Vitamin D: Promises on the Horizon and Challenges Ahead for Fighting Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
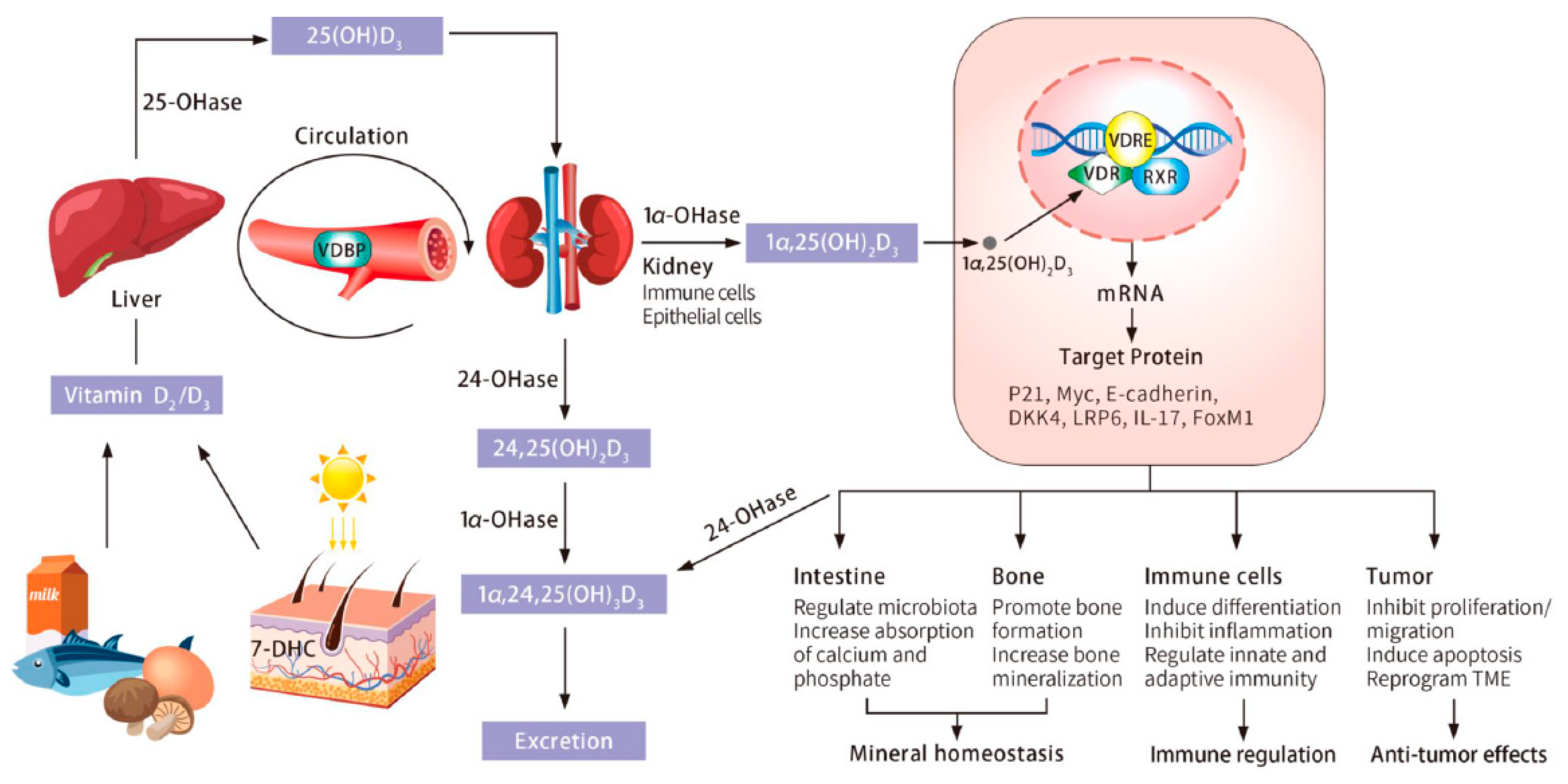
2. Vitamin D Sources, Metabolism, and Signaling
3. Vitamin D and Pancreatic Cancer Risk and Incidence
4. Vitamin D and Pancreatic Cancer Survival and Mortality
5. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Society, A.C. Cancer Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- Stromnes, I.M.; Hulbert, A.; Pierce, R.H.; Greenberg, P.D.; Hingorani, S.R. T-cell Localization, Activation, and Clonal Expansion in Human Pancreatic Ductal Adenocarcinoma. Cancer Immunol. Res. 2017, 5, 978–991. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Young, R.C. Value-Based Cancer Care. N. Engl. J. Med. 2015, 373, 2593–2595. [Google Scholar] [CrossRef]
- Golpour, A.; Bereswill, S.; Heimesaat, M.M. Antimicrobial and Immune-Modulatory Effects of Vitamin D Provide Promising Antibiotics-Independent Approaches to Tackle Bacterial Infections—Lessons Learnt from a Literature Survey. Eur. J. Microbiol. Immunol. 2019, 9, 80–87. [Google Scholar] [CrossRef]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Giovannucci, E. Vitamin D Status and Cancer Incidence, Survival, and Mortality. Adv. Exp. Med. Biol. 2020, 1268, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Skinner, H.G.; Michaud, D.S.; Giovannucci, E.; Willett, W.C.; Colditz, G.A.; Fuchs, C.S. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1688–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altieri, B.; Grant, W.B.; Della Casa, S.; Orio, F.; Pontecorvi, A.; Colao, A.; Sarno, G.; Muscogiuri, G. Vitamin D and pancreas: The role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit. Rev. Food Sci. Nutr. 2017, 57, 3472–3488. [Google Scholar] [CrossRef]
- Sluyter, J.D.; Manson, J.E.; Scragg, R. Vitamin D and Clinical Cancer Outcomes: A Review of Meta-Analyses. JBMR Plus 2021, 5, e10420. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Vieth, R.; Azad, A.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. A prospective nested case-control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res. 2006, 66, 10213–10219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loon, K.; Owzar, K.; Jiang, C.; Kindler, H.L.; Mulcahy, M.F.; Niedzwiecki, D.; O’Reilly, E.M.; Fuchs, C.; Innocenti, F.; Venook, A.P. 25-Hydroxyvitamin D levels and survival in advanced pancreatic cancer: Findings from CALGB 80303 (Alliance). J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.; Qian, Z.R.; Babic, A.; Morales-Oyarvide, V.; Rubinson, D.A.; Kraft, P.; Ng, K.; Bao, Y.; Giovannucci, E.L.; Ogino, S.; et al. Prediagnostic Plasma 25-Hydroxyvitamin D and Pancreatic Cancer Survival. J. Clin. Oncol. 2016, 34, 2899–2905. [Google Scholar] [CrossRef]
- Chirumbolo, S. Possible role of vitamin D3 on the adipocyte/fibroblast trans-differentiation mediated by pancreas cancer. Curr. Health Sci. J. 2015, 41, 5–10. [Google Scholar] [CrossRef]
- Kong, F.; Li, L.; Wang, G.; Deng, X.; Li, Z.; Kong, X. VDR signaling inhibits cancer-associated-fibroblasts’ release of exosomal miR-10a-5p and limits their supportive effects on pancreatic cancer cells. Gut 2019, 68, 950–951. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Z.; Gao, Y.; Xie, D.; Wei, D.; Cui, J.; Mishra, L.; Huang, S.; Zhang, Y.; Xie, K. Activation of vitamin D receptor signaling downregulates the expression of nuclear FOXM1 protein and suppresses pancreatic cancer cell stemness. Clin. Cancer Res. 2015, 21, 844–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukai, Y.; Eguchi, H. ASO Author Reflections: Regulation of Cancer-Associated Fibroblasts in Pancreatic Ductal Adenocarcinoma by Vitamin D Supplementation. Ann. Surg. Oncol. 2018, 25, 816–817. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Yamada, D.; Eguchi, H.; Iwagami, Y.; Asaoka, T.; Noda, T.; Kawamoto, K.; Gotoh, K.; Kobayashi, S.; Takeda, Y.; et al. Vitamin D Supplementation is a Promising Therapy for Pancreatic Ductal Adenocarcinoma in Conjunction with Current Chemoradiation Therapy. Ann. Surg. Oncol. 2018, 25, 1868–1879. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, S.; Naseem, I. Pancreatic cancer control: Is vitamin D the answer? Eur. J. Cancer Prev. 2016, 25, 188–195. [Google Scholar] [CrossRef]
- Wu, X.; Hu, W.; Lu, L.; Zhao, Y.; Zhou, Y.; Xiao, Z.; Zhang, L.; Zhang, H.; Li, X.; Li, W.; et al. Repurposing vitamin D for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm. Sin. B 2019, 9, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Dougan, M.; Tyan, K.; Giobbie-Hurder, A.; Blum, S.M.; Ishizuka, J.; Qazi, T.; Elias, R.; Vora, K.B.; Ruan, A.B.; et al. Vitamin D intake is associated with decreased risk of immune checkpoint inhibitor-induced colitis. Cancer 2020, 126, 3758–3767. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; National Academy Press: Washington, DC, USA, 2010.
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087s–1091s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Dowell, M.S.; Hale, C.A.; Bendich, A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J. Am. Coll. Nutr. 2003, 22, 142–146. [Google Scholar] [CrossRef]
- Serdar, M.A.; Batu Can, B.; Kilercik, M.; Durer, Z.A.; Aksungar, F.B.; Serteser, M.; Coskun, A.; Ozpinar, A.; Unsal, I. Analysis of Changes in Parathyroid Hormone and 25 (OH) Vitamin D Levels with Respect to Age, Gender and Season: A Data Mining Study. J. Med. Biochem. 2017, 36, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirumbolo, S. Commentary: Vitamin D and Pancreatic Cancer: A Pooled Analysis from the Pancreatic Cancer Case-Control Consortium. Front. Oncol. 2015, 5, 160. [Google Scholar] [CrossRef] [Green Version]
- Tsuprykov, O.; Chen, X.; Hocher, C.F.; Skoblo, R.; Lianghong, Y.; Hocher, B. Why should we measure free 25(OH) vitamin D? J. Steroid Biochem. Mol. Biol. 2018, 180, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D: Newer Concepts of Its Metabolism and Function at the Basic and Clinical Level. J. Endocr. Soc. 2020, 4, bvz038. [Google Scholar] [CrossRef]
- Carlberg, C.; Muñoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Dimitrov, V.; Barbier, C.; Ismailova, A.; Wang, Y.; Dmowski, K.; Salehi-Tabar, R.; Memari, B.; Groulx-Boivin, E.; White, J.H. Vitamin D-regulated Gene Expression Profiles: Species-specificity and Cell-specific Effects on Metabolism and Immunity. Endocrinology 2021, 162. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Mangin, M.; Sinha, R.; Fincher, K. Inflammation and vitamin D: The infection connection. Inflamm. Res. 2014, 63, 803–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef] [Green Version]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Alkan, A.; Köksoy, E.B. Vitamin D deficiency in cancer patients and predictors for screening (D-ONC study). Curr. Probl. Cancer 2019, 43, 421–428. [Google Scholar] [CrossRef]
- Calmarza, P.; Sanz París, A.; Prieto López, C.; Llorente Barrio, M.; Boj Carceller, D. Vitamin D levels in patients with recent cancer diagnosis. Nutr. Hosp. 2018, 35, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Seyedalipour, F.; Mansouri, A.; Vaezi, M.; Gholami, K.; Heidari, K.; Hadjibabaie, M.; Ghavamzadeh, A. High Prevalence of Vitamin D Deficiency in Newly Diagnosed Acute Myeloid Leukemia Patients and Its Adverse Outcome. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 209–216. [Google Scholar]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080s–1086s. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Sunlight “D” ilemma: Risk of skin cancer or bone disease and muscle weakness. Lancet 2001, 357, 4–6. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Heaney, R.P.; Holick, M.F.; Lips, P.; Meunier, P.J.; Vieth, R. Estimates of optimal vitamin D status. Osteoporos. Int. 2005, 16, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lovegrove, J.A.; Givens, D.I. 25(OH)D3-enriched or fortified foods are more efficient at tackling inadequate vitamin D status than vitamin D3. Proc. Nutr. Soc. 2018, 77, 282–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada-Gomez, J.M.; Bouillon, R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos. Int. 2018, 29, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Tangpricha, V. Vitamin D in food and supplements. Am. J. Clin. Nutr. 2012, 95, 1299–1300. [Google Scholar] [CrossRef] [Green Version]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripkovic, L.; Wilson, L.R.; Hart, K.; Johnsen, S.; de Lusignan, S.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Elliott, R.; et al. Daily supplementation with 15 μg vitamin D(2) compared with vitamin D(3) to increase wintertime 25-hydroxyvitamin D status in healthy South Asian and white European women: A 12-wk randomized, placebo-controlled food-fortification trial. Am. J. Clin. Nutr. 2017, 106, 481–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzaferro, S.; Goldsmith, D.; Larsson, T.E.; Massy, Z.A.; Cozzolino, M. Vitamin D metabolites and/or analogs: Which D for which patient? Curr. Vasc. Pharmacol. 2014, 12, 339–349. [Google Scholar] [CrossRef]
- Wu-Wong, J.R.; Tian, J.; Goltzman, D. Vitamin D analogs as therapeutic agents: A clinical study update. Curr. Opin. Investig. Drugs 2004, 5, 320–326. [Google Scholar]
- Barry, E.L.; Rees, J.R.; Peacock, J.L.; Mott, L.A.; Amos, C.I.; Bostick, R.M.; Figueiredo, J.C.; Ahnen, D.J.; Bresalier, R.S.; Burke, C.A.; et al. Genetic variants in CYP2R1, CYP24A1, and VDR modify the efficacy of vitamin D3 supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J. Clin. Endocrinol. Metab. 2014, 99, E2133–E2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Zanetti, R.; Ferlay, J. Cancer Incidence in Five Continents; IARC Scientific Publicaitons: Lyon, France, 2007; Volume 9. [Google Scholar]
- Mohr, S.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Garland, F.C. Ultraviolet B irradiance and vitamin D status are inversely associated with incidence rates of pancreatic cancer worldwide. Pancreas 2010, 39, 669–674. [Google Scholar] [CrossRef]
- Kinoshita, S.; Wagatsuma, Y.; Okada, M. Geographical distribution for malignant neoplasm of the pancreas in relation to selected climatic factors in Japan. Int. J. Health Geogr. 2007, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Giovannucci, E. Vitamin D and cancer incidence in the Harvard cohorts. Ann. Epidemiol. 2009, 19, 84–88. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Effect of interval between serum draw and follow-up period on relative risk of cancer incidence with respect to 25-hydroxyvitamin D level: Implications for meta-analyses and setting vitamin D guidelines. Derm. Endocrinol. 2011, 3, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Liu, Z.; Qin, H. Association between vitamin D and risk of colorectal cancer: A systematic review of prospective studies. J. Clin. Oncol. 2011, 29, 3775–3782. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S. Vitamin D and pancreatic cancer risk in the alpha-tocopherol, beta-carotene cancer prevention cohort. Cancer Res. 2006, 66, 9802–9803. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Nishihara, R.; Wang, M.; Chan, A.T.; Qian, Z.R.; Inamura, K.; Zhang, X.; Ng, K.; Kim, S.A.; Mima, K.; et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut 2016, 65, 296–304. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Hayes, R.B.; Horst, R.L.; Anderson, K.E.; Hollis, B.W.; Silverman, D.T. Serum vitamin D and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian screening trial. Cancer Res. 2009, 69, 1439–1447. [Google Scholar] [CrossRef] [Green Version]
- Wolpin, B.M.; Ng, K.; Bao, Y.; Kraft, P.; Stampfer, M.J.; Michaud, D.S.; Ma, J.; Buring, J.E.; Sesso, H.D.; Lee, I.M.; et al. Plasma 25-hydroxyvitamin D and risk of pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, X.; Sun, X.; Lu, S.; Liu, S. Vitamin intake and pancreatic cancer risk reduction: A meta-analysis of observational studies. Medicine 2018, 97, e0114. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.Z.; Chen, W.J.; Wu, J.; Chen, Y.; Wu, C.C.; Wang, Z.N. Plasma 25-hydroxyvitamin D levels, vitamin D intake, and pancreatic cancer risk or mortality: A meta-analysis. Oncotarget 2017, 8, 64395–64406. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, M.; Risch, H.A.; Bosetti, C.; Anderson, K.E.; Petersen, G.M.; Bamlet, W.R.; Cotterchio, M.; Cleary, S.P.; Ibiebele, T.I.; La Vecchia, C.; et al. Vitamin D and pancreatic cancer: A pooled analysis from the Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 2016, 27, 208. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Barry, E.L.; Mott, L.A.; Rees, J.R.; Sandler, R.S.; Snover, D.C.; Bostick, R.M.; Ivanova, A.; Cole, B.F.; Ahnen, D.J.; et al. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N. Engl. J. Med. 2015, 373, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Bassuk, S.S.; Buring, J.E. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J. Steroid Biochem. Mol. Biol. 2020, 198, 105522. [Google Scholar] [CrossRef]
- Barry, E.L.; Peacock, J.L.; Rees, J.R.; Bostick, R.M.; Robertson, D.J.; Bresalier, R.S.; Baron, J.A. Vitamin D Receptor Genotype, Vitamin D3 Supplementation, and Risk of Colorectal Adenomas: A Randomized Clinical Trial. JAMA Oncol. 2017, 3, 628–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haykal, T.; Samji, V.; Zayed, Y.; Gakhal, I.; Dhillon, H.; Kheiri, B.; Kerbage, J.; Veerapaneni, V.; Obeid, M.; Danish, R.; et al. The role of vitamin D supplementation for primary prevention of cancer: Meta-analysis of randomized controlled trials. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 480–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.R.I.; Xiong, Y. Influence of vitamin D on cancer risk and treatment: Why the variability? Trends Cancer Res. 2018, 13, 43–53. [Google Scholar]
- Grant, W.B.; Garland, C.F. The association of solar ultraviolet B (UVB) with reducing risk of cancer: Multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res. 2006, 26, 2687–2699. [Google Scholar]
- Eryilmaz, M.K.; Mutui, H.; Gunduz, S.; Uysal, M.; Musri, F.Y.; Coskun, H.S. More sunlight exposure may improve the overall survival in patients with pancreas cancer. J. Oncol. Sci. 2015, 2, 4. [Google Scholar]
- Cho, M.; Peddi, P.F.; Ding, K.; Chen, L.; Thomas, D.; Wang, J.; Lockhart, A.C.; Tan, B.; Wang-Gillam, A. Vitamin D deficiency and prognostics among patients with pancreatic adenocarcinoma. J. Transl. Med. 2013, 11, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, L.S.; Yilmaz, M.K.; Falkmer, U.G.; Poulsen, L.; Bøgsted, M.; Christensen, H.S.; Bojesen, S.E.; Jensen, B.V.; Chen, I.M.; Johansen, A.Z.; et al. Pre-treatment serum vitamin D deficiency is associated with increased inflammatory biomarkers and short overall survival in patients with pancreatic cancer. Eur. J. Cancer 2020, 144, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Cannon, T.L.; Ford, J.; Hester, D.; Trump, D.L. The Incidental Use of High-Dose Vitamin D3 in Pancreatic Cancer. Case Rep. Pancreat. Cancer 2016, 2, 32–35. [Google Scholar] [CrossRef] [Green Version]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Innocenti, F.; Owzar, K.; Jiang, C.; Etheridge, A.S.; Gordân, R.; Sibley, A.B.; Mulkey, F.; Niedzwiecki, D.; Glubb, D.; Neel, N.; et al. The vitamin D receptor gene as a determinant of survival in pancreatic cancer patients: Genomic analysis and experimental validation. PLoS ONE 2018, 13, e0202272. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, C.; Tong, Y.; Li, Y.; Gao, Y.; Hou, S.; Hao, M.; Han, X.; Wang, B.; Wang, Q.; et al. Novel Nonsecosteroidal Vitamin D Receptor Modulator Combined with Gemcitabine Enhances Pancreatic Cancer Therapy through Remodeling of the Tumor Microenvironment. J. Med. Chem. 2021, 64, 629–643. [Google Scholar] [CrossRef]
- Yu, W.D.; Ma, Y.; Flynn, G.; Muindi, J.R.; Kong, R.X.; Trump, D.L.; Johnson, C.S. Calcitriol enhances gemcitabine anti-tumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle 2010, 9, 3022–3029. [Google Scholar] [CrossRef] [Green Version]
- Akutsu, T.; Kitamura, H.; Himeiwa, S.; Kitada, S.; Akasu, T.; Urashima, M. Vitamin D and Cancer Survival: Does Vitamin D Supplementation Improve the Survival of Patients with Cancer? Curr. Oncol. Rep. 2020, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Crockett, S.D.; Barry, E.L.; Mott, L.A.; Ahnen, D.J.; Robertson, D.J.; Anderson, J.C.; Wallace, K.; Burke, C.A.; Bresalier, R.S.; Figueiredo, J.C.; et al. Calcium and vitamin D supplementation and increased risk of serrated polyps: Results from a randomised clinical trial. Gut 2019, 68, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Grant, W.B.; Giovannucci, E.L.; Lipkin, M.; Newmark, H.; Holick, M.F.; Garland, F.C. Vitamin D and prevention of breast cancer: Pooled analysis. J. Steroid Biochem. Mol. Biol. 2007, 103, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Maestro, M.A.; Molnár, F.; Carlberg, C. Vitamin D and Its Synthetic Analogs. J. Med. Chem. 2019, 62, 6854–6875. [Google Scholar] [CrossRef]
- Verstuyf, A.; Segaert, S.; Verlinden, L.; Bouillon, R.; Mathieu, C. Recent developments in the use of vitamin D analogues. Expert Opin. Investig. Drugs 2000, 9, 443–455. [Google Scholar] [CrossRef]
- Hummel, D.; Aggarwal, A.; Borka, K.; Bajna, E.; Kállay, E.; Horváth, H.C. The vitamin D system is deregulated in pancreatic diseases. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt B, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Reichrath, J.; Reichrath, S.; Vogt, T.; Römer, K. Crosstalk Between Vitamin D and p53 Signaling in Cancer: An Update. Adv. Exp. Med. Biol. 2020, 1268, 307–318. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef] [PubMed]
- Schizas, D.; Charalampakis, N.; Kole, C.; Economopoulou, P.; Koustas, E.; Gkotsis, E.; Ziogas, D.; Psyrri, A.; Karamouzis, M.V. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat. Rev. 2020, 86, 102016. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Trump, D.L. Vitamin D Receptor Signaling and Cancer. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1009–1038. [Google Scholar] [CrossRef] [PubMed]
- McAllister, F.; Bailey, J.M.; Alsina, J.; Nirschl, C.J.; Sharma, R.; Fan, H.; Rattigan, Y.; Roeser, J.C.; Lankapalli, R.H.; Zhang, H.; et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 2014, 25, 621–637. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zoltan, M.; Riquelme, E.; Xu, H.; Sahin, I.; Castro-Pando, S.; Montiel, M.F.; Chang, K.; Jiang, Z.; Ling, J.; et al. Immune Cell Production of Interleukin 17 Induces Stem Cell Features of Pancreatic Intraepithelial Neoplasia Cells. Gastroenterology 2018, 155, 210–223.e213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chandra, V.; Riquelme Sanchez, E.; Dutta, P.; Quesada, P.R.; Rakoski, A.; Zoltan, M.; Arora, N.; Baydogan, S.; Horne, W.; et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Fawaz, L.; Mrad, M.F.; Kazan, J.M.; Sayegh, S.; Akika, R.; Khoury, S.J. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin. Immunol. 2016, 166–167, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Pantalena, L.C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell. Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Luo, F.; Xing, J.C.; Zhang, F.; Xu, J.Z.; Zhang, Z.H. 1,25(OH)(2) D(3) inhibited Th17 cells differentiation via regulating the NF-κB activity and expression of IL-17. Cell Prolif. 2018, 51, e12461. [Google Scholar] [CrossRef] [Green Version]
- Karkeni, E.; Morin, S.O.; Bou Tayeh, B.; Goubard, A.; Josselin, E.; Castellano, R.; Fauriat, C.; Guittard, G.; Olive, D.; Nunès, J.A. Vitamin D Controls Tumor Growth and CD8+ T Cell Infiltration in Breast Cancer. Front. Immunol. 2019, 10, 1307. [Google Scholar] [CrossRef] [Green Version]
- Fleet, J.C.; Burcham, G.N.; Calvert, R.D.; Elzey, B.D.; Ratliff, T.L. 1α, 25 Dihydroxyvitamin D (1,25(OH)(2)D) inhibits the T cell suppressive function of myeloid derived suppressor cells (MDSC). J. Steroid Biochem. Mol. Biol. 2020, 198, 105557. [Google Scholar] [CrossRef] [PubMed]
- Gorchs, L.; Ahmed, S.; Mayer, C.; Knauf, A.; Fernández Moro, C.; Svensson, M.; Heuchel, R.; Rangelova, E.; Bergman, P.; Kaipe, H. The vitamin D analogue calcipotriol promotes an anti-tumorigenic phenotype of human pancreatic CAFs but reduces T cell mediated immunity. Sci. Rep. 2020, 10, 17444. [Google Scholar] [CrossRef]
- Prieux-Klotz, C.; Dior, M.; Damotte, D.; Dreanic, J.; Brieau, B.; Brezault, C.; Abitbol, V.; Chaussade, S.; Coriat, R. Immune Checkpoint Inhibitor-Induced Colitis: Diagnosis and Management. Target. Oncol. 2017, 12, 301–308. [Google Scholar] [CrossRef]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2020, 22, 362. [Google Scholar] [CrossRef]
- Cannell, J.J.; Grant, W.B.; Holick, M.F. Vitamin D and inflammation. Derm. Endocrinol. 2014, 6, e983401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R.; Theron, A.J.; Rapoport, B.L. Immunopathogenesis of Immune Checkpoint Inhibitor-Related Adverse Events: Roles of the Intestinal Microbiome and Th17 Cells. Front. Immunol. 2019, 10, 2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Sethi, V.; Vitiello, G.A.; Saxena, D.; Miller, G.; Dudeja, V. The Role of the Microbiome in Immunologic Development and its Implication For Pancreatic Cancer Immunotherapy. Gastroenterology 2019, 156, 2097–2115.e2092. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
- Poore, G.D.; Kopylova, E.; Zhu, Q.; Carpenter, C.; Fraraccio, S.; Wandro, S.; Kosciolek, T.; Janssen, S.; Metcalf, J.; Song, S.J.; et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 2020, 579, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e712. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, I.; Lu, R.; Zhang, Y.; Zhang, J.; Dai, Y.; Xia, Y.; Sun, J. Vitamin D receptor promotes healthy microbial metabolites and microbiome. Sci. Rep. 2020, 10, 7340. [Google Scholar] [CrossRef]
- Fakhoury, H.M.A.; Kvietys, P.R.; AlKattan, W.; Anouti, F.A.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef]
- Jin, D.; Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Dong, H.; Sun, J. Lack of Vitamin D Receptor Causes Dysbiosis and Changes the Functions of the Murine Intestinal Microbiome. Clin. Ther. 2015, 37, 996–1009.e1007. [Google Scholar] [CrossRef]
- Sun, J. Dietary vitamin D, vitamin D receptor, and microbiome. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 471–474. [Google Scholar] [CrossRef]
- Naderpoor, N.; Mousa, A.; Fernanda Gomez Arango, L.; Barrett, H.L.; Dekker Nitert, M.; de Courten, B. Effect of Vitamin D Supplementation on Faecal Microbiota: A Randomised Clinical Trial. Nutrients 2019, 11, 2888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The Effect of Various Doses of Oral Vitamin D(3) Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020, 40, 551–556. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Lu, R.; Wu, S.; Chatterjee, I.; Zhou, D.; Xia, Y.; Sun, J. Vitamin D Receptor Protects Against Dysbiosis and Tumorigenesis via the JAK/STAT Pathway in Intestine. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 729–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Dong, M.; Sheng, W.; Liu, Q.; Yu, D.; Dong, Q.; Li, Q.; Wang, J. Expression of vitamin D receptor as a potential prognostic factor and therapeutic target in pancreatic cancer. Histopathology 2015, 67, 386–397. [Google Scholar] [CrossRef] [PubMed]
- DeSmet, M.L.; Fleet, J.C. Constitutively active RAS signaling reduces 1,25 dihydroxyvitamin D-mediated gene transcription in intestinal epithelial cells by reducing vitamin D receptor expression. J. Steroid Biochem. Mol. Biol. 2017, 173, 194–201. [Google Scholar] [CrossRef]
| Protein Name | Gene Symbol | SNP Locus | Alleles | Location | Amino Acid Variant | Relation to Pancreatic Cancer (PC) | Reference PMID |
|---|---|---|---|---|---|---|---|
| Vitamin D binding protein | GC | rs2282679 | T>G | Intron | NA | No significant correlation | 26364161, 31467173 |
| rs4588 | G>A/G>T | Exon | T>M/T>K | No significant correlation | |||
| rs7041 | A>C/A>T | Exon | D>E | No significant correlation | |||
| rs1491711 | C>G | Intron | NA | Heterozygote is associated with PC risk | 23826131 | ||
| 25-hydroxyvitamin D-1 alpha hydroxylase | CYP27B1 | rs10877012 | G>C/G>T | 5′ promoter | NA | No association with increased risk for the development of PC | |
| rs4646536 | A>G | Intron | NA | No association with increased risk for the development of PC | |||
| rs703842 | A>C/A>G/A>T | 3′ UTR | NA | No significant correlation | 25799011 | ||
| rs1048691 | C>T | 3′ UTR | NA | No significant correlation | |||
| 25-hydroxyvitamin D 24-hydrolase | CYP24A1 | rs2585428 | C>T | Intron | NA | Significantly decrease the risk of PC | 29254801 |
| rs6127119 | C>T | Intron | NA | TT versus CC genotype is positively associated with PC risk a | 23826131 | ||
| Vitamin D receptor | VDR | rs2228570 | A>C/A>G/A>T | Exon | M>R / M>T / M>K | T allele associates with increased PC risk and tumor pathological differentiation b | 25616697, 33226370 |
| rs1544410 | C>A/C>G/C>T | Intron | NA | G allele associates with decreased PC risk and TNM classification c | 25616697, 32918214 | ||
| rs2853564 | G>A | Intron | NA | G allele associates with increased overall survival of PC patients d | 30107003 |
| Trial Identifier | Agents | Patient Condition | Patient Number | Study Phase | Design | Status | Location |
|---|---|---|---|---|---|---|---|
| NCT04617067 | Paricalcitol/Gemcitabine/Nab-paclitaxel | Advanced or metastatic PDAC | 43 | 2 | Single Group | Recruiting | Ireland |
| NCT04524702 | Paricalcitol/Gemcitabine/Hydroxychloroquine/Nab-paclitaxel | Advanced or metastatic PDAC | 21 | 2 | Single Group | Recruiting | United States |
| NCT04054362 | Paricalcitol/Paclitaxel protein bound/Cisplatin/Gemcitabine | Untreated metastatic PDAC | 14 | 2 | Non-Randomized | Recruiting | United Kingdom |
| NCT03883919 | Paricalcitol/5-FU/Leucovorin/Liposomal Irinotecan | Advanced PDAC progressed on Gemcitabine-based therapy | 20 | 1 | Non-Randomized | Recruiting | United States |
| NCT03520790 | Paricalcitol/Gemcitabine/Nab-paclitaxel | Untreated metastatic PDAC | 112 | 1/2 | Randomized | Active, not recruiting | United States |
| NCT03519308 | Paricalcitol/Nivolumab/Nab-Paclitaxel/Gemcitabine | Untreated resectable PDAC | 20 | 1a | Randomized | Recruiting | United States |
| NCT03415854 | Paricalcitol/Cisplatin/Paclitaxel Protein Bound/Gemcitabine | Untreated metastatic PDAC | 14 | 2 | Single Group | Active, not recruiting | United States |
| NCT03331562 | Paricalcitol/Pembrolizumab | Metastatic pancreatic cancer | 24 | 2 | Randomized | Completed | United States |
| NCT03138720 | Paricalcitol/Paclitaxel protein bound/Gemcitabine/Cisplatin | Resectable, borderline resectable, or locally advanced (unresectable) PDAC | 24 | 2 | Single Group | Recruiting | United States |
| NCT02930902 | Paricalcitol/Gemcitabine Hydrochloride/Nab-paclitaxel/Pembrolizumab | Resectable pancreatic cancer | 10 | 1b | Non-Randomized | Active, not recruiting | United States |
| NCT02754726 | Paricalcitol/Nivolumab/Albumin-bound paclitaxel/Cisplatin/Gemcitabine | Untreated metastatic PDAC | 10 | 2 | Single Group | Recruiting | United States |
| NCT03472833 | High-dose (4000 IU/day)/Standard-dose (800 IU/day) vitamin D3 | Pancreatic cancer with vitamin D deficiency | 60 | 3 | Randomized | Recruiting | Austria |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Wang, L.; Zuo, X.; Bresalier, R.S. Vitamin D: Promises on the Horizon and Challenges Ahead for Fighting Pancreatic Cancer. Cancers 2021, 13, 2716. https://doi.org/10.3390/cancers13112716
Wei D, Wang L, Zuo X, Bresalier RS. Vitamin D: Promises on the Horizon and Challenges Ahead for Fighting Pancreatic Cancer. Cancers. 2021; 13(11):2716. https://doi.org/10.3390/cancers13112716
Chicago/Turabian StyleWei, Daoyan, Liang Wang, Xiangsheng Zuo, and Robert S. Bresalier. 2021. "Vitamin D: Promises on the Horizon and Challenges Ahead for Fighting Pancreatic Cancer" Cancers 13, no. 11: 2716. https://doi.org/10.3390/cancers13112716
APA StyleWei, D., Wang, L., Zuo, X., & Bresalier, R. S. (2021). Vitamin D: Promises on the Horizon and Challenges Ahead for Fighting Pancreatic Cancer. Cancers, 13(11), 2716. https://doi.org/10.3390/cancers13112716






