell Activation and Cytotoxicity against Neoplastic Mast Cells. Blood Adv. 2018, 2, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Schmohl, J.U.; Felices, M.; Todhunter, D.; Taras, E.; Miller, J.S.; Vallera, D.A. Tetraspecific ScFv Construct Provides NK Cell Mediated ADCC and Self-Sustaining Stimuli via Insertion of IL-15 as a Cross-Linker. Oncotarget 2016, 7, 73830–73844. [Google Scholar] [CrossRef]
- Wiernik, A.; Foley, B.; Zhang, B.; Verneris, M.R.; Warlick, E.; Gleason, M.K.; Ross, J.A.; Luo, X.; Weisdorf, D.J.; Walcheck, B.; et al. Targeting Natural Killer Cells to Acute Myeloid Leukemia in Vitro with a CD16 × 33 Bispecific Killer Cell Engager and ADAM17 Inhibition. Clin. Cancer Res. 2013, 19, 3844–3855. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 20 April 2021).
- Chester, C.; Marabelle, A.; Houot, R.; Kohrt, H.E. Dual Antibody Therapy to Harness the Innate Anti-Tumor Immune Response to Enhance Antibody Targeting of Tumors. Curr. Opin. Immunol. 2015, 33, 1–8. [Google Scholar] [CrossRef]
- Inagaki, A.; Ishida, T.; Yano, H.; Ishii, T.; Kusumoto, S.; Ito, A.; Ri, M.; Mori, F.; Ding, J.; Komatsu, H.; et al. Expression of the ULBP Ligands for NKG2D by B-NHL Cells Plays an Important Role in Determining Their Susceptibility to Rituximab-Induced ADCC. Int. J. Cancer 2009, 125, 212–221. [Google Scholar] [CrossRef]
- Deguine, J.; Breart, B.; Lemaître, F.; Bousso, P. Cutting Edge: Tumor-Targeting Antibodies Enhance NKG2D-Mediated NK Cell Cytotoxicity by Stabilizing NK Cell-Tumor Cell Interactions. J. Immunol. 2012, 189, 5493–5497. [Google Scholar] [CrossRef] [Green Version]
- Turaj, A.H.; Cox, K.L.; Penfold, C.A.; French, R.R.; Mockridge, C.I.; Willoughby, J.E.; Tutt, A.L.; Griffiths, J.; Johnson, P.W.M.; Glennie, M.J.; et al. Augmentation of CD134 (OX40)-Dependent NK Anti-Tumour Activity Is Dependent on Antibody Cross-Linking. Sci. Rep. 2018, 8, 2278. [Google Scholar] [CrossRef] [Green Version]
- Chester, C.; Ambulkar, S.; Kohrt, H.E. 4-1BB Agonism: Adding the Accelerator to Cancer Immunotherapy. Cancer Immunol. Immunother. 2016, 65, 1243–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, R.M.; Trivedi, S.; Concha-Benavente, F.; Gibson, S.P.; Reeder, C.; Ferrone, S.; Ferris, R.L. CD137 Stimulation Enhances Cetuximab-Induced Natural Killer: Dendritic Cell Priming of Antitumor T-Cell Immunity in Patients with Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 707–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellner, C.; Günther, A.; Humpe, A.; Repp, R.; Klausz, K.; Derer, S.; Valerius, T.; Ritgen, M.; Brüggemann, M.; van de Winkel, J.G.; et al. Enhancing Natural Killer Cell-Mediated Lysis of Lymphoma Cells by Combining Therapeutic Antibodies with CD20-Specific Immunoligands Engaging NKG2D or NKp30. OncoImmunology 2016, 5, e1058459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Ferrari de Andrade, L.; Tay, R.E.; Pan, D.; Luoma, A.M.; Ito, Y.; Badrinath, S.; Tsoucas, D.; Franz, B.; May, K.F.; Harvey, C.J.; et al. Antibody-Mediated Inhibition of MICA and MICB Shedding Promotes NK Cell–Driven Tumor Immunity. Science 2018, 359, 1537–1542. [Google Scholar] [CrossRef] [Green Version]
- Borgerding, A.; Hasenkamp, J.; Engelke, M.; Burkhart, N.; Trümper, L.; Wienands, J.; Glass, B. B-Lymphoma Cells Escape Rituximab-Triggered Elimination by NK Cells through Increased HLA Class I Expression. Exp. Hematol. 2010, 38, 213–221. [Google Scholar] [CrossRef]
- Nijhof, I.S.; van Bueren, J.J.L.; van Kessel, B.; Andre, P.; Morel, Y.; Lokhorst, H.M.; van de Donk, N.W.C.J.; Parren, P.W.H.I.; Mutis, T. Daratumumab-Mediated Lysis of Primary Multiple Myeloma Cells Is Enhanced in Combination with the Human Anti-KIR Antibody IPH2102 and Lenalidomide. Haematologica 2015, 100, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.K.; Kung Sutherland, M.; Li, Y.; Zalevsky, J.; Schell, S.; Leung, W. Antibody-Dependent Cell-Mediated Cytotoxicity Overcomes NK Cell Resistance in MLL -Rearranged Leukemia Expressing Inhibitory KIR Ligands but Not Activating Ligands. Clin. Cancer Res. 2012, 18, 6296–6305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarek, N.; Le Luduec, J.B.; Gallagher, M.M.; Zheng, J.; Venstrom, J.M.; Chamberlain, E.; Modak, S.; Heller, G.; Dupont, B.; Cheung, N.K.V.; et al. Unlicensed NK Cells Target Neuroblastoma Following Anti-GD2 Antibody Treatment. J. Clin. Investig. 2012, 122, 3260–3270. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Lopez-Verges, S.; Pitcher, B.N.; Johnson, J.; Jung, S.H.; Zhou, L.; Hsu, K.; Czuczman, M.S.; Cheson, B.; Kaplan, L.; et al. CALGB 150905 (Alliance): Rituximab Broadens the Antilymphoma Response by Activating Unlicensed NK Cells. Cancer Immunol. Res. 2014, 2, 878–889. [Google Scholar] [CrossRef] [Green Version]
- Muntasell, A.; Cabo, M.; Servitja, S.; Tusquets, I.; Martínez-García, M.; Rovira, A.; Rojo, F.; Albanell, J.; López-Botet, M. Interplay between Natural Killer Cells and Anti-HER2 Antibodies: Perspectives for Breast Cancer Immunotherapy. Front. Immunol. 2017, 8, 1544. [Google Scholar] [CrossRef] [Green Version]
- Concha-Benavente, F.; Kansy, B.; Moskovitz, J.; Moy, J.; Chandran, U.; Ferris, R.L. PD-L1 Mediates Dysfunction in Activated PD-1+ NK Cells in Head and Neck Cancer Patients. Cancer Immunol. Res. 2018, 6, 1548–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Sunderland, A.; Zhou, Y.; Schulick, R.D.; Edil, B.H.; Zhu, Y. Blockade of CD112R and TIGIT Signaling Sensitizes Human Natural Killer Cell Functions. Cancer Immunol. Immunother. 2017, 66, 1367–1375. [Google Scholar] [CrossRef]
- Caruso, S.; De Angelis, B.; Carlomagno, S.; Del Bufalo, F.; Sivori, S.; Locatelli, F.; Quintarelli, C. NK Cells as Adoptive Cellular Therapy for Hematological Malignancies: Advantages and Hurdles. Semin. Hematol. 2020, 57, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Park, Y. Hitting the Complexity of the TIGIT-CD96-CD112R-CD226 Axis for next-Generation Cancer Immunotherapy. BMB Rep. 2021, 54, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, E.C.; Sheikhi, A.; Höppner, Y.D.; de Kok, P.; Yazdanpanah-Samani, M.; Spanholtz, J.; Verheul, H.M.W.; van der Vliet, H.J.; de Gruijl, T.D. Natural Killer Cells and Anti-Cancer Therapies: Reciprocal Effects on Immune Function and Therapeutic Response. Cancers 2021, 13, 711. [Google Scholar] [CrossRef]
- Wu, L.; Adams, M.; Carter, T.; Chen, R.; Muller, G.; Stirling, D.; Schafer, P.; Bartlett, J.B. Lenalidomide Enhances Natural Killer Cell and Monocyte-Mediated Antibody-Dependent Cellular Cytotoxicity of Rituximab-Treated CD20+ Tumor Cells. Clin. Cancer Res. 2008, 14, 4650–4657. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Hideshima, T.; Akiyama, M.; Podar, K.; Yasui, H.; Raje, N.; Kumar, S.; Chauhan, D.; Treon, S.P.; Richardson, P.; et al. Molecular Mechanisms Whereby Immunomodulatory Drugs Activate Natural Killer Cells: Clinical Application. Br. J. Haematol. 2005, 128, 192–203. [Google Scholar] [CrossRef]
- Gribben, J.G.; Fowler, N.; Morschhauser, F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. JCO 2015, 33, 2803–2811. [Google Scholar] [CrossRef] [Green Version]
- Yamshon, S.; Ruan, J. IMiDs New and Old. Curr. Hematol. Malig. Rep. 2019, 14, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.; Trisal, P.; Bjorklund, C.; Carrancio, S.; Toraño, E.G.; Guarinos, C.; Papazoglou, D.; Hagner, P.R.; Beldi-Ferchiou, A.; Tarte, K.; et al. Combination Lenalidomide-rituximab Immunotherapy Activates Anti-tumour Immunity and Induces Tumour Cell Death by Complementary Mechanisms of Action in Follicular Lymphoma. Br. J. Haematol. 2019, 185, 240–253. [Google Scholar] [CrossRef]
- Bertino, E.M.; McMichael, E.L.; Mo, X.; Trikha, P.; Davis, M.; Paul, B.; Grever, M.; Carson, W.E.; Otterson, G.A. A Phase I Trial to Evaluate Antibody-Dependent Cellular Cytotoxicity of Cetuximab and Lenalidomide in Advanced Colorectal and Head and Neck Cancer. Mol. Cancer Ther. 2016, 15, 2244–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villalba, M.; Alexia, C.; Bellin-Robert, A.; Fayd’herbe de Maudave, A.; Gitenay, D. Non-Genetically Improving the Natural Cytotoxicity of Natural Killer (NK) Cells. Front. Immunol. 2019, 10, 3026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachanova, V.; Sarhan, D.; DeFor, T.E.; Cooley, S.; Panoskaltsis-Mortari, A.; Blazar, B.R.; Curtsinger, J.M.; Burns, L.; Weisdorf, D.J.; Miller, J.S. Haploidentical Natural Killer Cells Induce Remissions in Non-Hodgkin Lymphoma Patients with Low Levels of Immune-Suppressor Cells. Cancer Immunol. Immunother. 2018, 67, 483–494. [Google Scholar] [CrossRef]
- Federico, S.M.; McCarville, M.B.; Shulkin, B.L.; Sondel, P.M.; Hank, J.A.; Hutson, P.; Meagher, M.; Shafer, A.; Ng, C.Y.; Leung, W.; et al. A Pilot Trial of Humanized Anti-GD2 Monoclonal Antibody (Hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Recurrent/Refractory Neuroblastoma. Clin. Cancer Res. 2017, 23, 6441–6449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modak, S.; Le Luduec, J.B.; Cheung, I.Y.; Goldman, D.A.; Ostrovnaya, I.; Doubrovina, E.; Basu, E.; Kushner, B.H.; Kramer, K.; Roberts, S.S.; et al. Adoptive Immunotherapy with Haploidentical Natural Killer Cells and Anti-GD2 Monoclonal Antibody M3F8 for Resistant Neuroblastoma: Results of a Phase I Study. OncoImmunology 2018, 7, e1461305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichocki, F.; Valamehr, B.; Bjordahl, R.; Zhang, B.; Rezner, B.; Rogers, P.; Gaidarova, S.; Moreno, S.; Tuininga, K.; Dougherty, P.; et al. GSK3 Inhibition Drives Maturation of NK Cells and Enhances Their Antitumor Activity. Cancer Res. 2017, 77, 5664–5675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naeimi Kararoudi, M.; Tullius, B.P.; Chakravarti, N.; Pomeroy, E.J.; Moriarity, B.S.; Beland, K.; Colamartino, A.B.L.; Haddad, E.; Chu, Y.; Cairo, M.S.; et al. Genetic and Epigenetic Modification of Human Primary NK Cells for Enhanced Antitumor Activity. Semin. Hematol. 2020, 57, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Gobius, I.; Souza-Fonseca-Guimaraes, F. Natural Killer Cell Engineering–a New Hope for Cancer Immunotherapy. Semin. Hematol. 2020, 57, 194–200. [Google Scholar] [CrossRef]
- Pomeroy, E.J.; Hunzeker, J.T.; Kluesner, M.G.; Lahr, W.S.; Smeester, B.A.; Crosby, M.R.; Lonetree, C.; Yamamoto, K.; Bendzick, L.; Miller, J.S.; et al. A Genetically Engineered Primary Human Natural Killer Cell Platform for Cancer Immunotherapy. Mol. Ther. 2020, 28, 52–63. [Google Scholar] [CrossRef]
- Afolabi, L.O.; Adeshakin, A.O.; Sani, M.M.; Bi, J.; Wan, X. Genetic Reprogramming for NK Cell Cancer Immunotherapy with CRISPR/Cas9. Immunology 2019, 158, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Chauhan, S.K.S.; Daly, J.; Natoni, A.; Fairfield, H.; Henderson, R.; Nolan, E.; Swan, D.; Hu, J.; Reagan, M.R.; et al. The CD38low Natural Killer Cell Line KHYG1 Transiently Expressing CD16F158V in Combination with Daratumumab Targets Multiple Myeloma Cells with Minimal Effector NK Cell Fratricide. Cancer Immunol. Immunother. 2020, 69, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, M.; Levy, E.; Karambelkar, A.; Li, L.; Reger, R.; Berg, M.; Peshwa, M.V.; Childs, R.W. Efficient MRNA-Based Genetic Engineering of Human NK Cells with High-Affinity CD16 and CCR7 Augments Rituximab-Induced ADCC against Lymphoma and Targets NK Cell Migration toward the Lymph Node-Associated Chemokine CCL19. Front. Immunol. 2016, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Jochems, C.; Hodge, J.W.; Fantini, M.; Fujii, R.; Morillon, Y.M.; Greiner, J.W.; Padget, M.R.; Tritsch, S.R.; Tsang, K.Y.; Campbell, K.S.; et al. NK Cell Line (HaNK) Expressing High Levels of Granzyme and Engineered to Express the High Affinity CD16 Allele. Oncotarget 2016, 7, 86359–86373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, K.M.; Hullsiek, R.; Mishra, H.K.; Mendez, D.C.; Li, Y.; Rogich, A.; Kaufman, D.S.; Wu, J.; Walcheck, B. Expression of a Recombinant High Affinity IgG Fc Receptor by Engineered NK Cells as a Docking Platform for Therapeutic MAbs to Target Cancer Cells. Front. Immunol. 2018, 9, 2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cambay, F.; Forest-Nault, C.; Dumoulin, L.; Seguin, A.; Henry, O.; Durocher, Y.; De Crescenzo, G. Glycosylation of Fcγ Receptors Influences Their Interaction with Various IgG1 Glycoforms. Mol. Immunol. 2020, 121, 144–158. [Google Scholar] [CrossRef]
- Zhu, H.; Blum, R.H.; Bjordahl, R.; Gaidarova, S.; Rogers, P.; Lee, T.T.; Abujarour, R.; Bonello, G.B.; Wu, J.; Tsai, P.F.; et al. Pluripotent Stem Cell-Derived NK Cells with High-Affinity Noncleavable CD16a Mediate Improved Antitumor Activity. Blood 2020, 135, 399–410. [Google Scholar] [CrossRef]
- Solocinski, K.; Padget, M.R.; Fabian, K.P.; Wolfson, B.; Cecchi, F.; Hembrough, T.; Benz, S.C.; Rabizadeh, S.; Soon-Shiong, P.; Schlom, J.; et al. Overcoming Hypoxia-Induced Functional Suppression of NK Cells. J. Immunother. Cancer 2020, 8, e000246. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, L.; Song, Y.; Zhang, Y.; Lin, D.; Hu, B.; Mei, Y.; Sandikin, D.; Liu, H. Augmented Anti-Tumor Activity of NK-92 Cells Expressing Chimeric Receptors of TGF-ΒR II and NKG2D. Cancer Immunol. Immunother. 2017, 66, 537–548. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Shankar, K.; Capitini, C.M.; Saha, K. Genome Engineering of Induced Pluripotent Stem Cells to Manufacture Natural Killer Cell Therapies. Stem Cell Res. Ther. 2020, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, J.P.; Mahmood, S.; Zhu, H.; Gaidarova, S.; Blum, R.; Bjordahl, R.; Cichocki, F.; Chu, H.; Bonello, G.; Lee, T.; et al. FT596: Translation of First-of-Kind Multi-Antigen Targeted Off-the-Shelf CAR-NK Cell with Engineered Persistence for the Treatment of B Cell Malignancies. Blood 2019, 134 (Suppl. 1). [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Béziat, V.; Liu, L.L.; Malmberg, J.A.; Ivarsson, M.A.; Sohlberg, E.; Björklund, A.T.; Retière, C.; Sverremark-Ekström, E.; Traherne, J.; Ljungman, P.; et al. NK Cell Responses to Cytomegalovirus Infection Lead to Stable Imprints in the Human KIR Repertoire and Involve Activating KIRs. Blood 2013, 121, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Gumá, M.; Angulo, A.; Vilches, C.; Gómez-Lozano, N.; Malats, N.; López-Botet, M. Imprint of Human Cytomegalovirus Infection on the NK Cell Receptor Repertoire. Blood 2004, 104, 3664–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlums, H.; Cichocki, F.; Tesi, B.; Theorell, J.; Beziat, V.; Holmes, T.D.; Han, H.; Chiang, S.C.C.; Foley, B.; Mattsson, K.; et al. Cytomegalovirus Infection Drives Adaptive Epigenetic Diversification of NK Cells with Altered Signaling and Effector Function. Immunity 2015, 42, 443–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Zhang, T.; Hwang, I.; Kim, A.; Nitschke, L.; Kim, M.; Scott, J.M.; Kamimura, Y.; Lanier, L.L.; Kim, S. Epigenetic Modification and Antibody-Dependent Expansion of Memory-like NK Cells in Human Cytomegalovirus-Infected Individuals. Immunity 2015, 42, 431–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, I.; Zhang, T.; Scott, J.M.; Kim, A.R.; Lee, T.; Kakarla, T.; Kim, A.; Sunwoo, J.B.; Kim, S. Identification of Human NK Cells That Are Deficient for Signaling Adaptor FcRγ and Specialized for Antibody-Dependent Immune Functions. Int. Immunol. 2012, 24, 793–802. [Google Scholar] [CrossRef]
- Kim, K.H.; Yu, H.T.; Hwang, I.; Park, S.; Park, S.H.; Kim, S.; Shin, E.C. Phenotypic and Functional Analysis of Human NK Cell Subpopulations According to the Expression of FcεRIγ and NKG2C. Front. Immunol. 2019, 10, 2865. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Scott, J.M.; Hwang, I.; Kim, S. Cutting Edge: Antibody-Dependent Memory-like NK Cells Distinguished by FcRγ Deficiency. J. Immunol. 2013, 190, 1402–1406. [Google Scholar] [CrossRef] [Green Version]
- Tesi, B.; Schlums, H.; Cichocki, F.; Bryceson, Y.T. Epigenetic Regulation of Adaptive NK Cell Diversification. Trends Immunol. 2016, 37, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Luetke-Eversloh, M.; Hammer, Q.; Durek, P.; Nordström, K.; Gasparoni, G.; Pink, M.; Hamann, A.; Walter, J.; Chang, H.D.; Dong, J.; et al. Human Cytomegalovirus Drives Epigenetic Imprinting of the IFNG Locus in NKG2Chi Natural Killer Cells. PLoS Pathog. 2014, 10, e1004441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, Q.; Rückert, T.; Romagnani, C. Natural Killer Cell Specificity for Viral Infections. Nat. Immunol. 2018, 19, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Paust, S.; Blish, C.A.; Reeves, R.K. Redefining Memory: Building the Case for Adaptive NK Cells. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Vergès, S.; Milush, J.M.; Schwartz, B.S.; Pando, M.J.; Jarjoura, J.; York, V.A.; Houchins, J.P.; Miller, S.; Kang, S.M.; Norris, P.J.; et al. Expansion of a Unique CD57+NKG2Chi Natural Killer Cell Subset during Acute Human Cytomegalovirus Infection. Proc. Natl. Acad. Sci. USA 2011, 108, 14725–14732. [Google Scholar] [CrossRef] [Green Version]
- Foley, B.; Cooley, S.; Verneris, M.R.; Pitt, M.; Curtsinger, J.; Luo, X.; Lopez-Vergès, S.; Lanier, L.L.; Weisdorf, D.; Miller, J.S. Cytomegalovirus Reactivation after Allogeneic Transplantation Promotes a Lasting Increase in Educated NKG2C+ Natural Killer Cells with Potent Function. Blood 2012, 119, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Foley, B.; Cooley, S.; Verneris, M.R.; Curtsinger, J.; Luo, X.; Waller, E.K.; Anasetti, C.; Weisdorf, D.; Miller, J.S. Human Cytomegalovirus (CMV)-Induced Memory-like NKG2C+ NK Cells Are Transplantable and Expand in Vivo in Response to Recipient CMV Antigen. J. Immunol. 2012, 189, 5082–5088. [Google Scholar] [CrossRef] [Green Version]
- Della Chiesa, M.; Falco, M.; Podestà, M.; Locatelli, F.; Moretta, L.; Frassoni, F.; Moretta, A. Phenotypic and Functional Heterogeneity of Human NK Cells Developing after Umbilical Cord Blood Transplantation: A Role for Human Cytomegalovirus? Blood 2012, 119, 399–410. [Google Scholar] [CrossRef]
- Muccio, L.; Bertaina, A.; Falco, M.; Pende, D.; Meazza, R.; Lopez-Botet, M.; Moretta, L.; Locatelli, F.; Moretta, A.; Della Chiesa, M. Analysis of Memory-like Natural Killer Cells in Human Cytomegalovirus-Infected Children Undergoing Aβ+T and B Cell-Depleted Hematopoietic Stem Cell Transplantation for Hematological Malignancies. Haematologica 2016, 101, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Amran, F.S.; Kramski, M.; Angelovich, T.A.; Elliott, J.; Hearps, A.C.; Price, P.; Jaworowski, A. An NK Cell Population Lacking FcRγ Is Expanded in Chronically Infected HIV Patients. J. Immunol. 2015, 194, 4688–4697. [Google Scholar] [CrossRef] [Green Version]
- Schuch, A.; Zecher, B.F.; Müller, P.; Correia, M.P.; Daul, F.; Rennert, C.; Tauber, C.; Schlitt, K.; Boettler, T.; Neumann-Haefelin, C.; et al. NK-cell responses are biased towards CD16-mediated effector functions in chronic hepatitis B virus infection. J. Hepatol. 2019, 70, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björkström, N.K.; Lindgren, T.; Stoltz, M.; Fauriat, C.; Braun, M.; Evander, M.; Michaëlsson, J.; Malmberg, K.J.; Klingström, J.; Ahlm, C.; et al. Rapid Expansion and Long-Term Persistence of Elevated NK Cell Numbers in Humans Infected with Hantavirus. J. Exp. Med. 2011, 208, 13–21. [Google Scholar] [CrossRef]
- Béziat, V.; Dalgard, O.; Asselah, T.; Halfon, P.; Bedossa, P.; Boudifa, A.; Hervier, B.; Theodorou, I.; Martinot, M.; Debré, P.; et al. CMV Drives Clonal Expansion of NKG2C + NK Cells Expressing Self-Specific KIRs in Chronic Hepatitis Patients: Innate Immunity. Eur. J. Immunol. 2012, 42, 447–457. [Google Scholar] [CrossRef]
- Hart, G.T.; Tran, T.M.; Theorell, J.; Schlums, H.; Arora, G.; Rajagopalan, S.; Sangala, A.D.J.; Welsh, K.J.; Traore, B.; Pierce, S.K.; et al. Adaptive NK Cells in People Exposed to Plasmodium Falciparum Correlate with Protection from Malaria. J. Exp. Med. 2019, 216, 1280–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumá, M.; Budt, M.; Sáez, A.; Brckalo, T.; Hengel, H.; Angulo, A.; López-Botet, M. Expansion of CD94/NKG2C+ NK Cells in Response to Human Cytomegalovirus-Infected Fibroblasts. Blood 2006, 107, 3624–3631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rölle, A.; Pollmann, J.; Ewen, E.M.; Le, V.T.K.; Halenius, A.; Hengel, H.; Cerwenka, A. IL-12-Producing Monocytes and HLA-E Control HCMV-Driven NKG2C+ NK Cell Expansion. J. Clin. Investig. 2014, 124, 5305–5316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, Q.; Rückert, T.; Borst, E.M.; Dunst, J.; Haubner, A.; Durek, P.; Heinrich, F.; Gasparoni, G.; Babic, M.; Tomic, A.; et al. Peptide-Specific Recognition of Human Cytomegalovirus Strains Controls Adaptive Natural Killer Cells. Nat. Immunol. 2018, 19, 453–463. [Google Scholar] [CrossRef]
- Liu, L.L.; Landskron, J.; Ask, E.H.; Enqvist, M.; Sohlberg, E.; Traherne, J.A.; Hammer, Q.; Goodridge, J.P.; Larsson, S.; Jayaraman, J.; et al. Critical Role of CD2 Co-Stimulation in Adaptive Natural Killer Cell Responses Revealed in NKG2C-Deficient Humans. Cell Rep. 2016, 15, 1088–1099. [Google Scholar] [CrossRef] [Green Version]
- Cichocki, F.; Cooley, S.; Davis, Z.; DeFor, T.E.; Schlums, H.; Zhang, B.; Brunstein, C.G.; Blazar, B.R.; Wagner, J.; Diamond, D.J.; et al. CD56dimCD57+NKG2C+ NK Cell Expansion Is Associated with Reduced Leukemia Relapse after Reduced Intensity HCT. Leukemia 2016, 30, 456–463. [Google Scholar] [CrossRef]
- Sarhan, D.; Hippen, K.L.; Lemire, A.; Hying, S.; Luo, X.; Lenvik, T.; Curtsinger, J.; Davis, Z.; Zhang, B.; Cooley, S.; et al. Adaptive NK Cells Resist Regulatory T-Cell Suppression Driven by IL37. Cancer Immunol. Res. 2018, 6, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Sarhan, D.; Cichocki, F.; Zhang, B.; Yingst, A.; Spellman, S.R.; Cooley, S.; Verneris, M.R.; Blazar, B.R.; Miller, J.S. Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells. Cancer Res. 2016, 76, 5696–5706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capuano, C.; Pighi, C.; Battella, S.; Santoni, A.; Palmieri, G.; Galandrini, R. Memory NK Cell Features Exploitable in Anticancer Immunotherapy. J. Immunol. Res. 2019, 2019, 8795673. [Google Scholar] [CrossRef]
- Capuano, C.; Battella, S.; Pighi, C.; Franchitti, L.; Turriziani, O.; Morrone, S.; Santoni, A.; Galandrini, R.; Palmieri, G. Tumor-Targeting Anti-CD20 Antibodies Mediate In Vitro Expansion of Memory Natural Killer Cells: Impact of CD16 Affinity Ligation Conditions and In Vivo Priming. Front. Immunol. 2018, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, K.H.; Lee, H.; Kim, C.G.; Chung, H.; Choi, Y.S.; Park, S.H.; Cheong, J.W.; Min, Y.H.; Shin, E.C.; et al. Adaptive Natural Killer Cells Facilitate Effector Functions of Daratumumab in Multiple Myeloma. Clin. Cancer Res. 2021, 27, 2947–2958. [Google Scholar] [CrossRef] [PubMed]
- Enqvist, M.; Jacobs, B.; Junlén, H.R.; Schaffer, M.; Melén, C.M.; Friberg, D.; Wahlin, B.E.; Malmberg, K.J. Systemic and Intra-Nodal Activation of NK Cells After Rituximab Monotherapy for Follicular Lymphoma. Front. Immunol. 2019, 10, 2085. [Google Scholar] [CrossRef] [PubMed]
- Corat, M.A.F.; Schlums, H.; Wu, C.; Theorell, J.; Espinoza, D.A.; Sellers, S.E.; Townsley, D.M.; Young, N.S.; Bryceson, Y.T.; Dunbar, C.E.; et al. Acquired Somatic Mutations in PNH Reveal Long-Term Maintenance of Adaptive NK Cells Independent of HSPCs. Blood 2017, 129, 1940–1946. [Google Scholar] [CrossRef] [Green Version]
- Schlums, H.; Jung, M.; Han, H.; Theorell, J.; Bigley, V.; Chiang, S.C.C.; Allan, D.S.J.; Davidson-Moncada, J.K.; Dickinson, R.E.; Holmes, T.D.; et al. Adaptive NK Cells Can Persist in Patients with GATA2 Mutation Depleted of Stem and Progenitor Cells. Blood 2017, 129, 1927–1939. [Google Scholar] [CrossRef] [Green Version]
- Cichocki, F.; Wu, C.Y.; Zhang, B.; Felices, M.; Tesi, B.; Tuininga, K.; Dougherty, P.; Taras, E.; Hinderlie, P.; Blazar, B.R.; et al. ARID5B Regulates Metabolic Programming in Human Adaptive NK Cells. J. Exp. Med. 2018, 215, 2379–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.L.; Béziat, V.; Oei, V.Y.S.; Pfefferle, A.; Schaffer, M.; Lehmann, S.; Hellström-Lindberg, E.; Söderhäll, S.; Heyman, M.; Grandér, D.; et al. Ex Vivo Expanded Adaptive NK Cells Effectively Kill Primary Acute Lymphoblastic Leukemia Cells. Cancer Immunol. Res. 2017, 5, 654–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oei, V.Y.S.; Siernicka, M.; Graczyk-Jarzynka, A.; Hoel, H.J.; Yang, W.; Palacios, D.; Almåsbak, H.; Bajor, M.; Clement, D.; Brandt, L.; et al. Intrinsic Functional Potential of NK-Cell Subsets Constrains Retargeting Driven by Chimeric Antigen Receptors. Cancer Immunol. Res. 2018, 6, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Scott, J.M.; Langguth, E.; Chang, H.; Park, P.H.; Kim, S. FcRγ Gene Editing Reprograms Conventional NK Cells to Display Key Features of Adaptive Human NK Cells. IScience 2020, 23, 101709. [Google Scholar] [CrossRef] [PubMed]

| Agent | Target | Malignancy | Study | ClinicalTrials.gov Identifier: Trial Number |
|---|---|---|---|---|
| NK cell engager | ||||
| AFM13 | CD16A, CD30 | HL | Phase 2 | NCT02321592 |
| CTCL | Phase 1/2 | NCT03192202 | ||
| HL | Phase 1 | NCT01221571 | ||
| PTCL; Transformed Mycosis Fungoides | Phase 2 | NCT04101331 | ||
| HL, in combination with anti-PD-1 antibody | Phase 1 | NCT02665650 | ||
| R/R ALCL, R/R B-Cell NHL, R/R Classic HL, R/R Mycosis Fungoides, R/R PTCL | Phase 1 | NCT04074746 | ||
| AFM24 | CD16A, EGFR | Advanced solid tumors | Phase 1/2 | NCT04259450 |
| GTB-3550 | CD16, IL-15R, CD33 | High-risk MDS; AML; Systemic Mastocytosis; Mast Cell Leukemia | Phase 1/2 | NCT03214666 |
| DF1001 | CD16, HER2 | Advanced stage HER2+ solid tumors, as monotherapy or in combination with anti-PD-1 mAb | Phase 1/2 | NCT04143711 |
| Antibody combination | ||||
| Rituximab plus Lirilumab | CD20, KIR | R/R or high-risk untreated patients with CLL | Phase 2 | NCT02481297 |
| Trastuzumab plus Monalizumab | HER2, NKG2A | Breast cancer | Phase 2 | NCT04307329 |
| Cetuximab plus Monalizumab | EGFR, NKG2A | Head and neck neoplasms | Phase 1/2 | NCT02643550 |
| Cetuximab plus Monalizumab | EGFR, NKG2A | HNSCC | Phase 3 | NCT04590963 |
| Cetuximab plus Pembrolizumab | EGFR, PD-1 | HNSCC, Lip SCC, Oral Cavity Cancer | Phase 2 | NCT03082534 |
| Cetuximab plus Nivolumab | EGFR, PD-1 | R/R or stage IV Head and Neck Squamous Cell Carcinoma, in combination with Paclitaxel | Phase 2 | NCT04282109 |
| Cetuximab plus Avelumab | EGFR, PD-L1 | Squamous Cell Anal Carcinoma | Phase 2 | NCT03944252 |
| Agent | Malignancy | Study | ClinicalTrials.gov Identifier: Trial Number |
|---|---|---|---|
| Autologous, in vitro-expanded NK cells | R/R neuroblastoma, in combination with dinutuximab anti-GD2 mAb and chemotherapy | Phase 1 | NCT04211675 |
| Autologous, in vitro-expanded NK cells (CELLPROTECT) | Post-transplant maintenance of MM, in combination with isatuximab anti-CD38 mAb | Phase 2 | NCT04558931 |
| Autologous, in vitro-expanded NK cells (SNK01) | Advanced/metastatic HER2- or EGFR-expressing cancers, in combination with trastuzumab or cetuximab anti-HER2 mAbs | Phase 1/2a | NCT04464967 |
| autologous, in vitro-expanded NK cells | R/R neuroblastoma, in combination with Ch14.18 anti-GD2 mAb and lenalidomide | Phase 1 | NCT02573896 |
| Third-party in vitro-expanded (IL-21) NK cells | R/R CTL or ATL, in combination with mogamulizumab anti-CCR4 mAb | Phase 1 | NCT04848064 |
| HLA-mismatched NK cells | High-risk neuroblastoma, in combination with Hu3F8 anti-GD2 mAb and IL-2 | Phase 1 | NCT02650648 |
| HLA-haploidentical in vitro-expanded NK cells | Neuroblastoma or R/R neuroblastoma, in combination with hu14.18-IL2 immunocytokine (anti-CD2 mAb linked to IL-2) | Phase 1 | NCT03209869 |
| iPSC-derived NK cells expressing high affinity, noncleavable CD16 (FT516) | R/R B cell lymphoma, in combination with rituximab or obinutuzumab anti-CD20 mAbs and IL-2 | Phase 1/1b | NCT04023071 |
| iPSC-derived NK cells expressing high affinity, noncleavable CD16 (FT516) | Recurrent gynecologic cancers, in combination with enoblituzumab anti-B7-H3 mAb and IL-2 | Phase 1 | NCT04630769 |
| iPSC-derived NK cells expressing high affinity, no-cleavable CD16 and IL-15 receptor fusion (IL-15RF), CD38 ko (FT538) | R/R MM, in combination with daratumumab anti-CD38 or elotuzumab anti-SLAMF7 mAbs | Phase 1 | NCT04614636 |
| Anti-CD19 CAR-transduced iPSC-derived NK cells, expressing high affinity, noncleavable CD16 and IL-15 receptor fusion (IL-15RF) (FT596) | R/R CLL or B cell lymphoma, in monotherapy or in combination with rituximab or obinutuzumab anti-CD20 mAbs | Phase 1 | NCT04245722 |
| Allogeneic, in vitro-expanded and terminally differentiated NK cells (FATE-NK100) | Advanced-stage solid tumors, in monotherapy or in combination with cetuximab or trastuzumab anti-HER2 mAbs | Phase 1 | NCT03319459 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capuano, C.; Pighi, C.; Battella, S.; De Federicis, D.; Galandrini, R.; Palmieri, G. Harnessing CD16-Mediated NK Cell Functions to Enhance Therapeutic Efficacy of Tumor-Targeting mAbs. Cancers 2021, 13, 2500. https://doi.org/10.3390/cancers13102500
Capuano C, Pighi C, Battella S, De Federicis D, Galandrini R, Palmieri G. Harnessing CD16-Mediated NK Cell Functions to Enhance Therapeutic Efficacy of Tumor-Targeting mAbs. Cancers. 2021; 13(10):2500. https://doi.org/10.3390/cancers13102500
Chicago/Turabian StyleCapuano, Cristina, Chiara Pighi, Simone Battella, Davide De Federicis, Ricciarda Galandrini, and Gabriella Palmieri. 2021. "Harnessing CD16-Mediated NK Cell Functions to Enhance Therapeutic Efficacy of Tumor-Targeting mAbs" Cancers 13, no. 10: 2500. https://doi.org/10.3390/cancers13102500
APA StyleCapuano, C., Pighi, C., Battella, S., De Federicis, D., Galandrini, R., & Palmieri, G. (2021). Harnessing CD16-Mediated NK Cell Functions to Enhance Therapeutic Efficacy of Tumor-Targeting mAbs. Cancers, 13(10), 2500. https://doi.org/10.3390/cancers13102500






