CT-Detected Subsolid Nodules: A Predictor of Lung Cancer Development at Another Location?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
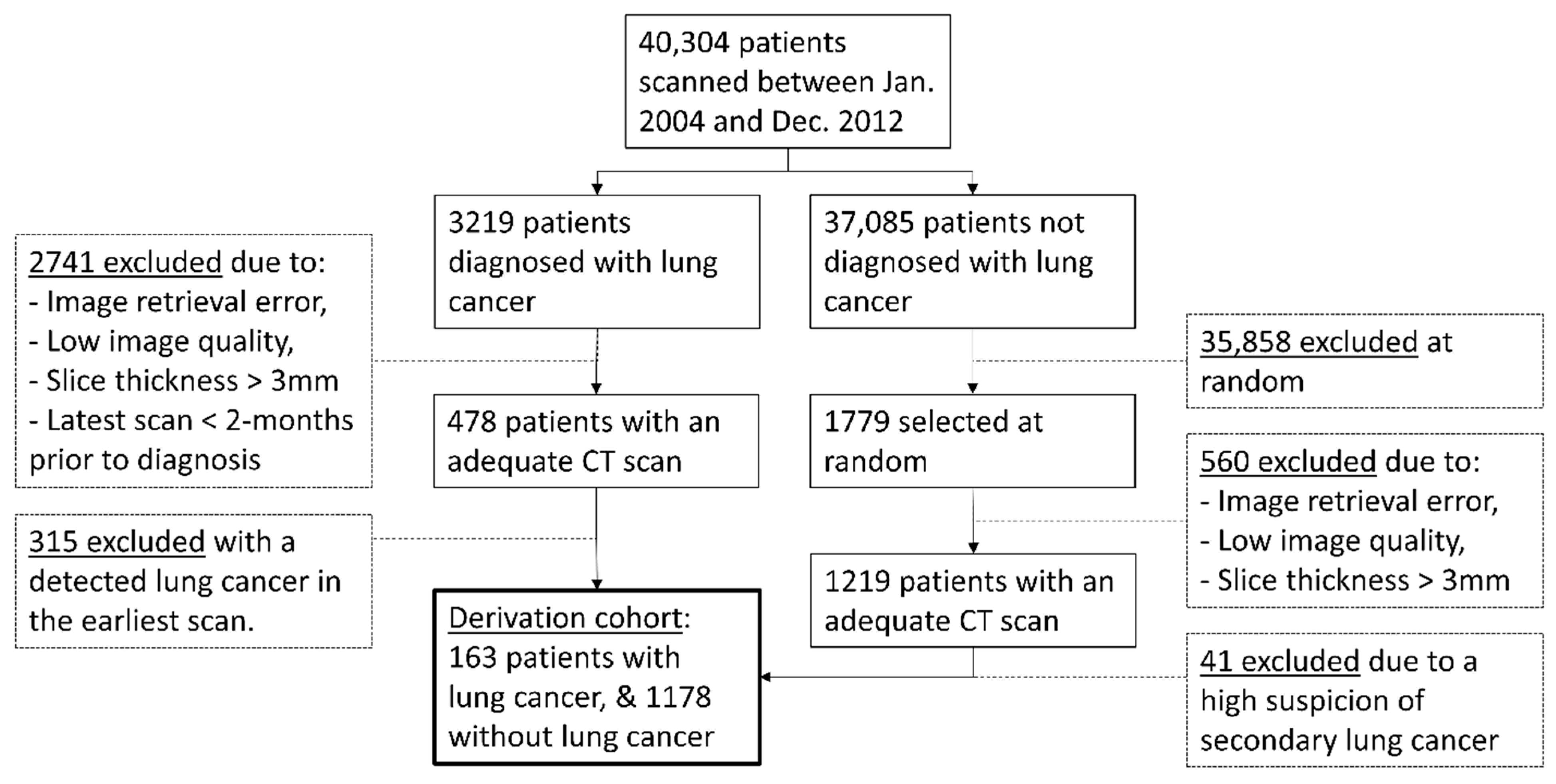
2.1. Study Population
2.2. CT Features
2.3. Statistical Analysis
3. Results
3.1. Univariable and Full Multivariable Analysis
3.2. Parsimonious Lung Cancer Incidence Risk Models
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Top 10 Causes of Death 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 6 May 2020).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.; Fornito, M.; Palazzolo, M.; Criscione, A.; Gangemi, M.; Borrata, F.; Vigneri, P.; Nardini, M.; Dunning, J. Ground glass opacities management in the lung cancer screening era. Ann. Transl. Med. 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.; Prokop, M.; Jacobs, C.; Capretti, G.; Sverzellati, N.; Ciompi, F.; van Ginneken, B.; Schaefer-Prokop, C.M.; Galeone, C.; Marchianò, A.; et al. Long-Term Active Surveillance of Screening Detected Subsolid Nodules is a Safe Strategy to Reduce Overtreatment. J. Thorac. Oncol. 2018, 13, 1454–1463. [Google Scholar] [CrossRef] [Green Version]
- Walter, J.E.; Heuvelmans, M.A.; Yousaf-Khan, U.; Dorrius, M.D.; Thunnissen, E.; Schermann, A.; Groen, H.J.; Van Der Aalst, C.M.; Nackaerts, K.; Vliegenthart, R.; et al. New Subsolid Pulmonary Nodules in Lung Cancer Screening: The NELSON Trial. J. Thorac. Oncol. 2018, 13, 1410–1414. [Google Scholar] [CrossRef] [Green Version]
- Henschke, C.I.; Yip, R.; Smith, J.P.; Wolf, A.S.; Flores, R.M.; Liang, M.; Salvatore, M.M.; Liu, Y.; Xu, D.M.; Yankelevitz, D.F.; et al. CT Screening for Lung Cancer: Part-Solid Nodules in Baseline and Annual Repeat Rounds. Am. J. Roentgenol. 2016, 207, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Yankelevitz, D.F.; Yip, R.; Smith, J.P.; Liang, M.; Liu, Y.; Xu, D.M.; Salvatore, M.M.; Wolf, A.S.; Flores, R.M.; Henschke, C.I.; et al. CT Screening for Lung Cancer: Nonsolid Nodules in Baseline and Annual Repeat Rounds. Radiology 2015, 277, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, M.M.; Palazzo, L.L.; Kong, C.Y.; Hunsaker, A.R. Cancer Risk in Subsolid Nodules in the National Lung Screening Trial. Radiology 2019, 293, 441–448. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, A.; Tammemagi, M.C.; Mayo, J.R.; Roberts, H.; Liu, G.; Soghrati, K.; Yasufuku, K.; Martel, S.; Laberge, F.; Gingras, M.; et al. Probability of Cancer in Pulmonary Nodules Detected on First Screening CT. N. Engl. J. Med. 2013, 369, 910–919. [Google Scholar] [CrossRef] [Green Version]
- Henschke, C.I.; Yankelevitz, D.F.; Mirtcheva, R.; McGuinness, G.; McCauley, D.; Miettinen, O.S. CT Screening for Lung Cancer. Am. J. Roentgenol. 2002, 178, 1053–1057. [Google Scholar] [CrossRef]
- Callister, M.E.J.; Baldwin, D.R.; Akram, A.R.; Barnard, S.; Cane, P.; Draffan, J.; Franks, K.; Gleeson, F.; Graham, R.; Malhotra, P.; et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules: Accredited by NICE. Thorax 2015, 70, ii1–ii54. [Google Scholar] [CrossRef] [Green Version]
- The American College of Radiology. Lung CT Screening Reporting & Data System v1.0 2014. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads (accessed on 29 April 2021).
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Schreuder, A.; Jacobs, C.; Scholten, E.T.; Van Ginneken, B.; Schaefer-Prokop, C.M.; Prokop, M. Typical CT Features of Intrapulmonary Lymph Nodes: A Review. Radiol. Cardiothorac. Imaging 2020, 2, e190159. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Ciompi, F.; Scholten, E.T.; Goo, J.M.; Prokop, M.; Jacobs, C.; Van Ginneken, B.; Schaefer-Prokop, C.M. Visual discrimination of screen-detected persistent from transient subsolid nodules: An observer study. PLoS ONE 2018, 13, e0191874. [Google Scholar] [CrossRef] [Green Version]
- De Hoop, B.; Van Ginneken, B.; Gietema, H.; Prokop, M. Pulmonary Perifissural Nodules on CT Scans: Rapid Growth Is Not a Predictor of Malignancy. Radiology 2012, 265, 611–616. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Therneau, T.M. A Package for Survival Analysis in R. 2020. Available online: https://CRAN.R-project.org/package=survival (accessed on 20 April 2021).
- Therneau, T.M.; Li, H. Computing the Cox model for case cohort designs. Lifetime Data Anal. 1999, 5, 99–112. [Google Scholar] [CrossRef]
- Zhang, Z. Multivariable fractional polynomial method for regression model. Ann. Transl. Med. 2016, 4, 174. [Google Scholar] [CrossRef]
- Royston, P.; Altman, D.G. Regression Using Fractional Polynomials of Continuous Covariates: Parsimonious Parametric Modelling. J. R. Stat. Soc. Ser. C Appl. Stat. 1994, 43, 429. [Google Scholar] [CrossRef]
- Schoenfeld, D. Chi-Squared Goodness-of-Fit Tests for the Proportional Hazards Regression Model. Biometrika 1980, 67, 145–153. [Google Scholar] [CrossRef]
- Schreuder, A.; Schaefer-Prokop, C.M.; Scholten, E.T.; Jacobs, C.; Prokop, M.; Van Ginneken, B. Lung cancer risk to personalise annual and biennial follow-up computed tomography screening. Thorax 2018, 73, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, A.; Jacobs, C.; Lessmann, N.; Broeders, M.J.; Silva, M.; Išgum, I.; de Jong, P.A.; Sverzellati, N.; Prokop, M.; Pastorino, U.; et al. Combining pulmonary and cardiac computed tomography biomarkers for disease-specific risk modelling in lung cancer screening. Eur. Respir. J. 2021, 2021, 2003386. [Google Scholar] [CrossRef] [PubMed]
- Sverzellati, N.; Randi, G.; Spagnolo, P.; Marchianò, A.; Silva, M.; Kuhnigk, J.-M.; Vecchia, C.L.; Zompatori, M.; Pastorino, U. Increased mean lung density: Another independent predictor of lung cancer? Eur. J. Radiol. 2013, 82, 1325–1331. [Google Scholar] [CrossRef]
- Honma, K.; Nelson, G.; Murray, J. Intrapulmonary lymph nodes in South African miners—An autopsy survey. Am. J. Ind. Med. 2007, 50, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, A.; Van Ginneken, B.; Scholten, E.T.; Jacobs, C.; Prokop, M.; Sverzellati, N.; Desai, S.R.; Devaraj, A.; Schaefer-Prokop, C.M. Classification of CT Pulmonary Opacities as Perifissural Nodules: Reader Variability. Radiology 2018, 288, 867–875. [Google Scholar] [CrossRef] [Green Version]
- Toumazis, I.; Bastani, M.; Han, S.S.; Plevritis, S.K. Risk-Based lung cancer screening: A systematic review. Lung Cancer 2020, 147, 154–186. [Google Scholar] [CrossRef] [PubMed]
- De Verdier, M.G. The Big Three Concept: A Way to Tackle the Health Care Crisis? Proc. Am. Thorac. Soc. 2008, 5, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, J.J.; Wisnivesky, J.P.; Henschke, C.I.; Yip, R.; Farooqi, A.O.; McCauley, D.I.; Chen, M.; Libby, D.M.; Smith, J.P.; Pasmantier, M.W.; et al. Emphysema Scores Predict Death from COPD and Lung Cancer. Chest 2012, 141, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Fan, K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events. Medicine 2018, 97, e10461. [Google Scholar] [CrossRef] [PubMed]
- Robbins, H.A.; Berg, C.D.; Cheung, L.C.; Chaturvedi, A.K.; Katki, H.A. Identification of Candidates for Longer Lung Cancer Screening Intervals Following a Negative Low-Dose Computed Tomography Result. J. Natl. Cancer Inst. 2019, 111, 996–999. [Google Scholar] [CrossRef]
- Lebrett, M.B.; Balata, H.; Evison, M.; Colligan, D.; Duerden, R.; Elton, P.; Greaves, M.; Howells, J.; Irion, K.; Karunaratne, D.; et al. Analysis of lung cancer risk model (PLCOM2012 and LLPv2) performance in a community-based lung cancer screening programme. Thorax 2020, 75, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Snowsill, T.; Yang, H.; Griffin, E.; Long, L.; Varley-Campbell, J.; Coelho, H.; Robinson, S.; Hyde, C. Low-dose computed tomography for lung cancer screening in high-risk populations: A systematic review and economic evaluation. Heal. Technol. Assess. 2018, 22, 1–276. [Google Scholar] [CrossRef]
- Kim, T.J.; Goo, J.M.; Lee, K.W.; Park, C.M.; Lee, H.J. Clinical, pathological and thin-section CT features of persistent multiple ground-glass opacity nodules: Comparison with solitary ground-glass opacity nodule. Lung Cancer 2009, 64, 171–178. [Google Scholar] [CrossRef] [PubMed]


| Variable | Type | Categories | Lung Cancer Patients (n = 163) | Lung-Cancer-Free Patients (n = 1178) |
|---|---|---|---|---|
| Control patient characteristics | ||||
| Age at earliest scan, years | Continuous | N/A | 64 (58–69) | 61 (52–70) |
| Sex (male) | Binary | 1 = Male; 0 = Female | 100 (61) | 685 (58) |
| Control CT features | ||||
| Emphysema | Binary | 1 = Mild, moderate, or severe; 0 = None | 88 (54) | 307 (26) |
| Bronchitis | Binary | 1 = Yes; 0 = No | 68 (42) | 287 (24) |
| Interstitial lung disease | Binary | 1 = Yes; 0 = No | 10 (6) | 19 (2) |
| Lymphadenopathy | Binary | 1 = Yes; 0 = No | 48 (29) | 198 (17) |
| Aortic calcifications | Binary | 1 = Mild, moderate, or severe; 0 = None | 107 (66) | 492 (42) |
| Coronary calcifications | Binary | 1 = Mild, moderate, or severe; 0 = None | 115 (71) | 582 (49) |
| Nodule present | Binary | 1 = Yes; 0 = No | 132 (81) | 522 (44) |
| Mean diameter of largest nodule, mm | Continuous | N/A | 9.0 (6.3–14.6) | 6.8 (5.0–12.4) |
| Nodule type variables | ||||
| Largest nodule is a SSN | Binary | 1 = Yes; 0 = No | 36/132 (27) | 102/522 (20) |
| Solid nodule present | Binary | 1 = Yes; 0 = No | 105 (64) | 365 (31) |
| Solid nodule count | Continuous | N/A | 1 (1–2) | 1 (1–3) |
| Part-solid nodule present | Binary | 1 = Yes; 0 = No | 18 (11) | 43 (4) |
| Part-solid nodule count | Continuous | N/A | 1 (1–1) | 1 (1–1) |
| Ground-glass nodule present | Binary | 1 = Yes; 0 = No | 31 (19) | 96 (8) |
| Ground-glass nodule count | Continuous | N/A | 1 (1–1) | 1 (1–2) |
| Perifissural nodule present | Binary | 1 = Yes; 0 = No | 16 (10) | 166 (14) |
| Perifissural nodule count | Continuous | N/A | 1 (1–1) | 1 (1–1) |
| Variable | Beta Coefficient | Hazard Ratio (95% Confidence Interval) | p Value |
|---|---|---|---|
| Patient characteristics | |||
| Age at earliest scan, per year (((x−39)/10)2) | 0.50255 | 1.65 (1.25 to 2.18) | <0.001 |
| Age at earliest scan, per year (((x−39)/10)3) | −0.13941 | 0.87 (0.82 to 0.93) | <0.001 |
| CT features excluding nodule type | |||
| Emphysema | 0.70903 | 2.03 (1.33 to 3.12) | 0.001 |
| Bronchitis | 0.39078 | 1.48 (0.95 to 2.29) | 0.08 |
| Interstitial lung disease | 1.24998 | 3.49 (1.36 to 8.97) | 0.009 |
| Lymphadenopathy | 0.54126 | 1.72 (1.06 to 2.93) | 0.028 |
| Aortic calcifications | 0.57696 | 1.78 (1.08 to 2.93) | 0.023 |
| Coronary calcifications | 0.51209 | 1.67 (1.04 to 2.69) | 0.035 |
| Nodule present | 1.12291 | 3.07 (1.54 to 6.13) | 0.001 |
| Nodule type information | |||
| Solid nodule count, per nodule | 1.39874 | 4.05 (1.30 to 12.63) | 0.016 |
| Solid nodule count, per nodule ((x + 1) × ln (x + 1)) | −0.68170 | 0.51 (0.31 to 0.83) | 0.007 |
| Part-solid nodule count, per nodule | 0.50634 | 1.66 (1.11 to 2.47) | 0.013 |
| Ground-glass nodule count, per nodule | 0.41971 | 1.52 (1.06 to 2.17) | 0.021 |
| Perifissural nodule present | −1.11383 | 0.33 (0.16 to 0.66) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreuder, A.; Prokop, M.; Scholten, E.T.; Mets, O.M.; Chung, K.; Mohamed Hoesein, F.A.A.; Jacobs, C.; Schaefer-Prokop, C.M. CT-Detected Subsolid Nodules: A Predictor of Lung Cancer Development at Another Location? Cancers 2021, 13, 2812. https://doi.org/10.3390/cancers13112812
Schreuder A, Prokop M, Scholten ET, Mets OM, Chung K, Mohamed Hoesein FAA, Jacobs C, Schaefer-Prokop CM. CT-Detected Subsolid Nodules: A Predictor of Lung Cancer Development at Another Location? Cancers. 2021; 13(11):2812. https://doi.org/10.3390/cancers13112812
Chicago/Turabian StyleSchreuder, Anton, Mathias Prokop, Ernst T. Scholten, Onno M. Mets, Kaman Chung, Firdaus A. A. Mohamed Hoesein, Colin Jacobs, and Cornelia M. Schaefer-Prokop. 2021. "CT-Detected Subsolid Nodules: A Predictor of Lung Cancer Development at Another Location?" Cancers 13, no. 11: 2812. https://doi.org/10.3390/cancers13112812
APA StyleSchreuder, A., Prokop, M., Scholten, E. T., Mets, O. M., Chung, K., Mohamed Hoesein, F. A. A., Jacobs, C., & Schaefer-Prokop, C. M. (2021). CT-Detected Subsolid Nodules: A Predictor of Lung Cancer Development at Another Location? Cancers, 13(11), 2812. https://doi.org/10.3390/cancers13112812







