Systemic Treatment Initiation in Classical and Endemic Kaposi’s Sarcoma: Risk Factors and Global Multi-State Modelling in a Monocentric Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Endpoints
2.3. Statistical Analysis
3. Results
3.1. Demographic Characteristics
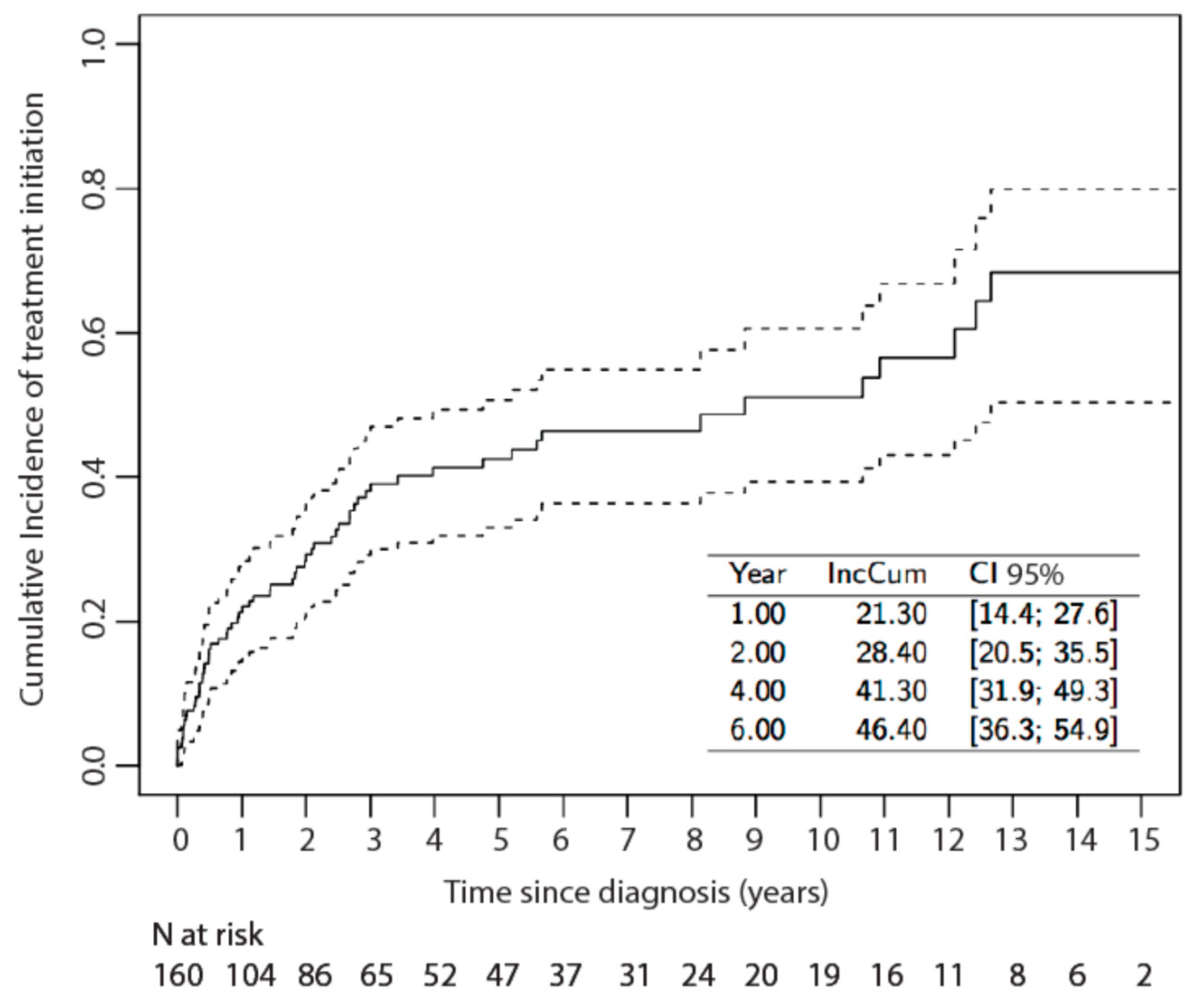
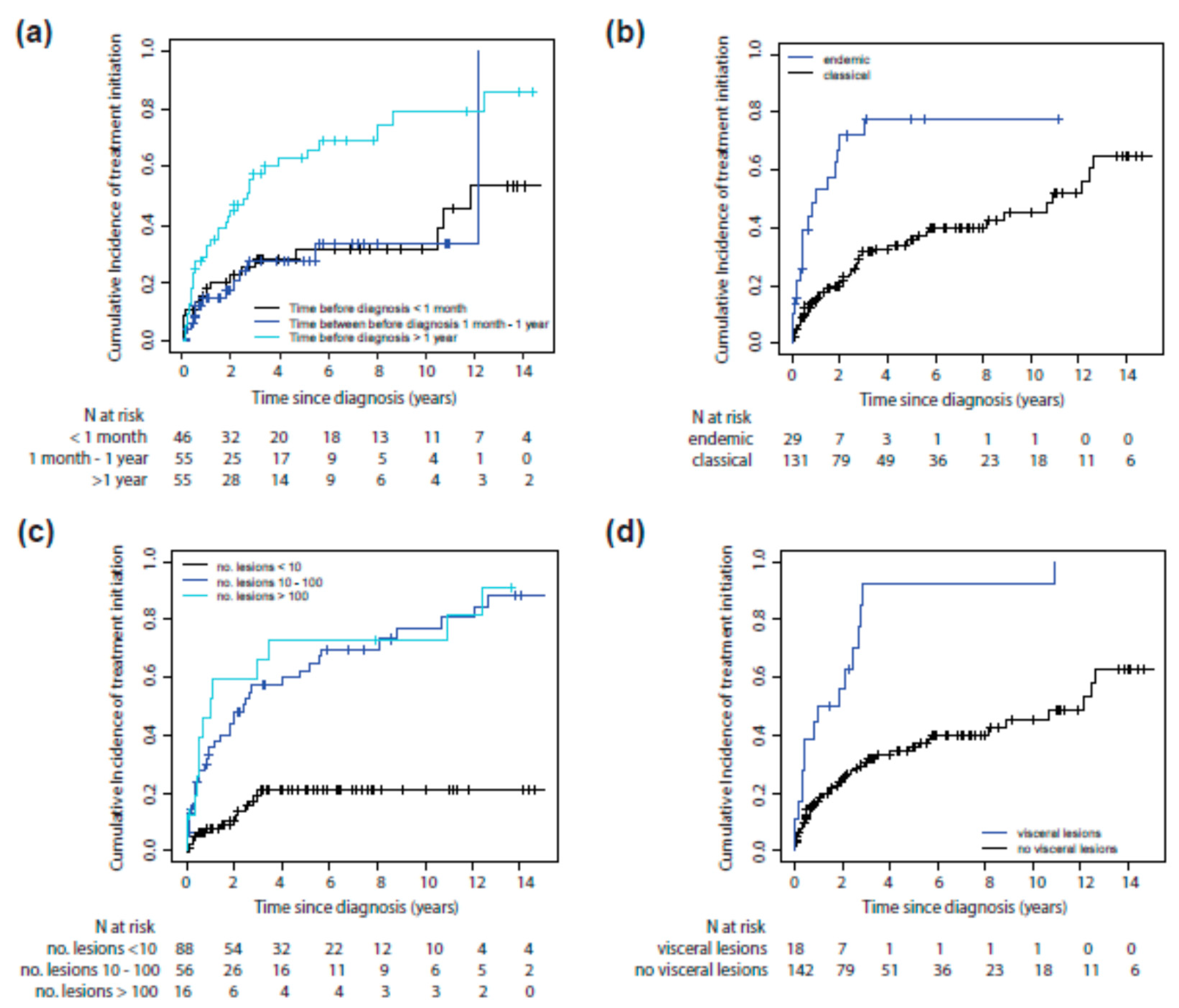
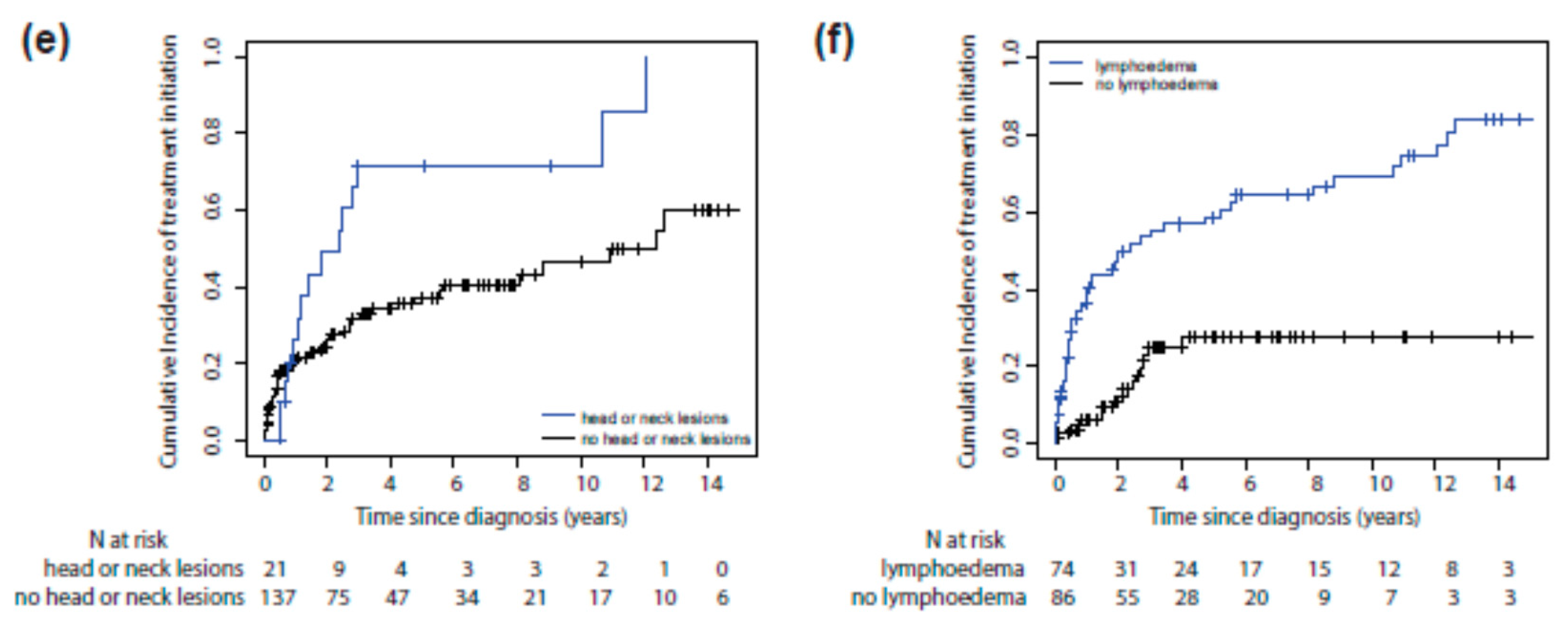
3.2. Risk Factors for Systemic Treatment Initiation
3.3. Therapeutic Response to KS Systemic Treatment
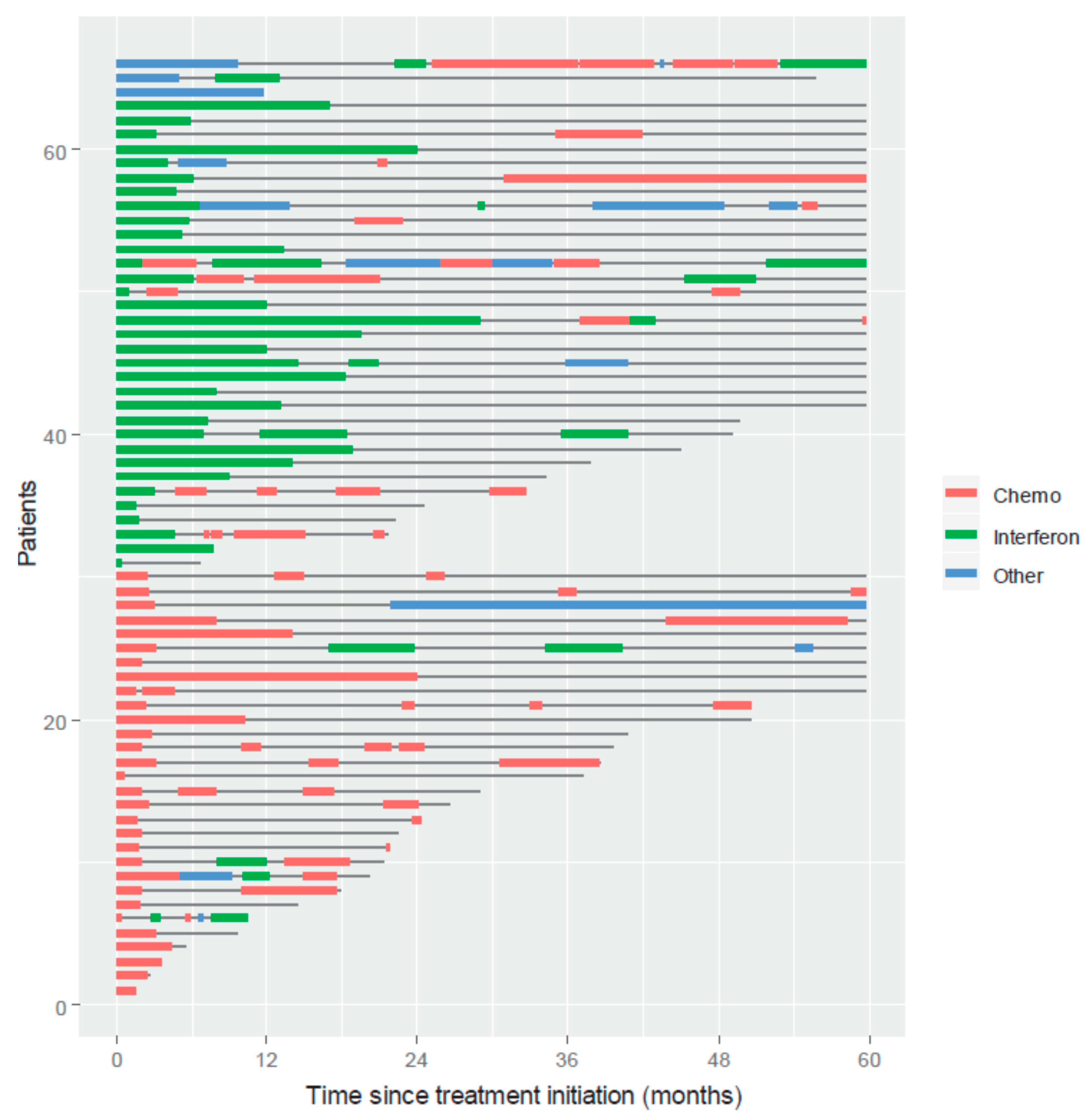
3.4. Treatment Free Time after KS Systemic Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| CR or PR (n = 53) | SD or PD (n = 11) | p-Value | |
|---|---|---|---|
| Age at treatment (years), median (IQR) | 64.5 (57.7; 73.4) | 58.3 (51.8; 74.1) | 0.48 |
| Time from diagnosis to systemic treatment (days), median (IQR) | 428 (142; 1026) | 306 (86; 830) | 0.54 |
| Subtype of Kaposi Sarcoma | 1 | ||
| classic | 39 (74%) | 8 (73%) | |
| endemic | 14 (26%) | 3 (27%) | |
| Type of systemic treatment | 0.11 | ||
| chemotherapy | 27 (51%) | 2 (18%) | |
| interferon | 24 (45%) | 8 (73%) | |
| other | 2 (4%) | 1 (9%) |
References
- Cottoni, F.; De Marco, R.; Montesu, M.A. Classical Kaposi’s sarcoma in north-east Sardinia: An overview from 1977 to 1991. Br. J. Cancer 1996, 73, 1132–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dal Maso, L.; Polesel, J.; Ascoli, V.; Zambon, P.; Budroni, M.; Ferretti, S.; Tumino, R.; Tagliabue, G.; Patriarca, S.; Federico, M.; et al. Classic Kaposi’s sarcoma in Italy, 1985–1998. Br. J. Cancer 2005, 92, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef] [Green Version]
- Lambert, M.; Gannage, M.; Karras, A.; Abel, M.; Legendre, C.; Kerob, D.; Agbalika, F.; Girard, P.-M.; Lebbe, C.; Caillat-Zucman, S. Differences in the frequency and function of HHV8-specific CD8 T cells between asymptomatic HHV8 infection and Kaposi sarcoma. Blood 2006, 108, 3871–3880. [Google Scholar] [CrossRef] [Green Version]
- Stratigos, J.D.; Potouridou, I.; Katoulis, A.C.; Hatziolou, E.; Christofidou, E.; Stratigos, A.; Hatzakis, A.; Stavrianeas, N.G. Classic Kaposi’s sarcoma in Greece: A clinico-epidemiological profile. Int. J. Dermatol. 1997, 36, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Nasti, G.; Martellotta, F.; Berretta, M.; Mena, M.; Fasan, M.; Perri, G.D.; Talamini, R.; Pagano, G.; Montroni, M.; Cinelli, R.; et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer 2003, 98, 2440–2446. [Google Scholar] [CrossRef]
- Frances, C. Kaposi’s sarcoma after renal transplantation. Nephrol. Dial. Transpl. 1998, 13, 2768–2773. [Google Scholar] [CrossRef] [Green Version]
- Lebbe, C.; Garbe, C.; Stratigos, A.J.; Harwood, C.; Peris, K.; Del Marmol, V.; Malvehy, J.; Zalaudek, I.; Hoeller, C.; Dummer, R.; et al. Diagnosis and treatment of Kaposi’s sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). Eur. J. Cancer 2019, 114, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Regnier-Rosencher, E.; Guillot, B.; Dupin, N. Treatments for classic Kaposi sarcoma: A systematic review of the literature. J. Am. Acad. Dermatol. 2013, 68, 313–331. [Google Scholar] [CrossRef]
- Mitsuyasu, R.T.; Groopman, J.E. Biology and therapy of Kaposi’s sarcoma. Semin. Oncol. 1984, 11, 53–59. [Google Scholar] [PubMed]
- Krigel, R.L.; Laubenstein, L.J.; Muggia, F.M. Kaposi’s sarcoma: A new staging classification. Cancer Treat Rep. 1983, 67, 531–534. [Google Scholar]
- Brambilla, L.; Boneschi, V.; Taglioni, M.; Ferrucci, S. Staging of classic Kaposi’s sarcoma: A useful tool for therapeutic choices. Eur. J. Dermatol. 2003, 13, 83–86. [Google Scholar]
- Gill, P.S.; Wernz, J.; Scadden, D.T.; Cohen, P.; Mukwaya, G.M.; von Roenn, J.H.; Jacobs, M.; Kempin, S.; Silverberg, I.; Gonzales, G.; et al. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi’s sarcoma. J. Clin. Oncol. 1996, 14, 2353–2364. [Google Scholar] [CrossRef] [PubMed]
- Northfelt, D.W.; Dezube, B.J.; Thommes, J.A.; Miller, B.J.; Fischl, M.A.; Friedman-Kien, A.; Kaplan, L.D.; Du Mond, C.; Mamelok, R.D.; Henry, D.H. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: Results of a randomized phase III clinical trial. J. Clin. Oncol. 1998, 16, 2445–2451. [Google Scholar] [CrossRef]
- Stewart, S.; Jablonowski, H.; Goebel, F.D.; Arasteh, K.; Spittle, M.; Rios, A.; Aboulafia, D.; Galleshaw, J.; Dezube, B.J. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J. Clin. Oncol. 1998, 16, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Lichterfeld, M.; Qurishi, N.; Hoffmann, C.; Hochdorfer, B.; Brockmeyer, N.H.; Arasteh, K.; Mauss, S.; Rockstroh, J.K. German Clinical AIDS Working Group (KAAD) Treatment of HIV-1-associated Kaposi’s sarcoma with pegylated liposomal doxorubicin and HAART simultaneously induces effective tumor remission and CD4+ T cell recovery. Infection 2005, 33, 140–147. [Google Scholar] [CrossRef]
- Cianfrocca, M.; Lee, S.; Von Roenn, J.; Tulpule, A.; Dezube, B.J.; Aboulafia, D.M.; Ambinder, R.F.; Lee, J.Y.; Krown, S.E.; Sparano, J.A. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: Evidence of symptom palliation from chemotherapy. Cancer 2010, 116, 3969–3977. [Google Scholar] [CrossRef] [Green Version]
- Gill, P.S.; Tulpule, A.; Espina, B.M.; Cabriales, S.; Bresnahan, J.; Ilaw, M.; Louie, S.; Gustafson, N.F.; Brown, M.A.; Orcutt, C.; et al. Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi’s sarcoma. J. Clin. Oncol. 1999, 17, 1876–1883. [Google Scholar] [CrossRef]
- Northfelt, D.W.; Dezube, B.J.; Thommes, J.A.; Levine, R.; Von Roenn, J.H.; Dosik, G.M.; Rios, A.; Krown, S.E.; DuMond, C.; Mamelok, R.D. Efficacy of pegylated-liposomal doxorubicin in the treatment of AIDS-related Kaposi’s sarcoma after failure of standard chemotherapy. J. Clin. Oncol. 1997, 15, 653–659. [Google Scholar] [CrossRef]
- Costa da Cunha, C.S.; Lebbe, C.; Rybojad, M.; Ferchal, F.; Rabian, C.; Vignon-Pennamen, M.D.; Calvo, F.; Morel, P. Long-term follow-up of non-HIV Kaposi’s sarcoma treated with low-dose recombinant interferon alfa-2b. Arch. Dermatol. 1996, 132, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Tur, E.; Brenner, S. Classic Kaposi’s sarcoma: Low-dose interferon alfa treatment. Dermatology 1998, 197, 37–42. [Google Scholar] [CrossRef]
- Pantanowitz, L.; Duke, W.H. Lymphoedematous variants of Kaposi’s sarcoma. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 118–120. [Google Scholar] [CrossRef]
- El-Mallawany, N.K.; Villiera, J.; Kamiyango, W.; Peckham-Gregory, E.C.; Scheurer, M.E.; Allen, C.E.; McAtee, C.L.; Legarreta, A.; Dittmer, D.P.; Kovarik, C.L.; et al. Endemic Kaposi sarcoma in HIV-negative children and adolescents: An evaluation of overlapping and distinct clinical features in comparison with HIV-related disease. Infect Agent Cancer 2018, 13, 33. [Google Scholar] [CrossRef]
- Chen, J.; Del Valle, L.; Lin, H.Y.; Plaisance-Bonstaff, K.; CraigForrest, J.; Post, S.R.; Qin, Z. Expression of PD-1 and PD-Ls in Kaposi’s sarcoma and regulation by oncogenic herpesvirus lytic reactivation. Virology 2019, 536, 16–19. [Google Scholar] [CrossRef]
- Uldrick, T.S.; Goncalves, P.H.; Abdul-Hay, M.; Claeys, A.J.; Emu, B.; Ernstoff, M.S.; Fling, S.P.; Fong, L.; Kaiser, J.C.; Lacroix, A.M.; et al. Assessment of the Safety of Pembrolizumab in Patients with HIV and Advanced Cancer-A Phase 1 Study. JAMA Oncol. 2019, 5, 1332–1339. [Google Scholar] [CrossRef]
- Delyon, J.; Bizot, A.; Battistella, M.; Madelaine, I.; Vercellino, L.; Lebbe, C. PD-1 blockade with nivolumab in endemic Kaposi sarcoma. Ann. Oncol. 2018, 29, 1067–1069. [Google Scholar] [CrossRef]
- Galanina, N.; Goodman, A.M.; Cohen, P.R.; Frampton, G.M.; Kurzrock, R. Successful Treatment of HIV-Associated Kaposi Sarcoma with Immune Checkpoint Blockade. Cancer Immunol. Res. 2018, 6, 1129–1135. [Google Scholar] [CrossRef] [Green Version]
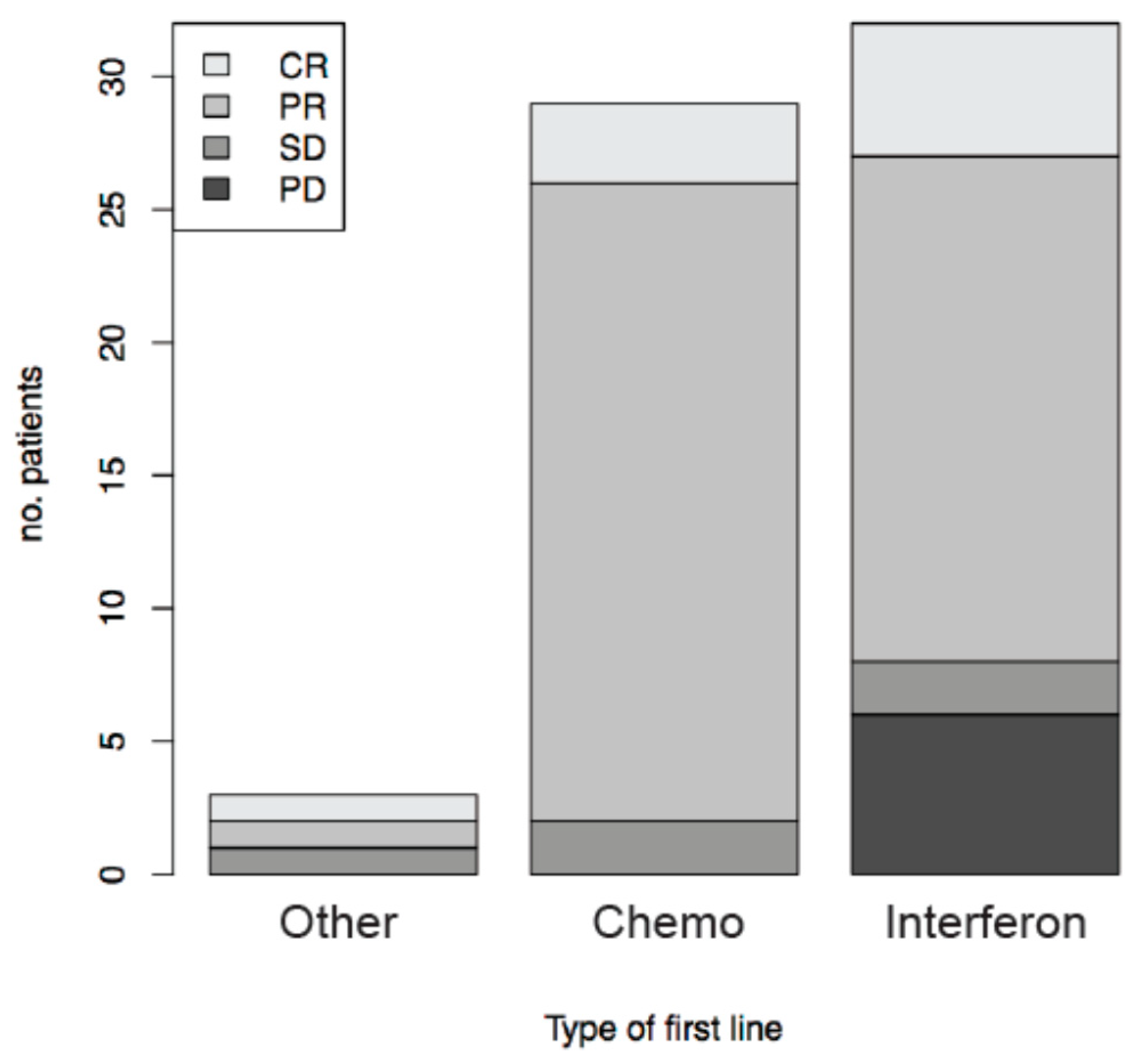

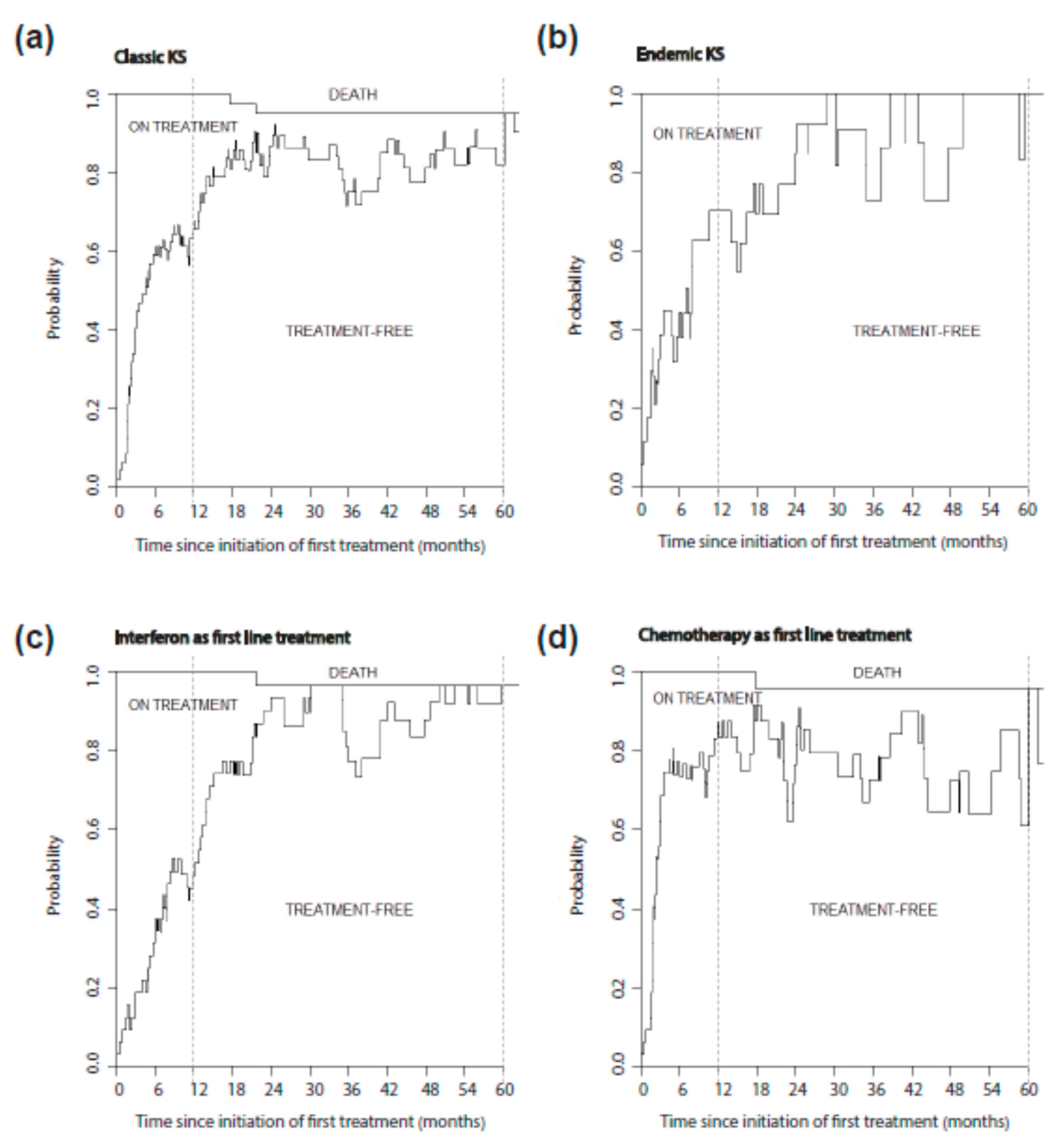
| Patients Characteristics (Total) | n = 160 |
|---|---|
| Age at diagnosis (years), median (IQR) | 62.6 (54.5; 72.4) |
| Gender | |
| female | 20 (12.5%) |
| male | 140 (87.5%) |
| Subtype of Kaposi Sarcoma | |
| classic | 131 (81.9%) |
| endemic | 29 (18.1%) |
| Number of lesions | |
| 0–10 | 88 (55%) |
| 10–100 | 56 (35%) |
| >100 | 16 (10%) |
| Disease localisation | |
| lower limbs | 146 (91.2%) |
| upper limbs | 61 (38.1%) |
| trunk | 32 (20.1%) |
| head or neck | 21 (13.3%) |
| mucosa | 12 (7.5%) |
| visceral | 18 (11.2%) |
| Painful lesions | |
| yes | 33 (21.2%) |
| no | 123 (78.8%) |
| Lymphedema | |
| yes | 74 (46.3%) |
| no | 86 (53.7%) |
| Serum LDH (n = 97) | |
| <UNL | 79 (81.4%) |
| >UNL | 18 (18.6%) |
| HHV8 PCR (n = 118) | |
| positive | 27 (22.9%) |
| negative | 91 (77.1%) |
| viral load (log), median (range) | 3.22 (2.50; 3.59) |
| Lymphocytes count (n = 117) | |
| >1500/mm3 | 60 (51.3%) |
| <1500/mm3 | 57 (48.7%) |
| CD4 count (nb/mm3), median (IQR) (n = 76) | 701 (507; 913) |
| Treatment | |
| observation | 22 (13.8%) |
| local | 72 (45%) |
| systemic | 66 (41.2%) |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | CI 95% | p-Value | HR | CI 95% | p-Value | |
| Age at diagnosis (years) | 1 | (0.98–1.02) | 8.62 × 10−1 | |||
| Time from first symptoms to diagnosis (months) | ||||||
| <1 m | 1 | 4.98 × 10−4 | 1 | 6.82 × 10−3 | ||
| 1–12 m | 0.98 | (0.47–2.05) | 2.19 | (0.98; 4.91) | ||
| >12 m | 2.61 | (1.44–4.72) | 2.7 | (1.41; 5.17) | ||
| Subtype of Kaposi Sarcoma | ||||||
| classic | 1 | 4.56 × 10−5 | 1 | . | 7.59 × 10−4 | |
| endemic | 3.61 | (2.07–6.3) | 3.29 | (1.71; 6.36) | ||
| Number of lesions | ||||||
| 0–10 | 1 | 2.26 × 10−9 | 1 | 1.27 × 10−4 | ||
| 10–100 | 5.2 | (2.82–9.6) | 3.64 | (1.81; 7.35) | ||
| >100 | 6.17 | (2.89–13.18) | 4.56 | (1.98; 10.54) | ||
| Disease localization | ||||||
| lower limbs no | 1 | 4.76 × 10−3 | ||||
| yes | 7.25 | (1.01–52.24) | ||||
| upper limbs no | 1 | 2.82 × 10−3 | ||||
| yes | 2.1 | (1.29–3.41) | ||||
| trunk no | 1 | 1.99 × 10−2 | ||||
| yes | 1.93 | (1.14–3.29) | ||||
| head or neck no | 1 | 1.12 × 10−2 | 1 | 2.34 × 10−2 | ||
| yes | 2.26 | (1.26–4.06) | 2.21 | (1.15; 4.25) | ||
| mucosa no | 1 | 8.50 × 10−2 | ||||
| yes | 1.96 | (0.97–3.97) | ||||
| visceral no | 1 | 2.32 × 10−5 | 1 | . | 3.98 × 10−2 | |
| yes | 4.1 | (2.3–7.31) | 2.11 | (1.06; 4.18) | ||
| Painful lesions | ||||||
| no | 1 | 1.17 × 10−3 | ||||
| yes | 2.41 | (1.45–4.02) | ||||
| Lymphedema | ||||||
| no | 1 | 2.52 × 10−7 | 1 | 5.62 × 10−3 | ||
| yes | 3.89 | (2.23–6.79) | 2.3 | (1.25; 4.26) | ||
| Serum LDH | ||||||
| <ULN | 1 | 3.42 × 10−3 | ||||
| >ULN | 2.76 | (1.47–5.17) | ||||
| Lymphocytes count | ||||||
| >1500/mm3 | 1 | 2.99 × 10−1 | ||||
| <1500/mm3 | 0.75 | (0.43–1.3) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benajiba, L.; Lambert, J.; La Selva, R.; Cochereau, D.; Baroudjian, B.; Roux, J.; Le Goff, J.; Pages, C.; Battistella, M.; Delyon, J.; et al. Systemic Treatment Initiation in Classical and Endemic Kaposi’s Sarcoma: Risk Factors and Global Multi-State Modelling in a Monocentric Cohort Study. Cancers 2021, 13, 2519. https://doi.org/10.3390/cancers13112519
Benajiba L, Lambert J, La Selva R, Cochereau D, Baroudjian B, Roux J, Le Goff J, Pages C, Battistella M, Delyon J, et al. Systemic Treatment Initiation in Classical and Endemic Kaposi’s Sarcoma: Risk Factors and Global Multi-State Modelling in a Monocentric Cohort Study. Cancers. 2021; 13(11):2519. https://doi.org/10.3390/cancers13112519
Chicago/Turabian StyleBenajiba, Lina, Jérôme Lambert, Roberta La Selva, Delphine Cochereau, Barouyr Baroudjian, Jennifer Roux, Jérôme Le Goff, Cécile Pages, Maxime Battistella, Julie Delyon, and et al. 2021. "Systemic Treatment Initiation in Classical and Endemic Kaposi’s Sarcoma: Risk Factors and Global Multi-State Modelling in a Monocentric Cohort Study" Cancers 13, no. 11: 2519. https://doi.org/10.3390/cancers13112519
APA StyleBenajiba, L., Lambert, J., La Selva, R., Cochereau, D., Baroudjian, B., Roux, J., Le Goff, J., Pages, C., Battistella, M., Delyon, J., & Lebbé, C. (2021). Systemic Treatment Initiation in Classical and Endemic Kaposi’s Sarcoma: Risk Factors and Global Multi-State Modelling in a Monocentric Cohort Study. Cancers, 13(11), 2519. https://doi.org/10.3390/cancers13112519









