Population Pharmacokinetics of Oxycodone and Metabolites in Patients with Cancer-Related Pain
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Pharmacokinetic Sample Collection
2.3. Determination of Oxycodone and Metabolites
2.4. Population Pharmacokinetic Analysis
2.5. Model Evaluation
2.6. Pharmacodynamic Analysis
3. Results
3.1. Patients
3.2. Population Pharmacokinetic Analysis
3.3. Pharmacodynamic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van den Beuken-van Everdingen, M.H.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom. Manage. 2016, 51, 1070–1090.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organisation. Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; World Health Organisation: Geneva, Switzerland, 2018. [Google Scholar]
- Rogers, E.; Mehta, S.; Shengelia, R.; Reid, M.C. Four Strategies for Managing Opioid-Induced Side Effects in Older Adults. Clin. Geriatr. 2013, 21, 1–15. [Google Scholar]
- Kinnunen, M.; Piirainen, P.; Kokki, H.; Lammi, P.; Kokki, M. Updated Clinical Pharmacokinetics and Pharmacodynamics of Oxycodone. Clin. Pharmacokinet. 2019, 58, 705–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saari, T.I.; Ihmsen, H.; Neuvonen, P.J.; Olkkola, K.T.; Schwilden, H. Oxycodone clearance is markedly reduced with advancing age: A population pharmacokinetic study. Br. J. Anaesth. 2012, 108, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Andreassen, T.N.; Klepstad, P.; Davies, A.; Bjordal, K.; Lundstrom, S.; Kaasa, S.; Dale, O. Influences on the pharmacokinetics of oxycodone: A multicentre cross-sectional study in 439 adult cancer patients. Eur. J. Clin. Pharmacol. 2011, 67, 493–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huddart, R.; Clarke, M.; Altman, R.B.; Klein, T.E. PharmGKB summary: Oxycodone pathway, pharmacokinetics. Pharm. Genom. 2018, 28, 230–237. [Google Scholar] [CrossRef]
- Lalovic, B.; Kharasch, E.; Hoffer, C.; Risler, L.; Liu-Chen, L.Y.; Shen, D.D. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: Role of circulating active metabolites. Clin. Pharmacol. Ther. 2006, 79, 461–479. [Google Scholar] [CrossRef]
- Mikus, G.; Klimas, R. Contribution of oxycodone and its metabolites to the analgesic effect. Br. J. Anaesth. 2014, 112, 944–945. [Google Scholar] [CrossRef] [Green Version]
- Stein, C.; Schäfer, M.; Hassan, A.H. Peripheral opioid receptors. Ann. Med. 1995, 27, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Mandema, J.W.; Kaiko, R.F.; Oshlack, B.; Reder, R.F.; Stanski, D.R. Characterization and validation of a pharmacokinetic model for controlled-release oxycodone. Br. J. Clin. Pharmacol. 1996, 42, 747–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Sun, D.; Palmisano, M.; Zhou, S. Slow drug delivery decreased total body clearance and altered bioavailability of immediate- and controlled-release oxycodone formulations. Pharmacol. Res. Perspect. 2016, 4, e00210. [Google Scholar] [CrossRef]
- Xu, X.S.; Etropolski, M.; Upmalis, D.; Okamoto, A.; Lin, R.; Nandy, P. Pharmacokinetic and pharmacodynamic modeling of opioid-induced gastrointestinal side effects in patients receiving tapentadol IR and oxycodone IR. Pharm. Res. 2012, 29, 2555–2564. [Google Scholar] [CrossRef]
- Ladebo, L.; Foster, D.J.R.; Abuhelwa, A.Y.; Upton, R.N.; Kongstad, K.T.; Drewes, A.M.; Christrup, L.L.; Olesen, A.E. Population pharmacokinetic-pharmacodynamic modelling of liquid and controlled-release formulations of oxycodone in healthy volunteers. Basic Clin. Pharmacol. Toxicol. 2020, 126, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Benziger, D.P.; Miotto, J.; Grandy, R.P.; Thomas, G.B.; Swanton, R.E.; Fitzmartin, R.D. A pharmacokinetic/pharmacodynamic study of controlled-release oxycodone. J. Pain Symptom Manag. 1997, 13, 75–82. [Google Scholar] [CrossRef]
- Lamminsalo, M.; Piirainen, P.; Kokki, H.; Knibbe, C.A.J.; Ranta, V.P.; Valitalo, P.; Kokki, M. Population pharmacokinetics of oxycodone in plasma and cerebrospinal fluid after epidural and intravenous administration. Expert Opin Drug Deliv. 2019, 16, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Kokubun, H.; Suzuki, A.; Takayanagi, R.; Yamada, Y.; Matoba, M.; Yago, K. Population pharmacokinetics of oxycodone in patients with cancer-related pain. J. Pain Palliat. Care Pharmacother. 2012, 26, 220–225. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Artino, A.R., Jr. Analyzing and interpreting data from likert-type scales. J. Grad. Med. Educ. 2013, 5, 541–542. [Google Scholar] [CrossRef] [Green Version]
- Lindbom, L.; Pihlgren, P.; Jonsson, E.N. PsN-Toolkit—A collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 2005, 79, 241–257. [Google Scholar] [CrossRef]
- Lindbom, L.; Ribbing, J.; Jonsson, E.N. Perl-speaks-NONMEM (PsN)—A Perl module for NONMEM related programming. Comput. Methods Programs Biomed. 2004, 75, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Keizer, R.J.; van Benten, M.; Beijnen, J.H.; Schellens, J.H.; Huitema, A.D. Pirana and PCluster: A modeling environment and cluster infrastructure for NONMEM. Comput. Methods Programs Biomed. 2011, 101, 72–79. [Google Scholar] [CrossRef]
- Jonsson, E.N.; Karlsson, M.O. Xpose—An S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 1999, 58, 51–64. [Google Scholar] [CrossRef]
- Beal, S.L. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 2001, 28, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.E.; Karlsson, M.O.; Dunne, A.; Ludden, T.M. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J. Pharmacokinet. Pharmacodyn. 2008, 35, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Joerger, M. Covariate pharmacokinetic model building in oncology and its potential clinical relevance. AAPS J. 2012, 14, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Comets, E.; Brendel, K.; Mentre, F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: The npde add-on package for R. Comput. Methods Programs Biomed. 2008, 90, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Upton, R.N.; Mould, D.R. Basic concepts in population modeling, simulation, and model-based drug development: Part 3 introduction to pharmacodynamic modeling methods. CPT Pharmacomet. Syst. Pharmacol. 2014, 3, e88. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models Usinglme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Watkins, P.B.; Murray, S.A.; Winkelman, L.G.; Heuman, D.M.; Wrighton, S.A.; Guzelian, P.S. Erythromycin breath test as an assay of glucocorticoid-inducible liver cytochromes P-450. Studies in rats and patients. J. Clin. Investig. 1989, 83, 688–697. [Google Scholar] [CrossRef] [Green Version]
- Kokki, M.; Valitalo, P.; Rasanen, I.; Aaltomaa, S.; Ojanpera, I.; Eskelinen, M.; Kokki, H. Absorption of different oral dosage forms of oxycodone in the elderly: A cross-over clinical trial in patients undergoing cystoscopy. Eur. J. Clin. Pharmacol. 2012, 68, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Geneesmiddeleninformatiebank, 2019. Summary of Product Characteristics Oxycodone Hydrochloride 5, 10, 20, 40 & 80 Prolonged Release Tablets. Available online: https://www.geneesmiddeleninformatiebank.nl/smpc/h100819_smpc.pdf (accessed on 1 June 2021).
- Romand, S.; Spaggiari, D.; Marsousi, N.; Samer, C.; Desmeules, J.; Daali, Y.; Rudaz, S. Characterization of oxycodone in vitro metabolism by human cytochromes P450 and UDP-glucuronosyltransferases. J. Pharm. Biomed. Anal. 2017, 144, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Kummer, O.; Hammann, F.; Moser, C.; Schaller, O.; Drewe, J.; Krahenbuhl, S. Effect of the inhibition of CYP3A4 or CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Eur. J. Clin. Pharmacol. 2011, 67, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cajanus, K.; Neuvonen, M.; Koskela, O.; Kaunisto, M.A.; Neuvonen, P.J.; Niemi, M.; Kalso, E. Analgesic Plasma Concentrations of Oxycodone After Surgery for Breast Cancer-Which Factors Matter? Clin. Pharmacol. Ther. 2018, 103, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Babalonis, S.; Lofwall, M.R.; Nuzzo, P.A.; Walsh, S.L. Pharmacodynamic effects of oral oxymorphone: Abuse liability, analgesic profile and direct physiologic effects in humans. Addict Biol. 2016, 21, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Hale, M.E.; Dvergsten, C.; Gimbel, J. Efficacy and safety of oxymorphone extended release in chronic low back pain: Results of a randomized, double-blind, placebo- and active-controlled phase III study. J. Pain. 2005, 6, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.W.; Bostrom, E.; Keizer, R.; Bjorkman, S.; Hammarlund-Udenaes, M. Oxymorphone active uptake at the blood-brain barrier and population modeling of its pharmacokinetic-pharmacodynamic relationship. J. Pharm. Sci. 2013, 102, 3320–3331. [Google Scholar] [CrossRef] [PubMed]
- Klimas, R.; Witticke, D.; El Fallah, S.; Mikus, G. Contribution of oxycodone and its metabolites to the overall analgesic effect after oxycodone administration. Expert Opin Drug Metab. Toxicol. 2013, 9, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Kokki, M.; Valitalo, P.; Kuusisto, M.; Ranta, V.P.; Raatikainen, K.; Hautajarvi, H.; Kokki, H. Central nervous system penetration of oxycodone after intravenous and epidural administration. Br. J. Anaesth. 2014, 112, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Bergstrand, M.; Karlsson, M.O. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009, 11, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Laugsand, E.A.; Sprangers, M.A.; Bjordal, K.; Skorpen, F.; Kaasa, S.; Klepstad, P. Health care providers underestimate symptom intensities of cancer patients: A multicenter European study. Health Qual Life Outcomes. 2010, 8, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
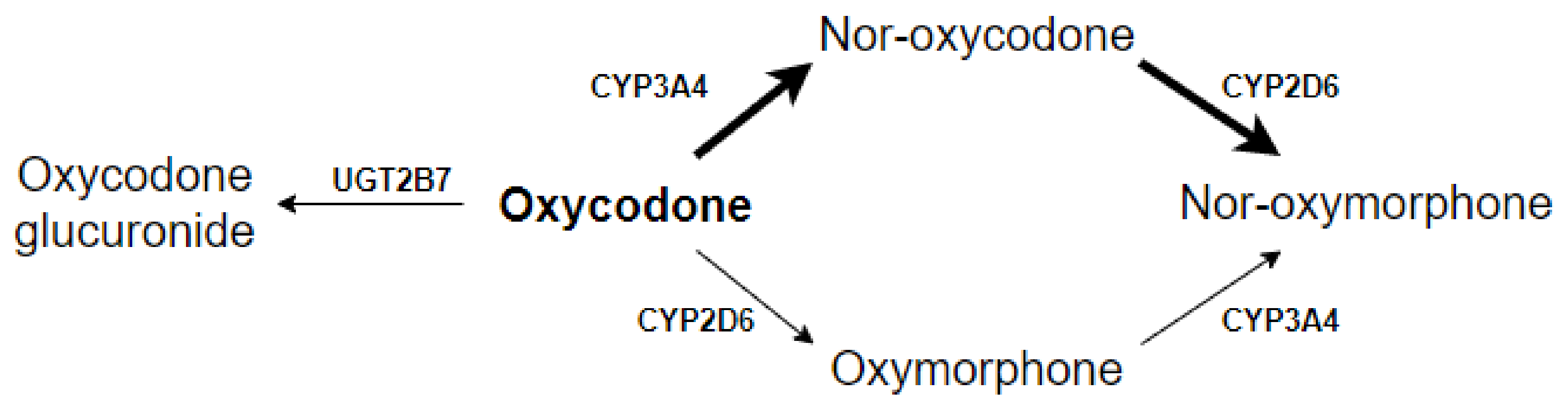
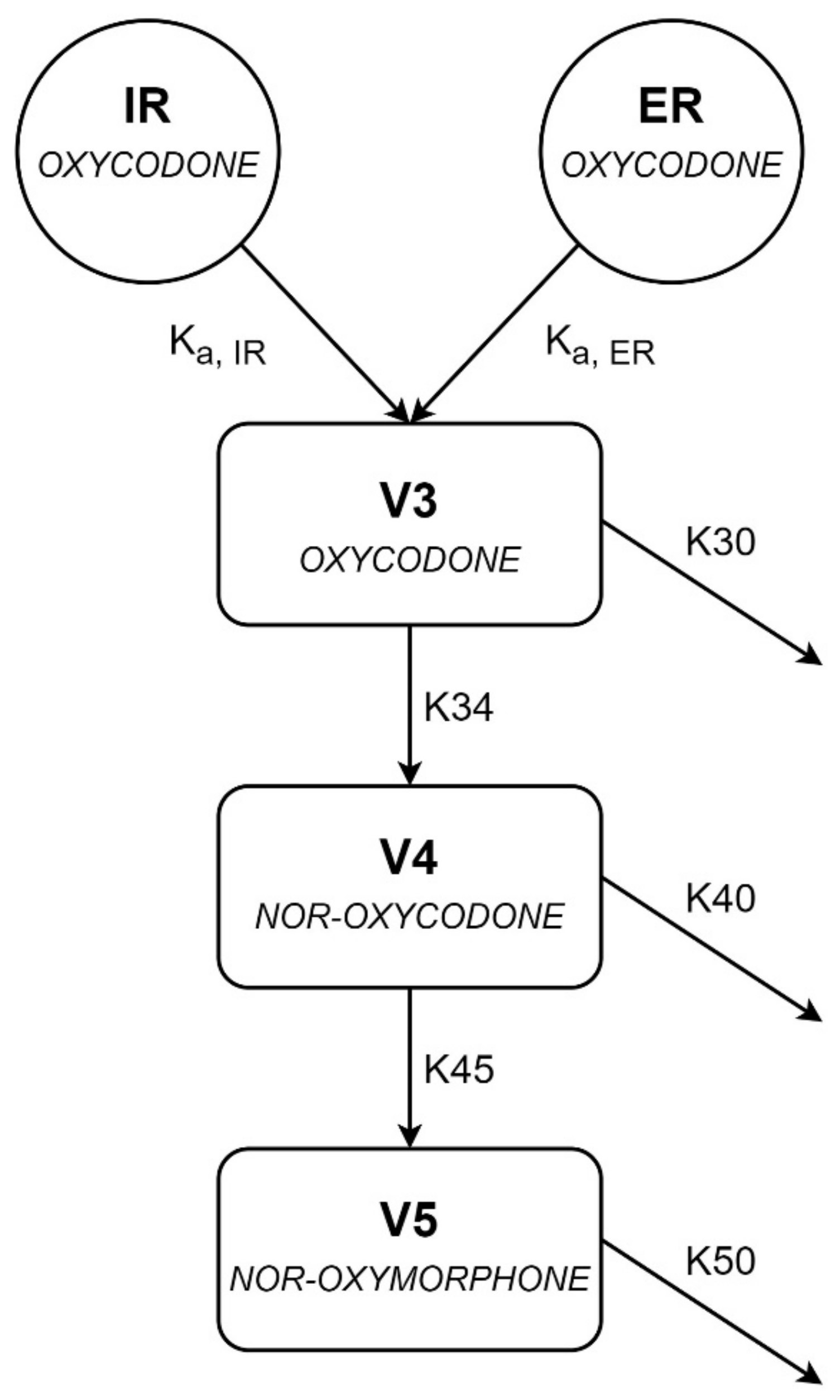

| Patient Characteristics (n = 28) | No./Median | %/Range |
|---|---|---|
| Sex (female) | 12 | 43% |
| Age (years) | 62.5 | 39–81 |
| Weight (kg) | 80.0 | 46–135 |
| Body Mass Index | 27.7 | 18.9–42.6 |
| Follow-up (hours) | 80.7 | 24.1–211.4 |
| No. of samples/patient | 12 | 4–51 |
| Dose at inclusion* | ||
| Immediate release (mg) | 5 | 0–25 |
| Extended release (mg) | 10 | 0–40 |
| Primary tumor | ||
| Urogenital | 9 | 32% |
| Breast | 5 | 18% |
| GIST/Soft tissue sarcoma | 4 | 14% |
| Melanoma | 3 | 11% |
| Other | 7 | 25% |
| WHO performance status | ||
| 0 | 0 | 0% |
| 1 | 8 | 29% |
| 2 | 9 | 32% |
| 3 | 4 | 14% |
| Unknown | 7 | 25% |
| eGFR (mL/min) | 86.0 | 37–>90 |
| Albumin (g/L) | 42.5 | 29–49 |
| CYP2D6 | ||
| EM | 12 | 43% |
| IM | 10 | 36% |
| Missing | 6 | 21% |
| CYP3A4*22 | ||
| *1/*1 | 22 | 79% |
| *1/*22 | 6 | 21% |
| *22/*22 | 0 | 0% |
| UGT2B7*2 | ||
| Wildtype | 6 | 21% |
| Heterozygous | 17 | 61% |
| Variant | 5 | 18% |
| Pain scores at inclusion | ||
| Average pain | 2 | 0–7 |
| Maximal pain | 6 | 0–10 |
| Parameter (Unit) | Parameter Estimate [Shrinkage] | RSE (%) | Bootstrap Median | 95% CI Bootstrap |
|---|---|---|---|---|
| Ka, IR (h−1) | 3.61 | 39.3 | 3.33 | (0.13–6.22) |
| Ka, ER23 (h−1) | 0.329 | 38.6 | 0.311 | (0.11–3.14) |
| V3/F (L) | 619 | 9.2 | 600 | (205–771) |
| K30 (h−1) | 0.012 | FIX | 0.012 | FIX |
| K34 (h−1) | 0.086 | 10.9 | 0.087 | (0.07–0.27) |
| V4/F (L) | 16.3 | FIX | 16.3 | FIX |
| K40 (h−1) | 3.28 | 30.1 | 3.22 | (1.53–4.99) |
| K45 (h−1) | 1.36 | 32.1 | 1.47 | (0.33–2.16) |
| V5/F (L) | 64.1 | FIX | 64.1 | FIX |
| K50 (h−1) | 1.97 | 35.9 | 2.00 | (0.47–3.59) |
| IIV | ||||
| K34 (CV%) | 35.7 [5.4] | 14.8 | 34.8 | (22.3–46.8) |
| K40 (CV%) | 98.7 [3.4] | 26.0 | 96.7 | (52.3–241) |
| K50 (CV%) | 81.7 [7.1] | 10.8 | 78.4 | (55.6–102) |
| Residual error | ||||
| Oxycodone | [8.6] | |||
| Proportional (%) | 39.7 | 13.4 | 38.7 | (28.8–51.3) |
| Additive (nM) | 0 | FIX | 0 | FIX |
| Nor-oxycodone | [5.4] | |||
| Proportional (%) | 16.7 | 18.5 | 16.5 | (9.78–23.3) |
| Additive (nM) | 3.34 | 40.4 | 3.10 | (0.77–6.34) |
| Nor-oxymorphone | [4.0] | |||
| Proportional (%) | 15.6 | 21.5 | 15.9 | (7.57–22.7) |
| Additive (nM) | 1.09 | 24.3 | 0.98 | (0.25–1.65) |
| Conditional number | 517.28 |
| Observations | Pain | Adverse Events | |
|---|---|---|---|
| Average | Maximal | Sum | |
| Oxycodone exposure (mM/hour) | 1.28 (−3.73–6.29) b | 0.58 (−5.74–6.89) b | −2.72 (−8.57–3.12) b |
| Nor-oxycodone exposure (mM/hour) | NA | NA | −3.29 (−10.62–4.04) b |
| Nor-oxymorphone exposure (mM/hour) | NA | NA | −12.13 (−39.43–15.18) b |
| Number of observations | 100 | 125 | 110 |
| R2 | 0.478 | 0.524 | 0.251 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agema, B.C.; Oosten, A.W.; Sassen, S.D.T.; Rietdijk, W.J.R.; van der Rijt, C.C.D.; Koch, B.C.P.; Mathijssen, R.H.J.; Koolen, S.L.W. Population Pharmacokinetics of Oxycodone and Metabolites in Patients with Cancer-Related Pain. Cancers 2021, 13, 2768. https://doi.org/10.3390/cancers13112768
Agema BC, Oosten AW, Sassen SDT, Rietdijk WJR, van der Rijt CCD, Koch BCP, Mathijssen RHJ, Koolen SLW. Population Pharmacokinetics of Oxycodone and Metabolites in Patients with Cancer-Related Pain. Cancers. 2021; 13(11):2768. https://doi.org/10.3390/cancers13112768
Chicago/Turabian StyleAgema, Bram C., Astrid W. Oosten, Sebastiaan D.T. Sassen, Wim J.R. Rietdijk, Carin C.D. van der Rijt, Birgit C.P. Koch, Ron H.J. Mathijssen, and Stijn L.W. Koolen. 2021. "Population Pharmacokinetics of Oxycodone and Metabolites in Patients with Cancer-Related Pain" Cancers 13, no. 11: 2768. https://doi.org/10.3390/cancers13112768
APA StyleAgema, B. C., Oosten, A. W., Sassen, S. D. T., Rietdijk, W. J. R., van der Rijt, C. C. D., Koch, B. C. P., Mathijssen, R. H. J., & Koolen, S. L. W. (2021). Population Pharmacokinetics of Oxycodone and Metabolites in Patients with Cancer-Related Pain. Cancers, 13(11), 2768. https://doi.org/10.3390/cancers13112768






